Evaluation of Adjuvant Antibiotic Loaded Injectable Bio-Composite Material in Diabetic Foot Osteomyelitis and Charcot Foot Reconstruction
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
2.2. Inclusion Criteria
2.3. Patient Groups
2.4. Surgical Management
2.5. Cerament Instillation
2.6. Outcomes
2.7. Governance and Approval
2.8. Statistical Analysis
3. Results
3.1. Procedures
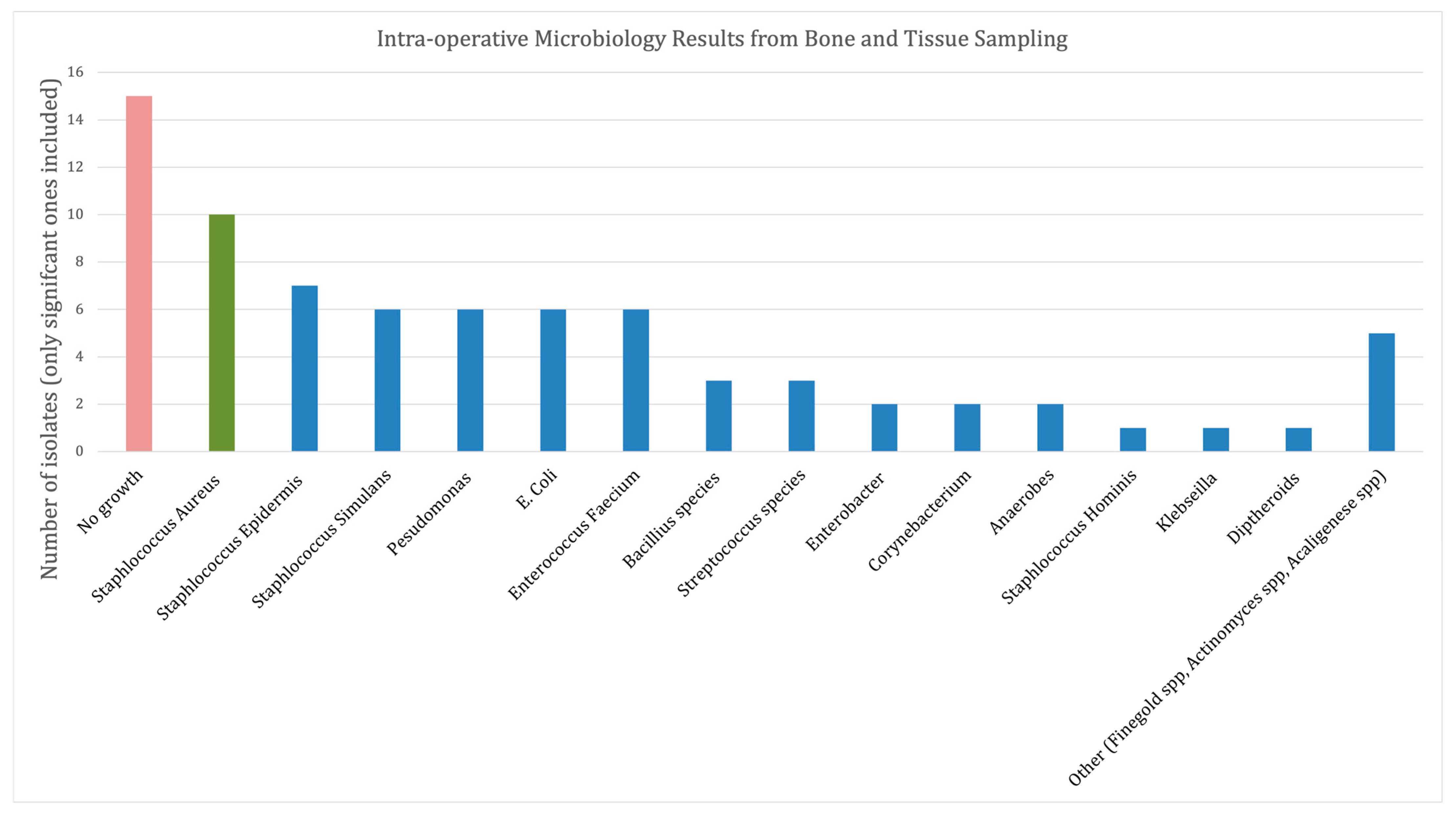
3.2. Microbiology
3.3. Cerament Use
4. Primary Outcome Measures
4.1. Infection Resolution and Ulcer Healing
4.2. Primary Bone Union
4.3. Post-Operative Infection Recurrence
5. Secondary Outcome Measures
5.1. Metalwork Infection
5.2. Ulcer Recurrence
5.3. Post-Operative Ambulation
5.4. Mortality
5.5. Limb Salvage
5.6. Further Procedures
6. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Take Home Message
- High proportion of patients achieving infection clearance and limb salvage in the surgical management of DFO with the use of antibiotic loaded Cerament;
- Cerament facilitates complex orthopaedic reconstruction in previously infected Charcot feet;
- Cerament usage allows shorter duration of systemic antibiotics following diabetic foot osteomyelitis surgery, even when the internal fixation metal work is used.
References
- Edmonds, M.; Manu, C.; Vas, P. The current burden of diabetic foot disease. J. Clin. Orthop. Trauma 2021, 17, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Evans, K.K.; Attinger, C.E.; Al-Attar, A.; Salgado, C.; Chu, C.K.; Mardini, S.; Neville, R. The importance of limb preservation in the diabetic population. J. Diabetes Complicat. 2011, 25, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Kroin, E.; Chaharbakhshi, E.O.; Schiff, A.; Pinzur, M.S. Improvement in Quality of Life Following Operative Correction of Midtarsal Charcot Foot Deformity. Foot Ankle Int. 2018, 39, 808–811. [Google Scholar] [CrossRef] [PubMed]
- Lazaro Martinez, J.L.; Garcia Alvarez, Y.; Tardaguila-Garcia, A.; Garcia Morales, E. Optimal management of diabetic foot osteomyelitis: Challenges and solutions. Diabetes Metab. Syndr. Obes. 2019, 12, 947–959. [Google Scholar] [CrossRef]
- Pinzur, M.S.; Schiff, A.P. Deformity and Clinical Outcomes Following Operative Correction of Charcot Foot: A New Classification With Implications for Treatment. Foot Ankle Int. 2018, 39, 265–270. [Google Scholar] [CrossRef]
- Markakis, K.; Faris, A.R.; Sharaf, H.; Faris, B.; Rees, S.; Bowling, F.L. Local Antibiotic Delivery Systems: Current and Future Applications for Diabetic Foot Infections. Int. J. Low Extrem. Wounds 2018, 17, 14–21. [Google Scholar] [CrossRef]
- McNally, M.A.; Ferguson, J.Y.; Lau, A.C.; Diefenbeck, M.; Scarborough, M.; Ramsden, A.J.; Atkins, B.L. Single-stage treatment of chronic osteomyelitis with a new absorbable, gentamicin-loaded, calcium sulphate/hydroxyapatite biocomposite: A prospective series of 100 cases. Bone Jt. J. 2016, 98, 1289–1296. [Google Scholar] [CrossRef]
- Niazi, N.S.; Drampalos, E.; Morrissey, N.; Jahangir, N.; Wee, A.; Pillai, A. Adjuvant antibiotic loaded bio composite in the management of diabetic foot osteomyelitis-A multicentre study. Foot 2019, 39, 22–27. [Google Scholar] [CrossRef]
- Vasukutty, N.; Mordecai, S.; Tarik, A.; Subramaniam, M.; Srinivasan, B. Limb salvage surgery in diabetic foot infection: Encouraging early results with a local antibiotic carrier. Diabet. Foot 2022, 25, 2. [Google Scholar]
- Bateman, A.H.; Bradford, S.; Hester, T.W.; Kubelka, I.; Tremlett, J.; Morris, V.; Pendry, E.; Kavarthapu, V.; Edmonds, M.E. Modern Orthopedic Inpatient Care of the Orthopedic Patient With Diabetic Foot Disease. Int. J. Low Extrem. Wounds 2015, 14, 384–392. [Google Scholar] [CrossRef]
- Ahluwalia, R.; Vainieri, E.; Tam, J.; Sait, S.; Sinha, A.; Manu, C.A.; Reichert, I.; Kavarthapu, V.; Edmonds, M.; Vas, P. Surgical Diabetic Foot Debridement: Improving Training and Practice Utilizing the Traffic Light Principle. Int. J. Low Extrem. Wounds 2019, 18, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Siebachmeyer, M.; Boddu, K.; Bilal, A.; Hester, T.W.; Hardwick, T.; Fox, T.P.; Edmonds, M.; Kavarthapu, V. Outcome of one-stage correction of deformities of the ankle and hindfoot and fusion in Charcot neuroarthropathy using a retrograde intramedullary hindfoot arthrodesis nail. Bone Jt. J. 2015, 97, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Vasukutty, N.; Jawalkar, H.; Anugraha, A.; Chekuri, R.; Ahluwalia, R.; Kavarthapu, V. Correction of ankle and hind foot deformity in Charcot neuroarthropathy using a retrograde hind foot nail-The Kings’ Experience. Foot Ankle Surg. 2018, 24, 406–410. [Google Scholar] [CrossRef]
- Kavarthapu, V.; Hester, T. Charcot hindfoot deformity reconstruction using a hindfoot nail—Surgical technique. J. Clin. Orthop. Trauma 2021, 16, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Kavarthapu, V.; Vris, A. Charcot midfoot reconstruction-surgical technique based on deformity patterns. Ann. Jt. 2020, 5, 28. [Google Scholar] [CrossRef]
- Kavarthapu, V.; Budair, B. Two-stage reconstruction of infected Charcot foot using internal fixation: A promising functional limb salvage technique. Bone Jt. J. 2021, 103, 1611–1618. [Google Scholar] [CrossRef]
- Drampalos, E.; Mohammad, H.R.; Kosmidis, C.; Balal, M.; Wong, J.; Pillai, A. Single stage treatment of diabetic calcaneal osteomyelitis with an absorbable gentamicin-loaded calcium sulphate/hydroxyapatite biocomposite: The Silo technique. Foot 2018, 34, 40–44. [Google Scholar] [CrossRef]
- Wee, J.; Thevendran, G. The role of orthobiologics in foot and ankle surgery: Allogenic bone grafts and bone graft substitutes. EFORT Open Rev. 2017, 2, 272–280. [Google Scholar] [CrossRef]
- Stravinskas, M.; Horstmann, P.; Ferguson, J.; Hettwer, W.; Nilsson, M.; Tarasevicius, S.; Petersen, M.M.; McNally, M.A.; Lidgren, L. Pharmacokinetics of gentamicin eluted from a regenerating bone graft substitute: In vitro and clinical release studies. Bone Jt. Res. 2016, 5, 427–435. [Google Scholar] [CrossRef]
- Stravinskas, M.; Nilsson, M.; Vitkauskiene, A.; Tarasevicius, S.; Lidgren, L. Vancomycin elution from a biphasic ceramic bone substitute. Bone Jt. Res. 2019, 8, 49–54. [Google Scholar] [CrossRef]
- Hutting, K.H.; Aan de Stegge, W.B.; van Netten, J.J.; Ten Cate, W.A.; Smeets, L.; Welten, G.; Scharn, D.M.; de Vries, J.P.M.; van Baal, J.G. Surgical Treatment of Diabetic Foot Ulcers Complicated by Osteomyelitis with Gentamicin-Loaded Calcium Sulphate-Hydroxyapatite Biocomposite. J. Clin. Med. 2021, 10, 371. [Google Scholar] [CrossRef] [PubMed]
- Wokhlu, A.; Vasukutty, N. Partial calcanectomy with antibiotic biocomposite injection for diabetes patients with heel ulcers and calcaneal osteomyelitis: A single stage treatment. Diabet. Foot 2021, 24, 2. [Google Scholar]
- Kummen, I.; Phyo, P.; Kavarthapu, V. Charcot foot reconstruction—How do hardware failure and non-union affect the clinical outcomes? Ann. Jt. 2020, 5, 25. [Google Scholar] [CrossRef]
- Nilsson, M.; Wang, J.S.; Wielanek, L.; Tanner, K.E.; Lidgren, L. Biodegradation and biocompatability of a calcium sulphate-hydroxyapatite bone substitute. J. Bone Jt. Surg. Br. 2004, 86, 120–125. [Google Scholar] [CrossRef]
- Lipsky, B.A.; Berendt, A.R.; Cornia, P.B.; Pile, J.C.; Peters, E.J.; Armstrong, D.G.; Deery, H.G.; Embil, J.M.; Joseph, W.S.; Karchmer, A.W. 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin. Infect. Dis. 2012, 54, e132–e173. [Google Scholar] [CrossRef] [PubMed]
- Lipsky, B.A.; Senneville, E.; Abbas, Z.G.; Aragon-Sanchez, J.; Diggle, M.; Embil, J.M.; Kono, S.; Lavery, L.A.; Malone, M.; van Asten, S.A.; et al. Guidelines on the diagnosis and treatment of foot infection in persons with diabetes (IWGDF 2019 update). Diabetes Metab. Res. Rev. 2020, 36 (Suppl. S1), e3280. [Google Scholar] [CrossRef]
- Dalla Paola, L.; Brocco, E.; Ceccacci, T.; Ninkovic, S.; Sorgentone, S.; Marinescu, M.G.; Volpe, A. Limb salvage in Charcot foot and ankle osteomyelitis: Combined use single stage/double stage of arthrodesis and external fixation. Foot Ankle Int. 2009, 30, 1065–1070. [Google Scholar] [CrossRef]
- Pinzur, M.S.; Gil, J.; Belmares, J. Treatment of osteomyelitis in charcot foot with single-stage resection of infection, correction of deformity, and maintenance with ring fixation. Foot Ankle Int. 2012, 33, 1069–1074. [Google Scholar] [CrossRef]
- Gariani, K.; Pham, T.T.; Kressmann, B.; Jornayvaz, F.R.; Gastaldi, G.; Stafylakis, D.; Philippe, J.; Lipsky, B.A.; Uckay, L. Three Weeks Versus Six Weeks of Antibiotic Therapy for Diabetic Foot Osteomyelitis: A Prospective, Randomized, Noninferiority Pilot Trial. Clin. Infect. Dis. 2021, 73, e1539–e1545. [Google Scholar] [CrossRef]
- Li, H.K.; Rombach, I.; Zambellas, R.; Walker, A.S.; McNally, M.A.; Atkins, B.L.; Lipsky, B.A.; Hughes, H.C.; Bose, D.; Kumin, M.; et al. Oral versus Intravenous Antibiotics for Bone and Joint Infection. N. Engl. J. Med. 2019, 380, 425–436. [Google Scholar] [CrossRef]
- Kelly, C.M.; Wilkins, R.M.; Gitelis, S.; Hartjen, C.; Watson, J.T.; Kim, P.T. The use of a surgical grade calcium sulfate as a bone graft substitute-Results of a multicenter trial. Clin. Orthop. Relat. Res. 2001, 382, 42–50. [Google Scholar] [CrossRef]
- Manas, A.B.; Taori, S.; Ahluwalia, R.; Slim, H.; Manu, C.; Rashid, H.; Kavarthapu, V.; Edmonds, M.; Vas, P.R.J. Admission Time Deep Swab Specimens Compared With Surgical Bone Sampling in Hospitalized Individuals With Diabetic Foot Osteomyelitis and Soft Tissue Infection. Int. J. Low Extrem. Wounds 2021, 20, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Jeffcoate, W.; Young, B. National Diabetic Foot Audit of England and Wales yields its first dividends. Diabet. Med. 2016, 33, 1464–1465. [Google Scholar] [CrossRef] [PubMed]
- Jeffcoate, W.J.; Vileikyte, L.; Boyko, E.J.; Armstrong, D.G.; Boulton, A.J.M. Current Challenges and Opportunities in the Prevention and Management of Diabetic Foot Ulcers. Diabetetes Care 2018, 41, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Vainieri, E.; Ahluwalia, R.; Slim, H.; Walton, D.; Manu, C.; Taori, S.; Wilkins, J.; Huang, D.Y.; Edmonds, M.; Rashid, H.; et al. Outcomes after Emergency Admission with a Diabetic Foot Attack Indicate a High Rate of Healing and Limb Salvage But Increased Mortality: 18-Month Follow-up Study. Exp. Clin. Endocrinol. Diabetes 2022, 130, 165–171. [Google Scholar] [CrossRef]
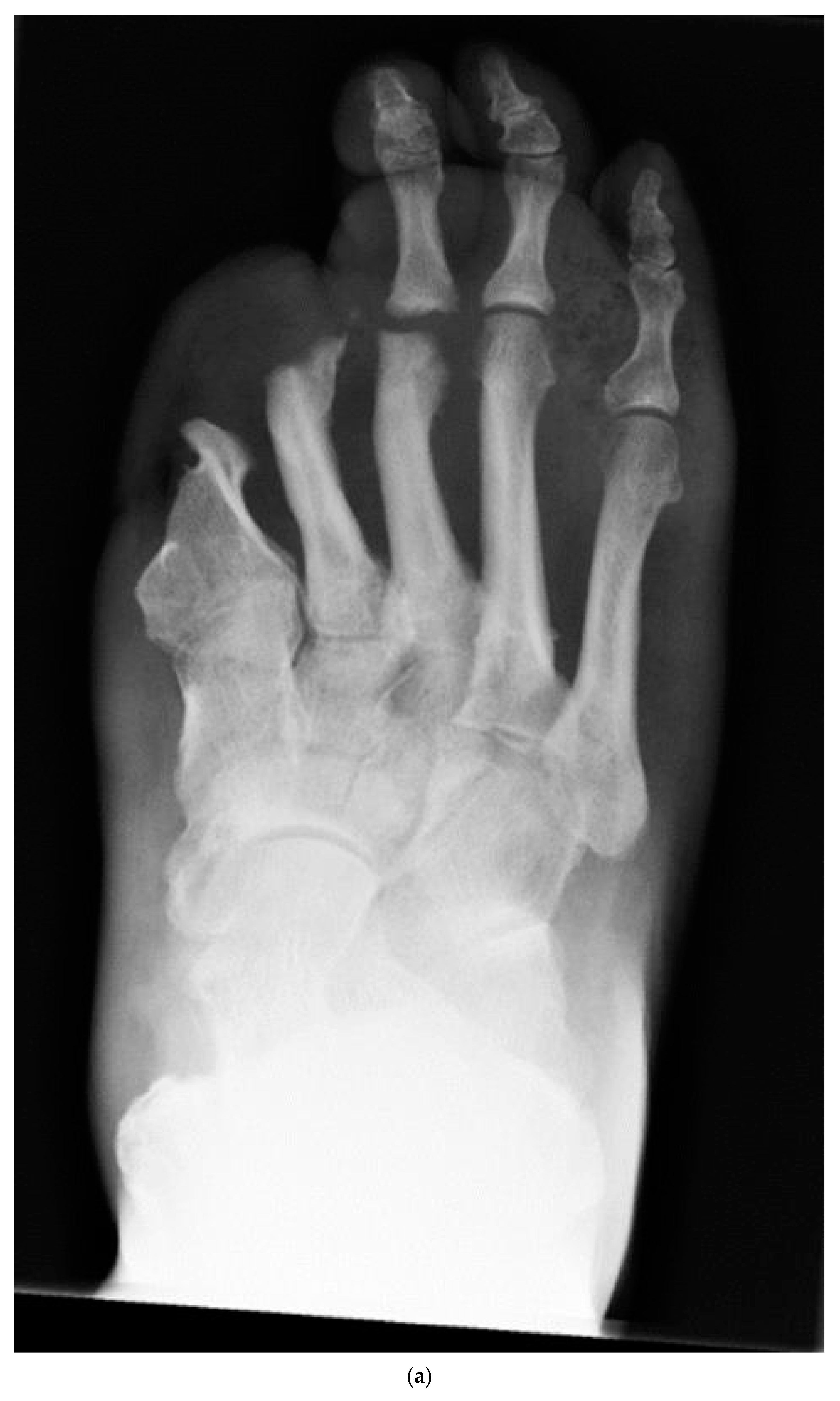
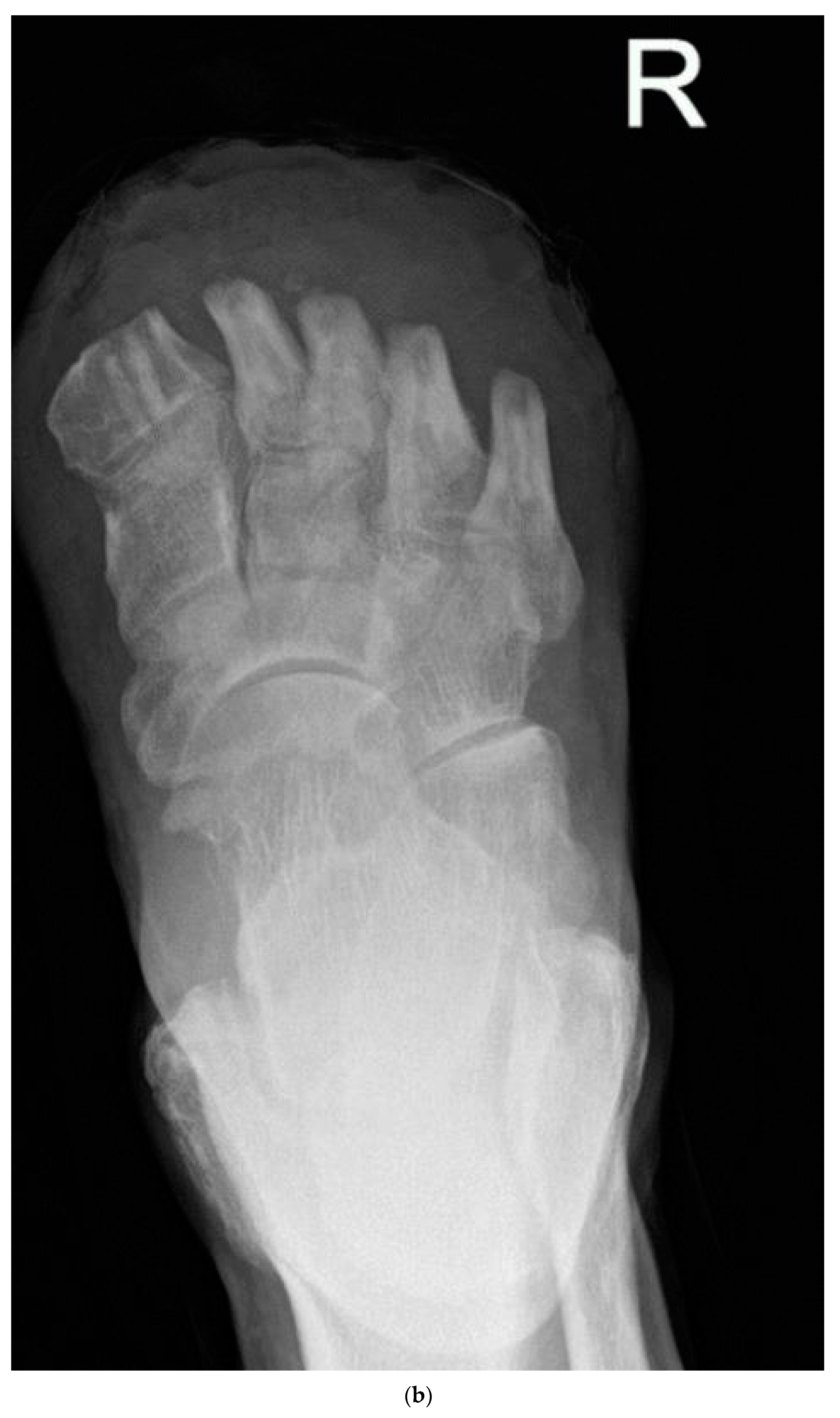
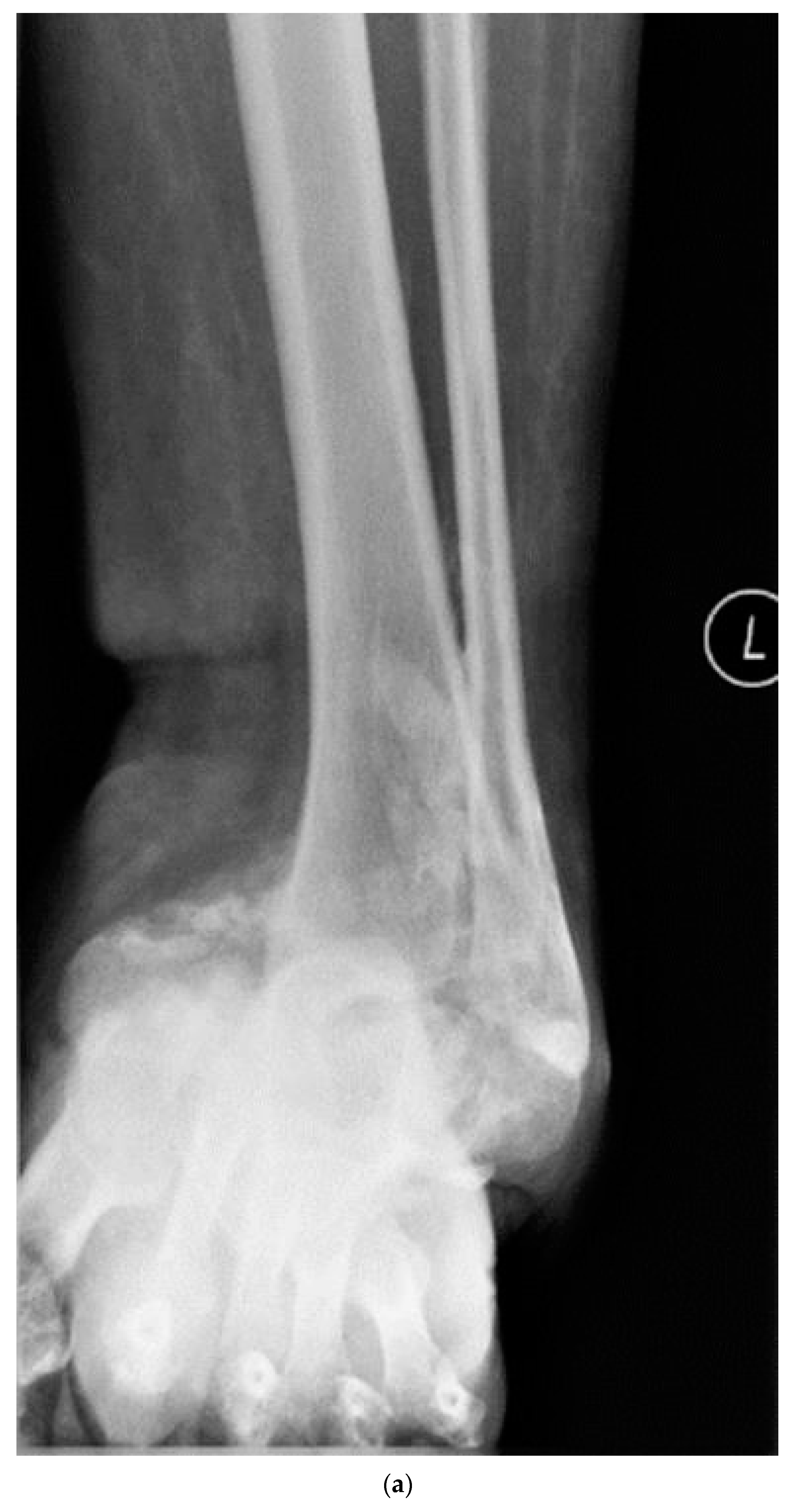

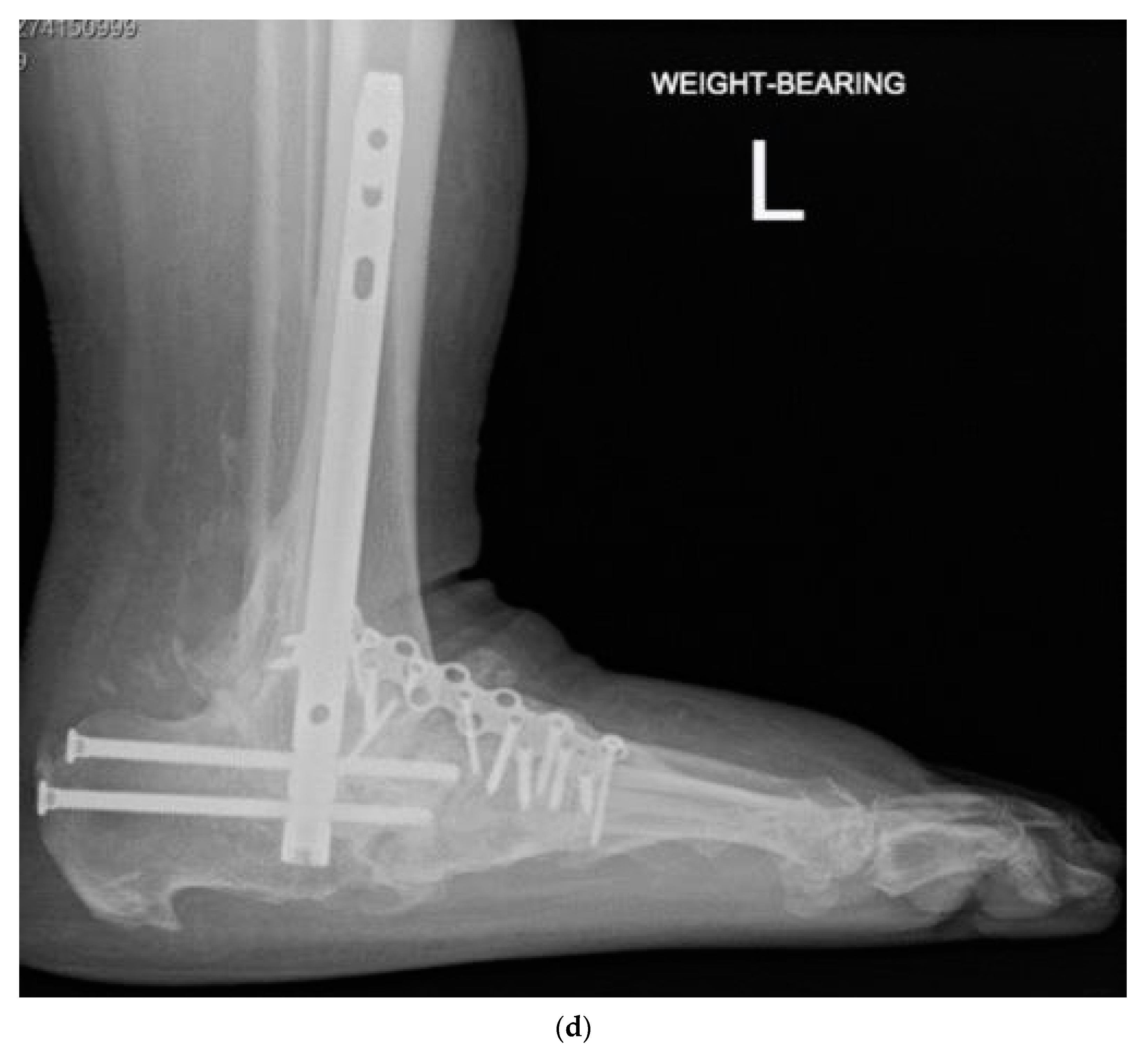
| Group 1 | Group 2a | Group 2b | One-Way ANOVA | |
|---|---|---|---|---|
| Number of patients (n=) | 17 | 19 | 17 | NS |
| Number of feet operated upon (n=) | 17 | 19 | 18 | NS |
| Number of patients lost to follow up | 0 | 1 | 0 | NS |
| Number of Males (n=) | 12 | 11 | 8 | NS |
| Mean age (years) | 55.7 | 55.7 | 55.8 | NS |
| Mean BMI (kg/m2) | 28.9 | 33.3 | 31.7 | NS |
| Number of pre–operative ulcers (n=) | 16 | 8 | 13 | NS |
| Mean ASA | 2.4 | 2.5 | 2.6 | NS |
| Retinopathy | 9 | 9 | 9 | NS |
| Nephropathy (eGFR <30 mL/min) | 4 | 7 | 6 | NS |
| Renal Dialysis | 0 | 2 | 2 | NS |
| Preceding revascularisation (n=) | 0 | 3 | 4 | NS |
| Pre-operative Mobility: | ||||
| Independent | 8 | 3 | 1 | p < 0.05 |
| Stick | 3 | 6 | 4 | NS |
| Wheelchair | 6 | 10 | 12 | NS |
| Group 1A | Group 2a | Group 2b | One-Way ANOVA | |
|---|---|---|---|---|
| Primary Bone Union | NA | 13 | 15 | NS |
| Non-Unions | NA | 6 | 3 | NS |
| Post-operative Deep Infection | None | None | 1 | NS |
| Non healing ulcer | 2 | None | None | NS |
| New Ulcer Formation (not at index site) | None | 2 | 3 | NS |
| Mortality within each group | 2 | 1 | 3 | NS |
| Mean post-operative Haemoglobin | 109 | 98 | 103 | NS |
| Post-operative ambulatory status: | NS | |||
| Independent in orthotic shoes | 10 | 13 | 13 | NS |
| Independent in a bivalve cast | 1 | 3 | 3 | NS |
| Partial weight bearing | 3 | 1 | 1 | NS |
| Non weight bearing/wheelchair | 1 | 1 | 1 | NS |
| Case | Single/Two Stage | Reconstruction Location | Smoking Status | Clinical Stability | Further Procedures |
|---|---|---|---|---|---|
| 1 | Single | Midfoot | Ex-Smoker | Stable | Removal of metalwork—bolt to prevent further ulceration |
| 2 | Single | Mid and hindfoot | No | Unstable | Exostectomy |
| 3 | Single | Midfoot | Ex-smoker | Stable | None |
| 4 | Single | Mid and hindfoot | No | Stable | None |
| 5 | Single | Hindfoot | Yes | Unstable | Removal of broken nail and revision hindfoot fusion |
| 6 | Single | Midfoot | No | Stable | None |
| 7 | Two stage | Mid and hindfoot | No | Unstable | Revision of hindfoot nail and ulcer debridement |
| 8 | Two stage | Mid and hindfoot | Yes | Stable | None |
| 9 | Two stage | Midfoot | Yes | Infected non union | Removal of metalwork and repeat stage 1 procedure due to deep infection |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kavarthapu, V.; Giddie, J.; Kommalapati, V.; Casey, J.; Bates, M.; Vas, P. Evaluation of Adjuvant Antibiotic Loaded Injectable Bio-Composite Material in Diabetic Foot Osteomyelitis and Charcot Foot Reconstruction. J. Clin. Med. 2023, 12, 3239. https://doi.org/10.3390/jcm12093239
Kavarthapu V, Giddie J, Kommalapati V, Casey J, Bates M, Vas P. Evaluation of Adjuvant Antibiotic Loaded Injectable Bio-Composite Material in Diabetic Foot Osteomyelitis and Charcot Foot Reconstruction. Journal of Clinical Medicine. 2023; 12(9):3239. https://doi.org/10.3390/jcm12093239
Chicago/Turabian StyleKavarthapu, Venu, Jasdeep Giddie, Varun Kommalapati, Joanne Casey, Maureen Bates, and Prashanth Vas. 2023. "Evaluation of Adjuvant Antibiotic Loaded Injectable Bio-Composite Material in Diabetic Foot Osteomyelitis and Charcot Foot Reconstruction" Journal of Clinical Medicine 12, no. 9: 3239. https://doi.org/10.3390/jcm12093239
APA StyleKavarthapu, V., Giddie, J., Kommalapati, V., Casey, J., Bates, M., & Vas, P. (2023). Evaluation of Adjuvant Antibiotic Loaded Injectable Bio-Composite Material in Diabetic Foot Osteomyelitis and Charcot Foot Reconstruction. Journal of Clinical Medicine, 12(9), 3239. https://doi.org/10.3390/jcm12093239






