Air Pollution and Perinatal Mental Health: A Comprehensive Overview
Abstract
1. Introduction
2. Materials and Methods
3. Results
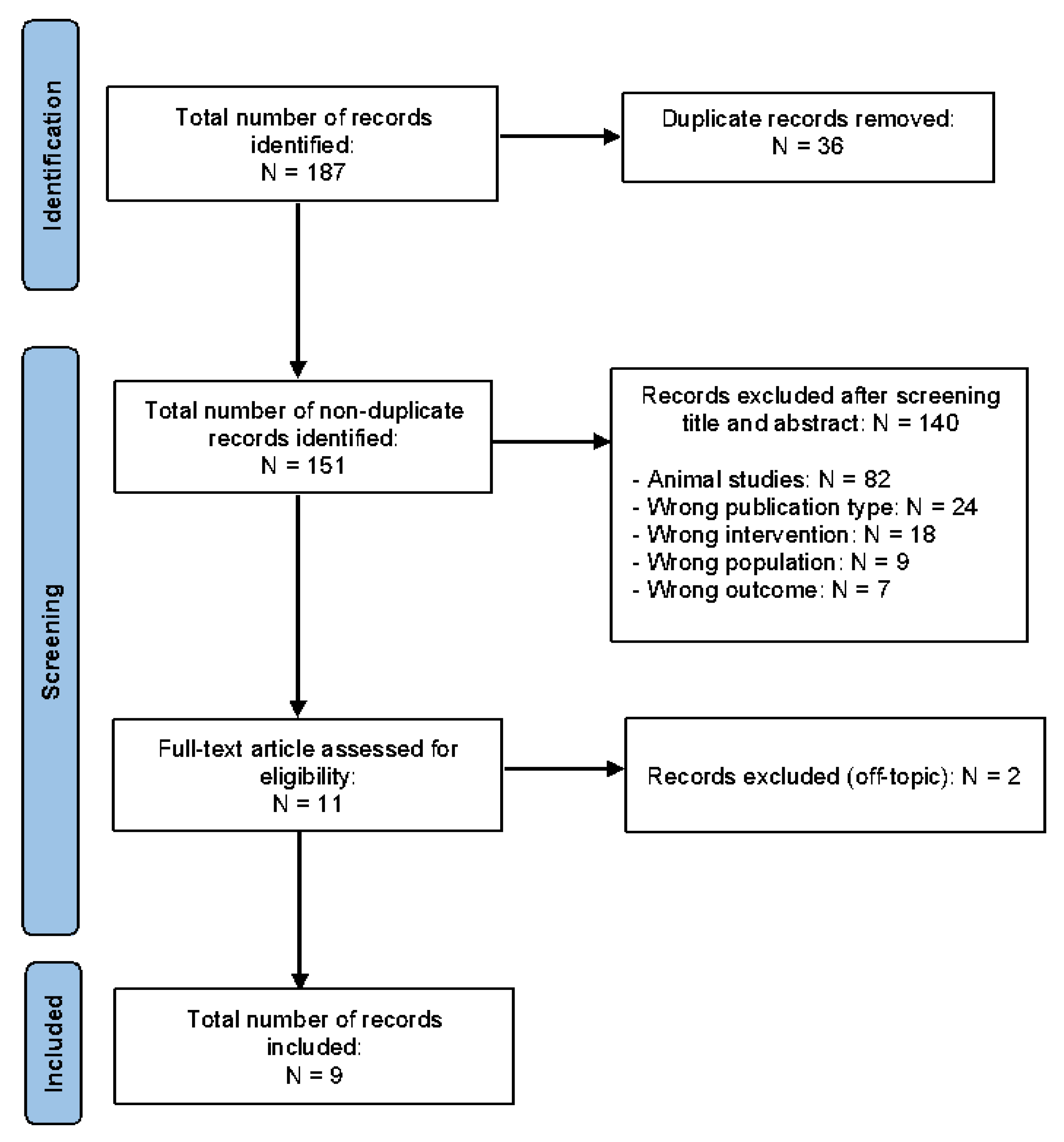
3.1. Literature Retrieval and Study Characteristics
3.2. Air Pollution Exposure and Maternal Depression
3.3. Air Pollution and Maternal Stress
3.4. Air Pollution and Other Mental Disorders
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tripathy, P. A Public Health Approach to Perinatal Mental Health: Improving Health and Wellbeing of Mothers and Babies. J. Gynecol. Obstet. Hum. Reprod. 2020, 49, 101747. [Google Scholar] [CrossRef]
- Rodriguez-Cabezas, L.; Clark, C. Psychiatric Emergencies in Pregnancy and Postpartum. Clin. Obstet. Gynecol. 2018, 61, 615–627. [Google Scholar] [CrossRef]
- D’Ascoli, P.T.; Alexander, G.R.; Petersen, D.J.; Kogan, M.D. Parental factors influencing patterns of prenatal care utilization. J. Perinatol. 1997, 17, 283–287. [Google Scholar]
- Barrow, K.; McGreal, A.; LiVecche, D.; Van Cleve, S.; Sikes, C.; Buoli, M.; Serati, M.; Bridges, C.C.; Ezeamama, A.; Barkin, J.L. Are Pediatric Providers On-Board With Current Recommendations Related to Maternal Mental Health Screening at Well-Child Visits in the State of Georgia? J. Am. Psychiatr. Nurses. Assoc. 2022, 28, 444–454. [Google Scholar] [CrossRef]
- Woody, C.A.; Ferrari, A.J.; Siskind, D.J.; Whiteford, H.A.; Harris, M.G. A Systematic Review and Meta-Regression of the Prevalence and Incidence of Perinatal Depression. J. Affect. Disord. 2017, 219, 86–92. [Google Scholar] [CrossRef]
- Silva, M.M.D.J.; Nogueira, D.A.; Clapis, M.J.; Leite, E.P.R.C. Anxiety in pregnancy: Prevalence and associated factors. Rev. Esc. Enferm. USP 2017, 51, e03253. [Google Scholar] [CrossRef] [PubMed]
- Bergink, V.; Rasgon, N.; Wisner, K.L. Postpartum Psychosis: Madness, Mania, and Melancholia in Motherhood. Am. J. Psychiatry 2016, 173, 1179–1188. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, M.W.; Wisner, K.L. Perinatal Mental Illness: Definition, Description and Aetiology. Best Pract. Res. Clin. Obstet. Gynaecol. 2014, 28, 3–12. [Google Scholar] [CrossRef]
- Buoli, M.; Grassi, S.; Iodice, S.; Carnevali, G.S.; Esposito, C.M.; Tarantini, L.; Barkin, J.L.; Bollati, V. The Role of Clock Genes in Perinatal Depression: The Light in the Darkness. Acta Psychiatr. Scand. 2019, 140, 382–384. [Google Scholar] [CrossRef] [PubMed]
- Carnevali, G.S.; Buoli, M. The Role of Epigenetics in Perinatal Depression: Are There Any Candidate Biomarkers? J. Affect. Disord. 2021, 280, 57–67. [Google Scholar] [CrossRef]
- Serati, M.; Redaelli, M.; Buoli, M.; Altamura, A.C. Perinatal Major Depression Biomarkers: A Systematic Review. J. Affect. Disord. 2016, 193, 391–404. [Google Scholar] [CrossRef] [PubMed]
- Cooklin, A.R.; Canterford, L.; Strazdins, L.; Nicholson, J.M. Employment Conditions and Maternal Postpartum Mental Health: Results from the Longitudinal Study of Australian Children. Arch. Womens Ment. Health 2011, 14, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Orru, H.; Ebi, K.L.; Forsberg, B. The Interplay of Climate Change and Air Pollution on Health. Curr. Environ. Health Rep. 2017, 4, 504–513. [Google Scholar] [CrossRef]
- Abbaffati, C. GBD 2019 Risk Factors Collaborators. Global burden of 87 risk factors in 204 countries and territories, 1990-2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1223–1249. [Google Scholar] [CrossRef]
- Cohen, A.J.; Brauer, M.; Burnett, R.; Anderson, H.R.; Frostad, J.; Estep, K.; Balakrishnan, K.; Brunekreef, B.; Dandona, L.; Dandona, R.; et al. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: An analysis of data from the Global Burden of Diseases Study 2015. Lancet 2017, 389, 1907–1918. [Google Scholar] [CrossRef]
- Bowe, B.; Xie, Y.; Yan, Y.; Al-Aly, Z. Burden of Cause-Specific Mortality Associated With PM 2.5 Air Pollution in the United States. JAMA Netw. Open 2019, 2, e1915834. [Google Scholar] [CrossRef]
- Delgado-Saborit, J.M.; Guercio, V.; Gowers, A.M.; Shaddick, G.; Fox, N.C.; Love, S. A critical review of the epidemiological evidence of effects of air pollution on dementia, cognitive function and cognitive decline in adult population. Sci. Total Environ. 2021, 757, 143734. [Google Scholar] [CrossRef]
- Buoli, M.; Grassi, S.; Caldiroli, A.; Carnevali, G.S.; Mucci, F.; Iodice, S.; Cantone, L.; Pergoli, L.; Bollati, V. Is There a Link between Air Pollution and Mental Disorders? Environ. Int. 2018, 118, 154–168. [Google Scholar] [CrossRef] [PubMed]
- Borroni, E.; Pesatori, A.C.; Bollati, V.; Buoli, M.; Carugno, M. Air Pollution Exposure and Depression: A Comprehensive Updated Systematic Review and Meta-Analysis. Environ. Pollut. 2022, 292, 118245. [Google Scholar] [CrossRef]
- Costa, L.G.; Cole, T.B.; Coburn, J.; Chang, Y.-C.; Dao, K.; Roqué, P.J. Neurotoxicity of Traffic-Related Air Pollution. NeuroToxicology 2017, 59, 133–139. [Google Scholar] [CrossRef]
- Monti, P.; Iodice, S.; Tarantini, L.; Sacchi, F.; Ferrari, L.; Ruscica, M.; Buoli, M.; Vigna, L.; Pesatori, A.C.; Bollati, V. Effects of PM Exposure on the Methylation of Clock Genes in A Population of Subjects with Overweight or Obesity. Int. J. Environ. Res. Public Health 2021, 18, 1122. [Google Scholar] [CrossRef]
- Sato, F.; Kohsaka, A.; Bhawal, U.; Muragaki, Y. Potential Roles of Dec and Bmal1 Genes in Interconnecting Circadian Clock and Energy Metabolism. Int. J. Mol. Sci. 2018, 19, 781. [Google Scholar] [CrossRef]
- Lopez-Minguez, J.; Gómez-Abellán, P.; Garaulet, M. Circadian rhythms, food timing and obesity. Proc. Nutr. Soc. 2016, 75, 501–511. [Google Scholar] [CrossRef]
- Borroni, E.; Pesatori, A.C.; Nosari, G.; Monti, P.; Ceresa, A.; Fedrizzi, P.; Bollati, V.; Buoli, M.; Carugno, M. Understanding the Interplay between Air Pollution, Biological Variables, and Major Depressive Disorder: Rationale and Study Protocol of the DeprAir Study. Int. J. Environ. Res. Public Health 2023, 20, 5196. [Google Scholar] [CrossRef]
- Iodice, S.; Di Paolo, M.; Barkin, J.L.; Tarantini, L.; Grassi, S.; Redaelli, M.; Serati, M.; Favalli, V.; Cirella, L.; Bollati, V.; et al. The Methylation of Clock Genes in Perinatal Depression: Which Role for Oxytocin? Front. Psychiatry 2021, 12, 734825. [Google Scholar] [CrossRef]
- Serati, M.; Esposito, C.M.; Grassi, S.; Bollati, V.; Barkin, J.L.; Buoli, M. The Association between Plasma ERVWE1 Concentrations and Affective Symptoms during Pregnancy: Is This a Friendly Alien? Int. J. Environ. Res. Public Health 2020, 17, 9217. [Google Scholar] [CrossRef]
- Polo-Kantola, P.; Aukia, L.; Karlsson, H.; Paavonen, E.J. Sleep quality during pregnancy: Associations with depressive and anxiety symptoms. Acta Obstet. Gynecol. Scand. 2017, 96, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Buemann, B. Oxytocin Release: A Remedy for Cerebral Inflammaging. Curr. Aging Sci. 2022, 15, 218–228. [Google Scholar] [CrossRef]
- Duthie, L.; Reynolds, R.M. Changes in the maternal hypothalamic-pituitary-adrenal axis in pregnancy and postpartum: Influences on maternal and fetal outcomes. Neuroendocrinology 2013, 98, 106–115. [Google Scholar] [CrossRef]
- Glynn, L.M.; Davis, E.P.; Sandman, C.A. New insights into the role of perinatal HPA-axis dysregulation in postpartum depression. Neuropeptides 2013, 47, 363–370. [Google Scholar] [CrossRef]
- Kritas, S.K.; Saggini, A.; Cerulli, G.; Caraffa, A.; Antinolfi, P.; Pantalone, A.; Rosati, M.; Tei, M.; Speziali, A.; Saggini, R.; et al. Corticotropin-releasing hormone, microglia and mental disorders. Int. J. Immunopathol. Pharmacol. 2014, 27, 163–167. [Google Scholar] [CrossRef]
- Frank, M.G.; Thompson, B.M.; Watkins, L.R.; Maier, S.F. Glucocorticoids mediate stress-induced priming of microglial pro-inflammatory responses. Brain Behav. Immun. 2012, 26, 337–345. [Google Scholar] [CrossRef]
- Leliavski, A.; Dumbell, R.; Ott, V.; Oster, H. Adrenal clocks and the role of adrenal hormones in the regulation of circadian physiology. J. Biol. Rhythm. 2015, 30, 20–34. [Google Scholar] [CrossRef]
- Snow, S.J.; Henriquez, A.R.; Costa, D.L.; Kodavanti, U.P. Neuroendocrine Regulation of Air Pollution Health Effects: Emerging Insights. Toxicol. Sci. 2018, 164, 9–20. [Google Scholar] [CrossRef]
- Pun, V.C.; Manjourides, J.; Suh, H. Association of Ambient Air Pollution with Depressive and Anxiety Symptoms in Older Adults: Results from the NSHAP Study. Environ. Health Perspect. 2017, 125, 342–348. [Google Scholar] [CrossRef]
- Sidebottom, A.C.; Hellerstedt, W.L.; Harrison, P.A.; Hennrikus, D. An examination of prenatal and postpartum depressive symptoms among women served by urban community health centers. Arch. Womens Ment. Health 2014, 17, 27–40. [Google Scholar] [CrossRef]
- Calderón-Garcidueñas, L.; Calderón-Garcidueñas, A.; Torres-Jardón, R.; Avila-Ramírez, J.; Kulesza, R.J.; Angiulli, A.D. Air Pollution and Your Brain: What Do You Need to Know Right Now. Prim. Health Care Res. Dev. 2015, 16, 329–345. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. The PRISMA Group Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Banti, S.; Mauri, M.; Oppo, A.; Borri, C.; Rambelli, C.; Ramacciotti, D.; Montagnani, M.S.; Camilleri, V.; Cortopassi, S.; Rucci, P.; et al. From the Third Month of Pregnancy to 1 Year Postpartum. Prevalence, Incidence, Recurrence, and New Onset of Depression. Results from the Perinatal Depression–Research & Screening Unit Study. Compr. Psychiatry 2011, 52, 343–351. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 Explanation and Elaboration: Updated Guidance and Exemplars for Reporting Systematic Reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Armijo-Olivo, S.; Stiles, C.R.; Hagen, N.A.; Biondo, P.D.; Cummings, G.G. Assessment of Study Quality for Systematic Reviews: A Comparison of the Cochrane Collaboration Risk of Bias Tool and the Effective Public Health Practice Project Quality Assessment Tool: Methodological Research: Quality Assessment for Systematic Reviews. J. Eval. Clin. Pract. 2012, 18, 12–18. [Google Scholar] [CrossRef]
- Örün, E.; Yalçın, S.S.; Aykut, O.; Orhan, G.; Morgil, G.K.; Yurdakök, K.; Uzun, R. Breast Milk Lead and Cadmium Levels from Suburban Areas of Ankara. Sci. Total Environ. 2011, 409, 2467–2472. [Google Scholar] [CrossRef]
- Sheffield, P.E.; Speranza, R.; Chiu, Y.-H.M.; Hsu, H.-H.L.; Curtin, P.C.; Renzetti, S.; Pajak, A.; Coull, B.; Schwartz, J.; Kloog, I.; et al. Association between Particulate Air Pollution Exposure during Pregnancy and Postpartum Maternal Psychological Functioning. PLoS ONE 2018, 13, e0195267. [Google Scholar] [CrossRef]
- Vuong, A.M.; Yolton, K.; Braun, J.M.; Sjodin, A.; Calafat, A.M.; Xu, Y.; Dietrich, K.N.; Lanphear, B.P.; Chen, A. Polybrominated Diphenyl Ether (PBDE) and Poly- and Perfluoroalkyl Substance (PFAS) Exposures during Pregnancy and Maternal Depression. Environ. Int. 2020, 139, 105694. [Google Scholar] [CrossRef]
- Bastain, T.M.; Chavez, T.; Habre, R.; Hernandez-Castro, I.; Grubbs, B.; Toledo-Corral, C.M.; Farzan, S.F.; Lurvey, N.; Lerner, D.; Eckel, S.P.; et al. Prenatal Ambient Air Pollution and Maternal Depression at 12 Months Postpartum in the MADRES Pregnancy Cohort. Environ. Health 2021, 20, 121. [Google Scholar] [CrossRef]
- Lamichhane, D.K.; Jung, D.-Y.; Shin, Y.-J.; Lee, K.-S.; Lee, S.-Y.; Ahn, K.; Kim, K.W.; Shin, Y.H.; Suh, D.I.; Hong, S.-J.; et al. Association between Ambient Air Pollution and Perceived Stress in Pregnant Women. Sci. Rep. 2021, 11, 23496. [Google Scholar] [CrossRef]
- Shih, P.; Wu, C.-D.; Chiang, T.; Chen, P.-C.; Su, T.-C.; Cheng, T.-J.; Chen, Y.-H.; Guo, Y.L. The Association between Postpartum Depression and Air Pollution during Pregnancy and Postpartum Period: A National Population Study in Taiwan. Environ. Res. Lett. 2021, 16, 084021. [Google Scholar] [CrossRef]
- Duan, C.-C.; Li, C.; Xu, J.-J.; He, Y.-C.; Xu, H.-L.; Zhang, D.; Yang, J.-Q.; Yu, J.-L.; Zeng, W.-T.; Wang, Y.; et al. Association between Prenatal Exposure to Ambient Air Pollutants and Postpartum Depressive Symptoms: A Multi-City Cohort Study. Environ. Res. 2022, 209, 112786. [Google Scholar] [CrossRef]
- Niedzwiecki, M.M.; Rosa, M.J.; Solano-González, M.; Kloog, I.; Just, A.C.; Martínez-Medina, S.; Schnaas, L.; Tamayo-Ortiz, M.; Wright, R.O.; Téllez-Rojo, M.M.; et al. Particulate Air Pollution Exposure during Pregnancy and Postpartum Depression Symptoms in Women in Mexico City. Environ. Int. 2020, 134, 105325. [Google Scholar] [CrossRef]
- Zhang, Y.; Tayarani, M.; Wang, S.; Liu, Y.; Sharma, M.; Joly, R.; RoyChoudhury, A.; Hermann, A.; Gao, O.H.; Pathak, J. Identifying Urban Built Environment Factors in Pregnancy Care and Maternal Mental Health Outcomes. BMC Pregnancy Childbirth 2021, 21, 599. [Google Scholar] [CrossRef]
- Jin, M.; Yin, J.; Zheng, Y.; Shen, X.; Li, L.; Jin, M. Pollution Characteristics and Sources of Polybrominated Diphenyl Ethers in Indoor Air and Dustfall Measured in University Laboratories in Hangzhou, China. Sci. Total Environ. 2018, 624, 201–209. [Google Scholar] [CrossRef]
- Jin, M.; Zhang, S.; He, J.; Lu, Z.; Zhou, S.; Ye, N. Polybrominated Diphenyl Ethers from Automobile Microenvironment: Occurrence, Sources, and Exposure Assessment. Sci. Total Environ. 2021, 781, 146658. [Google Scholar] [CrossRef]
- Beristain-Montiel, E.; Villalobos-Pietrini, R.; Nuñez-Vilchis, A.; Arias-Loaiza, G.E.; Hernández-Paniagua, I.Y.; Amador-Muñoz, O. Polybrominated Diphenyl Ethers and Organochloride Pesticides in the Organic Matter of Air Suspended Particles in Mexico Valley: A Diagnostic to Evaluate Public Policies. Environ. Pollut. 2020, 267, 115637. [Google Scholar] [CrossRef]
- Wang, Q.; Ruan, Y.; Zhao, Z.; Zhang, L.; Hua, X.; Jin, L.; Chen, H.; Wang, Y.; Yao, Y.; Lam, P.K.S.; et al. Per- and Polyfluoroalkyl Substances (PFAS) in the Three-North Shelter Forest in Northern China: First Survey on the Effects of Forests on the Behavior of PFAS. J. Hazard. Mater. 2022, 427, 128157. [Google Scholar] [CrossRef]
- Grandjean, P.; Timmermann, C.A.G.; Kruse, M.; Nielsen, F.; Vinholt, P.J.; Boding, L.; Heilmann, C.; Mølbak, K. Severity of COVID-19 at Elevated Exposure to Perfluorinated Alkylates. PLoS ONE 2020, 15, e0244815. [Google Scholar] [CrossRef]
- Makey, C.M.; McClean, M.D.; Braverman, L.E.; Pearce, E.N.; He, X.-M.; Sjödin, A.; Weinberg, J.M.; Webster, T.F. Polybrominated Diphenyl Ether Exposure and Thyroid Function Tests in North American Adults. Environ. Health Perspect. 2016, 124, 420–425. [Google Scholar] [CrossRef]
- Cerquiglini Monteriolo, S.; D’Innocenzio, F. Environmental pollution due to lead and cadmium: Data from air sampling. Ann. Ist. Super. Sanita 1985, 21, 11–17. [Google Scholar]
- Absalon, D.; Ślesak, B. The Effects of Changes in Cadmium and Lead Air Pollution on Cancer Incidence in Children. Sci. Total Environ. 2010, 408, 4420–4428. [Google Scholar] [CrossRef]
- Meng, X.; Jiang, J.; Chen, T.; Zhang, Z.; Lu, B.; Liu, C.; Xue, L.; Chen, J.; Herrmann, H.; Li, X. Chemical drivers of ozone change in extreme temperatures in eastern China. Sci. Total Environ. 2023, 874, 162424. [Google Scholar] [CrossRef]
- Kosaka, H.; Uozumi, M.; Tyuma, I. The Interaction between Nitrogen Oxides and Hemoglobin and Endothelium-Derived Relaxing Factor. Free Radic. Biol. Med. 1989, 7, 653–658. [Google Scholar] [CrossRef]
- Wu, T.; Xu, S.; Chen, B.; Bao, L.; Ma, J.; Han, W.; Xu, A.; Yu, K.N.; Wu, L.; Chen, S. Ambient PM2.5 Exposure Causes Cellular Senescence via DNA Damage, Micronuclei Formation, and CGAS Activation. Nanotoxicology 2022, 16, 757–775. [Google Scholar] [CrossRef] [PubMed]
- Jayaraj, R.L.; Rodriguez, E.A.; Wang, Y.; Block, M.L. Outdoor Ambient Air Pollution and Neurodegenerative Diseases: The Neuroinflammation Hypothesis. Curr. Environ. Health Rep. 2017, 4, 166–179. [Google Scholar] [CrossRef] [PubMed]
- Altamura, A.C.; Buoli, M.; Pozzoli, S. Role of Immunological Factors in the Pathophysiology and Diagnosis of Bipolar Disorder: Comparison with Schizophrenia: A Comprehensive Review. Psychiatr. Clin. Neurosci. 2014, 68, 21–36. [Google Scholar] [CrossRef]
- Cugurra, A.; Mamuladze, T.; Rustenhoven, J.; Dykstra, T.; Beroshvili, G.; Greenberg, Z.J.; Baker, W.; Papadopoulos, Z.; Drieu, A.; Blackburn, S.; et al. Skull and Vertebral Bone Marrow Are Myeloid Cell Reservoirs for the Meninges and CNS Parenchyma. Science 2021, 373, eabf7844. [Google Scholar] [CrossRef]
- Pope, C.A.; Bhatnagar, A.; McCracken, J.P.; Abplanalp, W.; Conklin, D.J.; O’toole, T. Exposure to Fine Particulate Air Pollution Is Associated With Endothelial Injury and Systemic Inflammation. Circ. Res. 2016, 119, 1204–1214. [Google Scholar] [CrossRef]
- Tsai, D.H.; Riediker, M.; Berchet, A.; Paccaud, F.; Waeber, G.; Vollenweider, P.; Bochud, M. Effects of short- and long-term exposures to particulate matter on inflammatory marker levels in the general population. Environ. Sci. Pollut. Res. Int. 2019, 26, 19697–19704. [Google Scholar] [CrossRef]
- Sawyer, K.M. The Role of Inflammation in the Pathogenesis of Perinatal Depression and Offspring Outcomes. Brain Behav. Immun. Health 2021, 18, 100390. [Google Scholar] [CrossRef] [PubMed]
- Hermon, N.; Wainstock, T.; Sheiner, E.; Golan, A.; Walfisch, A. Impact of Maternal Depression on Perinatal Outcomes in Hospitalized Women—A Prospective Study. Arch. Womens Ment. Health 2019, 22, 85–91. [Google Scholar] [CrossRef]
- Buoli, M.; Grassi, S.; Di Paolo, M.; Redaelli, M.; Bollati, V. Is Perinatal Major Depression Affecting Obstetrical Outcomes? Commentary on “Impact of Maternal Depression on Perinatal Outcome in Hospitalized Women-a Prospective Study”. Arch. Womens Ment. Health 2020, 23, 595–596. [Google Scholar] [CrossRef]
- Kristiansson, M.; Sörman, K.; Tekwe, C.; Calderón-Garcidueñas, L. Urban Air Pollution, Poverty, Violence and Health—Neurological and Immunological Aspects as Mediating Factors. Environ. Res. 2015, 140, 511–513. [Google Scholar] [CrossRef]
- Burke, C.S.; Susser, L.C.; Hermann, A.D. GABAA dysregulation as an explanatory model for late-onset postpartum depression associated with weaning and resumption of menstruation. Arch. Womens Ment. Health 2019, 22, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.X.; Sampaio, P.; Rasga, C.; Martiniano, H.; Faria, C.; Café, C.; Oliveira, A.; Duque, F.; Oliveira, G.; Sousa, L.; et al. Evidence for an association of prenatal exposure to particulate matter with clinical severity of Autism Spectrum Disorder. Environ Res. 2023, 228, 115795. [Google Scholar] [CrossRef] [PubMed]
- Aguglia, A.; Serafini, G.; Escelsior, A.; Canepa, G.; Amore, M.; Maina, G. Maximum Temperature and Solar Radiation as Predictors of Bipolar Patient Admission in an Emergency Psychiatric Ward. Int. J. Environ. Res. Public Health 2019, 16, 1140. [Google Scholar] [CrossRef] [PubMed]
- Barkin, J.L.; Philipsborn, R.P.; Curry, C.L.; Upadhyay, S.; Geller, P.A.; Pardon, M.; Dimmock, J.; Bridges, C.C.; Sikes, C.A.; Kondracki, A.J.; et al. Climate Change Is an Emerging Threat to Perinatal Mental Health. J. Am. Psychiatr. Nurses Assoc. epub ahead of print. 2022. [Google Scholar] [CrossRef]
- Brauer, M.; Freedman, G.; Frostad, J.; van Donkelaar, A.; Martin, R.V.; Dentener, F.; van Dingenen, R.; Estep, K.; Amini, H.; Apte, J.S.; et al. Ambient Air Pollution Exposure Estimation for the Global Burden of Disease 2013. Environ. Sci. Technol. 2015, 50, 79–88. [Google Scholar] [CrossRef]
- Heslehurst, N.; Brown, H.; Pemu, A.; Coleman, H.; Rankin, J. Perinatal health outcomes and care among asylum seekers and refugees: A systematic review of systematic reviews. BMC Med. 2018, 16, 89. [Google Scholar] [CrossRef]
- Meltzer-Brody, S.; Howard, L.M.; Bergink, V.; Vigod, S.; Jones, I.; Munk-Olsen, T.; Honikman, S.; Milgrom, J. Postpartum psychiatric disorders. Nat. Rev. Dis. Primers. 2018, 4, 18022. [Google Scholar] [CrossRef]
| Study (Country) | Design | Quality (EPHPP) | Study Participants | Psychometric Tools | Psychiatric Disorders | Perinatal Window | Pollutants | Source of Exposure Assessment | Adjustment Variables | Effect Size (Cohen’s d) | Main Results |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Duan et al., 2022 [48] (China) | Multi-city prospective cohort study | Moderate | 10,209 pregnant women in 5 hospitals from Shangai, Hangzhou and Shaoxing (October 2019–February 2021) | EPDS (cut-off score: 10 and 13) at 6 weeks postpartum | PPD | Pregnancy–6 weeks postpartum | PM2.5 PM10 SO2 CO NO2 O3 | Local ambient monitoring stations | Socio-demographic variables, obstetric variables, season, city, daily temperature | Entire pregnancy: PM10: 0.21 (for a 10 μg/m3 increase) CO: 0.46 (for a 0.1 μg/m3 increase) NO2: 0.27 (for a 10 μg/m3 increase) 2nd trimester: SO2: 0.05 (for 1 mg/m3 increase) | Exposure to PM10, CO and NO2 during the whole pregnancy is associated with an increased risk of developing depression at 6 weeks postpartum SO2 exposure during the second trimester increases the risk of PPD |
| Bastain et al., 2021 [45] (USA) | Prospective cohort study | Moderate | 180 women from the MADRES project cohort in Los Angeles, California (2015–2020) | CES-D scale (cut-off score: 16) at 12 months postpartum | Maternal depression | Pregnancy–12 months postpartum | PM2.5 PM10 NO2 O3 | Local ambient monitoring stations | Socio-demographic variables, history of depression, air conditioning use, average temperature, study recruitment site | Entire pregnancy: NO2: 0.39 2nd trimester: PM2.5: 0.24 NO2: 0.53 | Exposure to NO2 during the whole pregnancy is associated with an increased risk of developing depression at 12 months postpartum Exposure to NO2 and PM2.5 during the second trimester is associated with an increased risk of developing depression at 12 months postpartum |
| Lamichhane et al., 2021 [46] (Republic of Korea) | Prospective cohort study | Moderate | 2153 pregnant women followed up in different medical centers in the Seoul metropolitan area (2007–2015) | PSS scale assessed at the 36th week of pregnancy (third trimester) | Prenatal maternal stress (increase in PSS scores) | Pregnancy | PM2.5 PM10 NO2 O3 | LUR models | Socio-demographic variables, obstetric characteristics, medical comorbidities, maternal smoking, alcohol during pregnancy | Entire pregnancy: PM2.5: 0.93 PM10: 1.32 3rd trimester: O3: 0.75 | During the whole pregnancy IQR increases in exposure to PM2.5 and PM10 were associated with 0.37- and 0.54-point increases in PSS scores During the third trimester IQR increases in exposure to O3 were associated with 0.30-point increases in PSS scores |
| Shih et al., 2021 [47] (Taiwan) | Prospective cohort study | Weak | 21,188 mother-infant pairs from Taiwan Birth Cohort Study-TBCS (2005) | 36-Item Short Form Survey administered 6 months after childbirth | PPD | Pregnancy–6 months postpartum | PM2.5 CO NO2 | Hybrid Kringing-LUR and LUR-based machine learning models | Socio-demographic variables, obstetrical variables, breastfeeding, infant general health status, perinatal smoking or smoking history, perinatal alcohol consumption, ambient temperature | 1st trimester: NO2: 0.01 per IQR increases in exposure (10.67 ppb) | PPD occurrence was significantly related to exposure to NO2 during first trimester of pregnancy (early pregnancy) |
| Zhang et al., 2021 [50] (USA) | Retrospective observational study | Weak | EHR data on 8949 pregnant women from an urban academic medical center in New York City (2015–2017) | PPD diagnosis within 1 year after childbirth based on SNOMED codes | PPD | Pregnancy–12 months postpartum | PM2.5O3 | LUR models | Socio-demographic variables, clinical problems, medication prescriptions, built environment, prenatal care variation, pregnancy characteristics and outcomes | NA | Women who experienced a prenatal care pattern with highest rates of PPD were more likely to reside in neighbourhoods with lower air pollutant concentration |
| Niedzwiecki et al., 2020 [49] (Mexico) | Retrospective cohort study | Moderate | 509 mothers from the PROGRESS study in Mexico City (July 2007–February 2011) | EPDS (cut-off score: 13) administered during pregnancy, at 1 and 6 months postpartum | PPD | Pregnancy–6 months postpartum | PM2.5 | LUR models | Socio-demographic variables, negative life events during pregnancy, environmental tobacco smoke, birth season | 0.26 | A 5 μg/m3 change in PM2.5 average exposure during pregnancy was associated with increased PPD risk at 6 months |
| Vuong et al., 2020 [44] (USA) | Prospective cohort study | Moderate | 377 women from the HOME study conducted in Cincinnati, Ohio (March 2003–February 2006) | BDI-II at 20-week gestation and 7 times postpartum (4 weeks, 1,2,3,4,5 and 8 years) | Maternal Depression | Pregnancy–12 months postpartum | PBDEs (BDE-28, -47, -99, -100, -153 and ƩPBDEs) PFAS (PFOA, PFOS, PFHxS, PFNA) | PBDEs and PFAS blood levels at 16 ± 3 weeks of gestation were collected, then chromatography and mass spectrometry analysis were performed | Socio-demographic variables, self-reported marijuana use during pregnancy, serum cotinine (tobacco use or environmental smoke exposure), serum ƩPCBS, maternal IQ | Estimated score differences in BDI scores at 4 weeks after delivery by 10-fold increases in serum PBDE concentrations (ng/g lipid) during pregnancy: BDE-28: 0.65 (NS) BDE-4: 0.23 BDE-99: 0.80 (inverse association) BDE-100: 0.12 (inverse association) BDE-153: 0.49 ∑PBDEs: 0.41 (inverse association) Estimated score differences in BDI scores at 4 weeks after delivery by 1-ln unit increases in serum PFAS concentrations(ng/mL) during pregnancy: PFOA: 1.69 PFOS: 1.56 PFHxS: 0.59 (NS) PFNA: 1.34 (NS) Estimated score differences in BDI scores at 1 year after delivery by 10-fold increases in serum PBDE concentrations (ng/g lipid) during pregnancy: BDE-28: 2.80 (NS) BDE-47: 2.61 (NS) BDE-99: 2.07 (NS) BDE-100: 2.93 (NS) BDE-153: 2.44 (NS) ∑PBDEs: 2.68 (NS) Estimated score differences in BDI scores at 1 year after delivery by 1-ln unit increases in serum PFAS concentrations(ng/mL) during pregnancy: PFOA: 0.85 (NS) PFOS: 0.78 PFHxS: 0.39 (NS) PFNA: 0.41 (inverse association-NS) | PBDEs and PFAs blood levels during pregnancy were found to contribute to severity of depressive symptoms after delivery |
| Sheffield et al., 2018 [43] (USA) | Prospective cohort study | Moderate | 557 mothers who delivered at ≥ 37 weeks of gestation from the ACCESS project cohort (2002–2007) | EPDS (cut-off score: 13) at 6 and 12 months postpartum | PPD | Pregnancy–12 months postpartum | PM2.5 | Data from U.S. Environmental Protection Agency (EPA) | Socio-demographic variables, prenatal smoking, season of delivery | NA | Increased PM2.5 exposure in mid-pregnancy (second trimester) was associated with severity of depressive and anhedonia symptoms, particularly in Black women |
| Örun et al., 2011 [42] (Turkey) | Prospective cohort study | Moderate | 144 mothers residing in a suburban area who delivered in Ankara (July-September 2006) | EPDS scale (cut-off score: 13) | PPD | 2 months postpartum | Pb Cd | Pb and Cd levels in breast milk at 2 months postpartum were determined by ICP-MS | Maternal and infant characteristics | Pb: 0.11 Cd: 0.10 | No correlation was found between breast milk Pb and Cd levels and EPDS scores |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Surace, T.; Quitadamo, C.; Caldiroli, A.; Capuzzi, E.; Colmegna, F.; Nosari, G.; Borroni, E.; Fedrizzi, L.; Bollati, V.; Pesatori, A.C.; et al. Air Pollution and Perinatal Mental Health: A Comprehensive Overview. J. Clin. Med. 2023, 12, 3146. https://doi.org/10.3390/jcm12093146
Surace T, Quitadamo C, Caldiroli A, Capuzzi E, Colmegna F, Nosari G, Borroni E, Fedrizzi L, Bollati V, Pesatori AC, et al. Air Pollution and Perinatal Mental Health: A Comprehensive Overview. Journal of Clinical Medicine. 2023; 12(9):3146. https://doi.org/10.3390/jcm12093146
Chicago/Turabian StyleSurace, Teresa, Cecilia Quitadamo, Alice Caldiroli, Enrico Capuzzi, Fabrizia Colmegna, Guido Nosari, Elisa Borroni, Luca Fedrizzi, Valentina Bollati, Angela Cecilia Pesatori, and et al. 2023. "Air Pollution and Perinatal Mental Health: A Comprehensive Overview" Journal of Clinical Medicine 12, no. 9: 3146. https://doi.org/10.3390/jcm12093146
APA StyleSurace, T., Quitadamo, C., Caldiroli, A., Capuzzi, E., Colmegna, F., Nosari, G., Borroni, E., Fedrizzi, L., Bollati, V., Pesatori, A. C., Carugno, M., Clerici, M., & Buoli, M. (2023). Air Pollution and Perinatal Mental Health: A Comprehensive Overview. Journal of Clinical Medicine, 12(9), 3146. https://doi.org/10.3390/jcm12093146











