Abstract
Urticaria is a condition characterized by the development of itchy wheals (hives), angioedema, or both. The pathophysiology of chronic spontaneous urticaria (CSU) is still poorly understood. It is suggested that there is no dominant and independent mechanism of CSU; however, there are different immunological and non-immunological abnormalities that act simultaneously or/and follow each other resulting in clinical symptoms. The latest hypothesis points out that mast cells (MCs) to be activated via autoantibodies in autoallergic or autoimmune mechanism mediators released from degranulated MCs are responsible for the vasoactive and neurospecific effect in CSU. According to many clinical observations, it is suggested that psychological stress can be both a triggering factor in the onset of CSU and a modulating one in the course of the disease and therapy effectiveness. Of importance, the mechanistic background of the psychological stress response in the skin has not yet been fully elucidated. However, of note, a variety of inflammatory mediators, neuropeptides, and neurotransmitters facilitate this phenomenon. This review presents recent findings on the neuro–immuno–psychological aspects of CSU, highlighting an emerging role of neuro–immune interactions. It also points out the usefulness of psychological tools employment for the baseline diagnosis of perceived stress level and the presence of its symptoms. Furthermore, it proposes the implementation of non-invasive interventions to reduce psychological stress and anxiety. A bio–psycho–social approach including psychological support and patient education seems to be as important as traditional pharmacotherapy for CSU. It facilitates the effective control of active disease and a prolonged remission time in this disease.
1. Introduction
1.1. Characteristics and Epidemiology of Chronic Urticaria
Chronic urticaria (CU) is a condition characterized by the development of itchy wheals (hives), angioedema, or both, with reoccurring symptoms for more than six weeks [1]. The lifetime prevalence of all types of urticaria is usually below 10% according to different reports, whereas CU develops only in approximately one-fourth of these individuals. Of the total CU patients, one-third suffer from both hives and angioedema, 30–40% present isolated hives, and approximately 10% show isolated angioedema. The natural history of the disease has a very wide range. Approximately half of the patients follow a three-month self-limited evolution, and within a year the disease resolves in almost 80% of them. However, in more than 10% of the patients, a disease duration of 5 years or longer is expected. Females are affected at least twice as often as males, and most patients are over 20 years of age. In children, the prevalence varies from less than 1% to almost 5%, largely depending on the methodology employed by the researchers [2].
The purpose of this review is to summarize the findings on the neuro–immuno–psychological aspects of urticaria, regarding the bio–psycho–social model of patient care. The pathophysiology of urticaria is still poorly understood, although better knowledge of abnormalities in neuroimmune cutaneous response is the key to understanding the stress-related mechanism in this condition.
1.2. Current Insight into the CSU Mechanism
The pathomechanism of chronic spontaneous urticaria (CSU) is not well established. It is suggested that there is no dominant and independent mechanism of CSU; however, there are different immunological and non-immunological abnormalities that act mutually or/and follow each other resulting in clinical symptoms of CSU [3]. Undoubtedly, for all types of urticaria, the major players of the disease and wheal formulation are mast cells (MCs) and mediators released from these cells. Still, antihistamines constitute the first-line therapy in CSU; however, they are not equally effective in all cases [4]. Light and electron microscopy revealed degranulated MCs in the dermis following wheal appearance. According to some reports, increased numbers of skin MCs are detected in lesional and non-lesional skin in CSU and other types of urticaria; however, the results concerning the disposition of dermal MCs in urticarial patients are somewhat conflicting [3,5]. The activation of dermal MCs leads to immediate cell degranulation and the release of preformed mediators stored in granules, such as histamine, heparin, serotonin, chymase, tryptase tumor necrosis factor-α (TNF-α), nerve growth factor (NGF), and many others. Activated MCs also secrete de novo synthetized arachidonic acid metabolites including leukotrienes (LTs), prostaglandins (PGs), and a platelet activation factor (PAF) into the tissue. A wide range of cytokines and chemotactic agents including interleukins (ILs) such as IL-1, IL-4, IL-5, IL-6, IL-8, IL-10, IL-31, IL-33, macrophage inflammatory protein-1 (MIP-1), granulocyte–macrophage colony-stimulating factor (GM-CSF), transforming growth factor β (TGF-β), vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), TNF-α, and C–C chemokine ligand 2 and 5 (CCL2 and CCL5) are also released upon the MCs’ activation [5,6,7,8]. The vasoactive properties of the MCs’ mediators induce increased vasodilatation, an up-regulation of adhesion molecule expression, higher vascular permeability, and plasma extravasation. As a consequence, inflammatory cell/protein accumulation is observed in the affected skin. Importantly, some of the MCs’ mediators including substance P (SP), NGF, and vasoactive intestinal peptide (VIP) can interact with peripheral nerve endings and activate sensory nerves. Cross-talk between MCs and neuronal tissue is the fundamental point of the stress-induced skin neurogenic inflammation further discussed below. The abundance of neutrophils, eosinophils, basophils, CD4+ lymphocytes, as well as monocytes is detected in urticarial wheal. Furthermore, alternations in serum circulating T cell subtypes and their activities were observed in CSU vs. healthy subjects (imbalance in the proinflammatory Th17 cells and Treg cells). Skin biopsies revealed a higher expression of IL-4, IL-5, IL-25, IL-33, and thymic stromal lymphopoietin (TSLP) in urticarial wheal and non-lesional skin. These cytokines can promote a Th2-related response as well as amplify chronic inflammation and angiogenesis. On the other hand, an elevated expression of interferon γ (IFN-γ) in affected skin was detected, indicating a mixed Th1- and Th2-skewed polarization of skin immune response in CSU [3,4,5,9,10,11].
It is not entirely understood (i) what is the major stimulator of MC activation in the onset of CSU and (ii) which MC receptors are mainly involved in CSU-related activation. The proposed hypothesis points at MCs’ activation via autoantibodies, i.e., IgE specific to self-antigen (autoallergic mechanism) or IgG1/IgG3/IgM specific to IgE or its high-affinity receptor FcεRI (autoimmune mechanism). The autoimmune nature of the disease is supported by a deficit in T regulatory cell (Treg) activity in CSU. Tregs are generally responsible for suppressing the autoreactive immune response. It is worth remembering that FcεRI-mediated stimulation is not the only possible mechanism of MC activation considering the varied repertoire of MCs’ receptors. According to the stress-induced reaction in the skin, MC receptors specific to neuropeptides, neurotransmitters, and hormones are of special importance. There is a growing body of research on the potential role of other factors in CSU pathological cascade reaction, i.e., mediators other than histamine recruited inflammatory cells (e.g., basophils, neutrophils, and eosinophils) and skin-related cells, especially keratinocytes as a source of proinflammatory cytokines and activation of the coagulation system. The genetic background of CSU was also confirmed (alternations in HLA-DR4 and HLA-DQ8 gene expression) [3,4,12]. Interestingly, the urticarial lesion was reported to be the second most common skin-related symptom of COVID-19, which could also indicate the potential role of infectious agents in the course of CSU [13].
1.3. Urticaria affects the Quality of Life and Psychological Functioning
CSU is a stress-modulated condition in which the outcome of conventional treatment is often suboptimal. A bio–psycho–social approach including psychological support and patient education seems to be as important as traditional pharmacotherapy. It facilitates effective control and a prolonged remission time of the disease. During the COVID-19 pandemic and massive isolation, excessive fear, stress, and anxiety among people were reported. Patients suffering from chronic conditions, such as CSU, additionally experienced insufficient control of the disease. Beyaz et al. [14] have documented that urticarial activity score 7 (UAS7) during the pandemic time was higher compared to the pre-pandemic period. Furthermore, authors have observed that UAS7 was positively correlated with the Fear of COVID–19 Scale (FCV-19), depression, anxiety, and stress subscale score.
The association with psychological stress indicates the potential role of the neuroendocrine system in the etiopathogenesis of urticaria. However, it is still poorly understood how psychological stress interferes with the skin immune system in CSU. Although the role of psychological factors in development and aggravation is not fully confirmed, the psychosocial impact of CSU is widely accepted [15,16,17,18,19,20,21,22]. The struggle of chronic disease, prolonged treatment, and multiple consultations with different practitioners (often resulting in fatigue, work absence, or lowered school performance) [16,17] leads to a deterioration in psychological functioning. Patients suffering from urticaria manifest significantly higher scores in somatization, obsessive–compulsive disorder, interpersonal sensitivity and depression, anxiety, and stress levels [18,19,20,21]. A positive correlation between the severity of the disease and poor psychological wellness or lower quality of life is also frequently reported [22].
The wellbeing and quality of life of CSU patients is also affected by their quality of sleep. Subjective symptoms such as itching and pain can have a significant impact on sleep quality in patients with chronic skin diseases [23]. At the same time, sleep alterations (i.e., shortening of sleep; frequent awakenings during the night) lead to further deterioration in quality of life together with fatigue and frustration. In addition, patients with CSU are more likely to suffer from sleep disorders such as obstructive sleep apnea syndrome or sleep-disordered breathing [24,25]. As a consequence, there is an even greater psychological burden on the patient and, as a consequence, a higher risk of an exacerbation of CSU symptoms.
2. Psychological and Biological Aspects of Stress Reaction
2.1. Neurobiology of Stress Reaction
The regulation of the body adaptive response to stress involves mainly the hypothalamic–pituitary–adrenal (HPA) axis, and the sympathetic–adrenomedullary (SAM) system. The HPA axis plays a key role in maintaining body homeostasis and the body response to stress. Stress results in a corticoliberin-releasing hormone (CRH) release from the hypothalamus. This information is then transmitted to the anterior lobe of the pituitary gland, where the secretion of the adrenocorticotropic hormone (ACTH) takes place. This phenomenon leads to the stimulation of cortisol release into the blood from the adrenal cortex. An increased cortisol level leads to the inhibition of CRH and ACTH secretion by a negative feedback loop [26,27]. The importance of the HPA axis is mainly based on the action of cortisol. In stressful situations, cortisol is secreted as a body defense response. Cortisol reduces the inflammatory response, stimulates gluconeogenesis, and is responsible for protecting the body from an excessive immune response [26]. The SAM system represents another major immunoregulatory system. In general, SAM activation mediates short-term effects with rapid responses, whereas the HPA axis activation leads to short- and long-term effects [28,29]. Interactions of these two major stress systems (SAM and HPA) occur at several levels, functioning cooperatively and/or sequentially, acting in opposite ways in most visceral organ targets.
Furthermore, the response to physical and/or psychological stressors involves a rapid physiological adaptation mediated mainly by catecholamines. An epinephrine (E) and norepinephrine (NE) release from the adrenal medulla causes general physiological changes in order to prepare the body for a “fight-or-flight” reaction. E and NE effects include maintaining alertness, metabolic actions (increased glucose via glycogenolysis and gluconeogenesis, lipolysis, increased oxygen consumption, and thermogenesis), and cardiovascular actions [28].
The growing body of evidence shows the significant role of brain and gut interactions in stress response. The brain–gut axis consists in bidirectional communication between the central and the enteric nervous system and the intestinal one. The brain–gut axis creates a link between the emotional and cognitive centers of the brain with peripheral gut functions [30]. Mast cells are important effectors of the brain–gut axis. MCs translate the stress signals that have been transmitted through the brain–gut axis into the release of proinflammatory mediators. The latter can stimulate nerve endings and further affect afferent nerve terminals and change their perception, affect intestinal motility, increase intestinal hyperpermeability, and in susceptible individuals modulate the inflammation. Interestingly, MCs secrete a wide range of neurotransmitters and proinflammatory cytokines and express surface receptors for CRH demonstrating an important link between these cells and the stress response [31,32]. Furthermore, gut microbiota may participate in the modulation of the brain–gut axis and thus in the stress response. It was shown that microbiota composition may change as a result of the stress response leading to gut dysbiosis. The latter is especially noticed when permanent or repetitive exposure to stress situations is encountered. On the other hand, the individual stress response and the ability to adapt to stressful factors may depend on gut microbiota composition. That is why the treatment and care of patient suffering from stress-related diseases such as CSU should also focus on the restoration of gut microbiota composition. Applying a diet “friendly” to the gut microbiome or using specific oral probiotic strains, mainly the Lactobacillus family also called psychobiotics should be considered. Of note, more and more data, including clinical trials, indicate the benefits of psychobiotics supplementation (probiotics and prebiotics) on human cognitive function and the reduction of cortisol response. Research concerning the gut–microbiota–brain axis is a promising direction with great opportunities, although still much remains to be discovered in this field [33,34,35].
2.2. Stress as a Transaction; Acute and Chronic Stress
Psychological stress is strongly linked to the individual experience of difficult situations in everyday life encountered by all representatives of Homo sapiens. The situation of illness, i.e., the situation of being sick, including CSU, can be treated by the individual as a stressor and thus leads to psychological strain. The important point is that the psychological stress response to CSU will not be significantly different from stress experienced in other situations. Therefore, the mechanism described further on is universal and additionally reflects the process of stress in CSU patients. However, what is important for the emergence of a stress response is not the mere occurrence of the situation itself, but the course of the so-called stress transaction, which is the result of the Homo sapien’s relationship with the environment. The stress transaction takes place in two phases: the primary and secondary appraisals. The primary one involves an evaluation of the situation and identification of potentially stressful (threatening or difficult) events. The secondary appraisal involves an analysis of the demands and resources available to the individual in this particular situation. If in the course of these assessments, which are often unconscious and automatic in nature, the situation is judged to be potentially threatening and the individual resources are deemed insufficient to meet the demands of the situation, a stress response will occur, and the level of psychological stress will increase. It should be noted that this process is subjective in nature, and the same situation can be assessed in different ways depending on the course of the stress transaction. Throughout the process, it is also important for the individual to identify the possibility of taking action to remove the cause of the stress or its consequences: the implementation of a coping process [36].
According to Lazarus and Folkman’s theory, coping is a constantly changing cognitive and behavioral effort to manage specific external and internal demands evaluated by an individual as stressful, i.e., burdening or exceeding his or her own resources. Each individual has its repertoire of preferred coping styles, among which problem-focused (e.g., planning; active coping), emotion-focused (e.g., positive interpretation), or avoidance-focused (e.g., denial) styles can be distinguished. Problem-focused and emotion-focused strategies are complementary, and their employment leads to effective behavior in a stressful situation. If an individual has a positive attitude in a stressful situation and is able to regulate the level of arousal (emotion-focused coping) it helps to motivate and makes it easier to take action to eliminate the problem (problem-focused coping) [37].
Of importance, stress is not an explicitly negative and harmful phenomenon. It can be differentiated into positive stress (eustress) and negative stress (distress). The prerequisite for eustress is that the stressful situation is treated as a challenge and that the demands of the environment are assessed in favorable–positive terms, which inspires enthusiasm, hope, and motivation for positive change. Distress is the result of evaluating the stressful situation as threatening. It leads to impaired functioning, described most often as angry, tiring, excessive, prolonged, and thus harmful. It is a type of reaction that makes it difficult to perform tasks and control emotions, negatively affecting health and well-being [38]. It is also important to distinguish between acute stress, which is short-lived, occurs as a result of single situations or random events, and is usually adaptive, and chronic stress, which results from the experience of persistent difficult life situations or multiple episodes of acute stress. The experience of chronic stress can lead to permanent changes in the nervous system and results in a significant deterioration of psychological functioning, leading to psychological depression, anxiety, and somatic disorders [39].
2.3. Methods of Stress Level Diagnosis
Due to the highly subjective nature of the stress transaction, it is impossible to determine a universal level of stress occurring as a result of the disease for all the patients. Physiological, biochemical, and psychological methods can be used to measure the level of stress. All of the above methods have their advantages and disadvantages.
Physiological stress measurement mainly focuses on monitoring parameters such as heart rate, blood pressure, respiration rate, and changes in skin electrical resistance. The use of these measures will be particularly useful in acute stress situations but is not recommended in clinical practice and the evaluation of the actual stress level experienced by chronically ill patients [40].
Biochemical assessment involves mainly the evaluation of adrenal cortical hormones in the blood, urine, or saliva. An increase in catecholamines indicates the effects of short-term stress. An elevated concentration of corticoids (e.g., cortisol) and a low level of dehydroepiandrosterone sulfate (DHEA-S) accompanies chronic stress. However, the literature data on serum cortisol levels in CSU are sparse and conflicting [40].
Psychological methods to evaluate stress levels are of a questionnaire’s nature. They examine various aspects directly or indirectly related to stress. Popular questionnaires that directly indicate patient stress levels are the following: Perceived Stress Scale (PSS10), which measures subjective feelings of stress and the Depression, Anxiety Stress Scale (DASS) which is for measuring the intensity of perceived symptoms of depression, anxiety, and stress [41]. Of importance, due to the subjective nature of the stress transaction, we do not recommend the use of scales based on indicating experienced stressful situations such as the Social Readjustment Rating Scale (SRRS) when working with chronically ill patients.
Apart from questionnaires based on the direct measuring of stress levels, additional methods can be introduced to further enhance the evaluation of psychological functioning. The Coping Orientation to Problems Experienced (COPE) [37] and the Coping Inventory for Stressful Situations (CISS) [42] allow an assessment of the individual preference toward the use of different coping styles. The above questionnaires are used to assess tendencies to take actions that lead to effective coping with stress. Furthermore, the Hospital Anxiety and Depression Scale (HADS) [43] and the State and Trait Anxiety Inventory (STAI) [44] measure the level of emotions and emotional states typically associated with chronic stress, i.e., depression and anxiety. The aforementioned tools are used both for research purposes and in clinical practice. It is worth mentioning that due to various regulations worldwide, access to some of the mentioned questionnaires may be restricted to psychologists and psychiatrists only.
3. Stress-Induced Skin Reaction in the Course of CSU
3.1. Stress and Skin Diseases
Many clinical observations confirm that there is a link between the onset and exacerbation of inflammatory dermatoses and a high exposure to psychological stress [45]. Regardless of the urticaria type, increased psychological stress is closely related to worsening and more frequent relapses of the disease [46]. Bidirectional interactions between psychological stress and chronic inflammatory skin diseases are presented in Figure 1. Of note, patients suffering from CSU are especially vulnerable to stress-induced alternations because the triggering agent of the disease remains unclear; therefore, signs and symptoms appear unexpectedly and spontaneously during life activities exerting continuous tension on the patient. Such a situation creates a vicious circle for the patients. On the one hand, illness itself leads to a worsening of the psychological functioning of the patient, and on the other hand, illness itself is exacerbated by psychological factors related to emotions and stress. It is also worth noting that most studies show a correlation between disease severity and psychological factors, without indicating what is the effect and what is the cause [14,47,48]. This means that stress reduction activities can lead to CSU symptoms’ alleviation. Therapeutical support and caring executed by an interdisciplinary medical team could lead to improvements in both patients’ skin disease severity and psychological functioning thus breaking the aforementioned vicious circle [49].
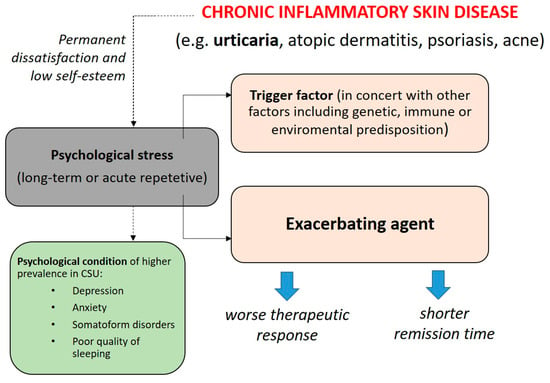
Figure 1.
The hypothesis of bidirectional interactions between psychological stress in chronic skin diseases including chronic spontaneous urticaria (CSU) [12,50].
Long-term or frequent exposure to psychological stress affects every human organ, including the skin. The mechanistic background of the psychological stress response in the skin has not yet been well documented. Importantly, skin “battered” by dermatological inflammatory problems (e.g., urticaria, atopic dermatitis, psoriasis, and acne) is especially susceptible to stress-mediated influence. The reactive threshold of the skin response is lower than in healthy skin, probably due to a constant accumulation and arousal of immune cells, proinflammatory cytokines and chemokines in the lesional skin and non-lesional skin [3,51]. This phenomenon was also confirmed in the course of CSU. Furthermore, serum markers of systemic inflammation including C-reactive protein (CRP), IL-1β, IL-6, IL-18, TNF-α, IFN-γ, VEGF, and matrix metalloproteinase-9 (MMP-9) are in a higher concentration as compared to healthy controls. In CSU, this phenomenon can cause stress-induced exacerbations in the course of the disease [4,50,52,53,54]. Importantly, the cell receptor expression is not always constitutive. That is why a particular receptor may appear in response to an inflammable-specific milieu. For example, an elevated expression of corticotropin-releasing hormone receptor 1 (CRH-1R) is observed in CSU patients as compared to healthy individuals, indicating that CSU may be exacerbated by psychological stress via HPA axis stimulation [55]. CSU’s relation to stress was also linked to the activation of the opioid system in the chronic stage of the disease. Of importance, an elevated level of β-endorphins in CU patients vs. healthy subjects was also documented [56].
3.2. HPA Axis and CSU
The early stage of an acute stress reaction results in the temporary stimulation of the central HPA axis, which leads to stress hormone secretion, including CRH, ACTH, and cortisol. Cortisol is a natural glucocorticoid that acts as a specific inhibitor of the excessive immune response. After adaptation to an acute stressor, the HPA axis returns to normal activity due to a negative feedback mechanism mediated mainly by cortisol. It is suggested that some cytokines such as IL-6 and IL-18 may also play an important role in HPA axis modulation and control [57]. It is noteworthy that chronic stress or repetitive episodes of acute stress results in the dysregulation of the HPA axis which in turn leads to a lack of negative feedback control (fatigue/exhaustion of HPA). This may explain the increased levels of CRH, IL-1β, IL-6, IL-18, and other proinflammatory agents during the long-term exposition to stress that is observed in CSU [11,50,52]. Bearing in mind the systemic increase of some proinflammatory factors in CSU, it should be noted that brain homeostasis may be also disrupted in CSU patients causing an altered reaction to stress. However, more research data are needed to gain better knowledge on the mechanistic background of this phenomenon. It was observed that peripheral inflammation and MCs’ activation can alter the blood–brain barrier permeability (BBB). BBB is formed by endothelial cells, a basement membrane, astrocytes, and pericytes that separate the central nervous system (CNS) from peripheral circulation. Under normal physiological conditions, the BBB restricts the entry of peripheral immune cells into the CNS through a low expression of leukocyte adhesion molecules. However, in pathological conditions such as an inflammable-specific milieu, the above situation is interrupted. As a consequence, BBB becomes hyperpermeable through loss/alterations of its integrity. It should be underlined that this phenomenon also refers to the gut permeability-causing imbalance in microbiome and dysfunction of the local immune system. Inflammatory factors secreted by immune cells, as well as reactive oxygen species (ROS) and MMPs promote immune cells’ migration into the CNS and increased BBB permeability development. Thus, another vicious cycle could be formed [58].
Interestingly, the results of serum cortisol concentration in CSU patients compared to healthy subjects are inconsistent. Many studies have found no difference between CSU patients and controls according to basal cortisol levels [57,59]. On the other hand, Varghese et al. [52] have indicated lower cortisol levels in patients with CSU compared to controls. This result was negatively correlated with urticaria severity measured by UAS7, stress level, and disease duration. Vurgun et al. [57] have not found any difference in terms of cortisol concentration between the control group and CSU patients. However, the authors revealed a lower level of DHEA-S and higher cortisol/DHEA-S ratio in CSU patients. They further concluded that examining the ratio of cortisol/DHEA-S rather than hormones levels alone may be a useful tool for the evaluation of the HPA axis functions in an individual.
The skin appears to have the equivalent of an HPA axis with a similar cascade of hormonal response. Receptors for CRH (CRH-R1 and CRH-R2), urocortin, cortisol (glucocorticoid receptor-GR), ACTH (melanocortin receptors MC1R and MC2R), α-melanocyte-stimulating hormone (α-MSH), and β-endorphin are expressed on normal skin cells. Furthermore, an elevated expression of CRH-1R was observed in urticarial lesions. CRH-R1 is a major stress-related receptor constitutively expressed on the cells located in the epidermis, dermis, and subcutis, including MCs [54]. Acute stress and the intradermal administration of CRH stimulate skin MCs and increase vascular permeability in a CRH-R-dependent manner [55]. The association of CRH with mast cell degranulation has been also proven in rats [60]. CRH may also induce IL-6 and IL-18 release from keratinocytes and MCs [61]. Moreover, both CRH and ACTH were shown to activate basophils in humans [62]. Importantly, a skin cell can secrete stress-related hormones by itself and thus participate in local skin inflammation [54]. It should be emphasized that the skin HPA axis has its own, unique regulatory mechanism driven by cross-talk between nerve endings, resident skin cells/structures, and immune cells recruited from surrounding vessels (neuro–immune–cutaneous circuit). That is why a stress-induced skin reaction is difficult to interpret, especially if it concerns the reaction of the affected skin such as in CSU.
Konstantinou et al. [11] in their meta-analysis documented that current studies concerning stress-related responses in the course of CSU are focused mainly on (i) the assessment of the serum concentration of specific mediators including neuropeptides and pro-inflammatory agents, such as IL-18, CRP, neuropeptide Y (NPY), SP, stem cell factor (SCF), VIP, calcitonin gene-related peptide (CGRP), and NGF and (ii) the observation of the skin wheal and flare reaction after an injection of stress-related hormones and mediators, additionally before and after a specific CSU treatment.
Many researchers have observed some differences in the serum levels of neuropeptides and hormones in CSU patients compared to healthy controls. The results concerning SP serum concentration seem to be the most consistent. Basak et al. [63] documented that SP and stem cell factor (SCF) but not CGRP, VIP, NPY, and NGF were elevated in CSU patients as compared to healthy subjects. Metz et al. [64] have also reported that serum SP concentration was higher in CSU patients, and furthermore, the SP level correlated with the severity of CSU. Research performed by Zheng et al. [65] revealed that SP serum concentration was higher in CSU patients, and these subjects have an elevated number of basophils expressing NK-1R (receptor for SP) compared to controls. Furthermore, basophil stimulation with SP was stronger in CSU patients. Other studies revealed that CU patients have stronger wheal and flare reactions mediated through an injection with CGRP and SP [66]. SP is a stress-related pro-inflammatory neuropeptide that is released from cutaneous peripheral nerve endings. SP is the key mediator in connecting the brain to the hair follicle by stimulating mast cell degranulation and increasing macrophage infiltration [67]. Increased levels and cutaneous response to SP may lead to wheal and flares formation [68]. SP can also induce the expression of functional CRH receptor-1 in human mast cells [69]. SP may coordinate the interaction between the HPA axis and the sympathetic nervous system and can be a possible link between stress response and urticaria. SP is a crucial mediator in skin neurogenic inflammation. Receptors specific for SP are expressed on MCs (NK-1R and MRGPRX2), and this expression is up-regulated in severe CU [51]. It is suggested that SP may act as a trigger factor in the course of CSU; however, more research is still needed.
3.3. Neuro–Immuno–Cutaneous Circuit and Its Potential Implication in CSU
There is a link between the immune and neuroendocrine system and the skin. Neuronal tissue including autonomic and sensory nerves is widely distributed over all skin layers remaining in direct contact with resident skin and newly recruited skin cells such as leukocytes. Skin cells respond to nerve-induced stimulation via specific neuropeptides receptors. Elements of the neuro–immune–cutaneous circuit share many common mediators, i.e., hormones, cytokines, neuropeptides, and neurotransmitters. Skin cells and nerves become a target for many stress-related mediators; however, they themselves secrete various neurohormonal factors as well. This mutual communication actively regulates tissue inflammation and the maintenance of homeostasis under physiological conditions. However, in some skin pathologies including urticaria, cutaneous neurogenic inflammation develops. Many studies documented that wheal and flare reaction mediated by the intradermal injection of neuropeptides is significantly larger and longer lasting in patients with CU. The concept of neurogenic inflammation includes chronic increased vascular permeability, leukocyte infiltration, and protein extravasation caused mainly by neuropeptides released due to local and systemic HPA axis stimulation and acting in concert with proinflammatory agents [7,70]. Skin neurogenic inflammation reflects clinical observations that psychological stress may influence the course of inflammatory skin disease.
The role of MCs in the immunopathogenesis of CSU is unquestionable. Not without reason, dermal mast cells are regarded as a link in the neuro–immune–cutaneous axis also in CSU. The most crucial features of MCs’ biology are presented in Figure 2. It is worth remembering that every stimulation of MCs that leads to cell degranulation is inextricably linked not only to vasoactive and proinflammatory effects but additionally to sensory nerve stimulation.
According to stress-related skin response, the wide spectrum of MCs’ cell receptors with a high affinity to neuropeptides, neurotransmitters, neurokinins, and hormones are of particular interest. The main MCs’ neurohormonal specific receptors are presented in Table 1. Some of these receptors are additionally expressed on cutaneous nerves including those specific to histamine, NGF, SP, CGRP, and neurokinin-1. Interestingly, MCs’ expression of mas-related G protein-coupled X2 receptor (MRGPRX2/MrgX2) is elevated in CSU patients as compared to healthy individuals. Taking into account that the serum concentration of SP (main ligand of MrgX2) is also higher in CSU patients, controlling MrgX2-induced cell activation seems to be of high importance in the effective treatment of the different types of urticaria [5,70]. Currently used biological drugs affect mainly IgE and decrease free IgE levels. Subsequently, IgE receptors (FcεRI) on cells are down-regulated. A complete response rate of omalizumab ranges from 26% to 83% as demonstrated in several studies including XCUISITE, ASTERIA, and the recent ligelizumab trial. Thus, there is a need to search and aim at new etiopathological pathways such as those related to neurohormonal factors in order to implement new biological treatment options in CSU [71].

Table 1.
Main receptors expressed on MCs involved in skin stress-related response [5,72,73].
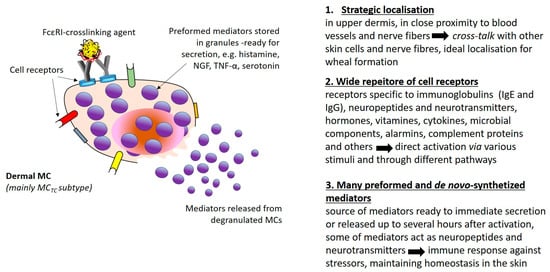
Figure 2.
Mast cell as a link in the neuro–immune–cutaneous axis [72].
Although nerve fibers remain in direct contact with many skin cells and structures (e.g., keratinocytes, sebocytes, fibroblasts, and hair follicles), their communication with MCs is well-documented and seems to be the main component of the stress-related neurogenic inflammation detected in affected skin [5,7,50]. Observations on the direct interaction between mast cells and local skin nerve fibers in human and animal models show that neural stimulation similar to stress-related leads to the secretion of neuropeptides capable of triggering MCs [15]. SP, CGRP, VIP, and neurokinins A and B (NKA and NKB) are of special importance regarding stress-related dermal MCs’ activation mediated by neurons. Moreover, many mediators secreted by MCs, such as histamine, ACTH, CRH, β-endorphins, neurotensin (NT), SP, NGF, VIP, and TNF-α may not only stimulate cutaneous nerves but also act in an autocrine manner on MCs’ activity and releasability [73]. Figure 3 demonstrates the possible cross-talk between dermal MCs and cutaneous nerve fibers in the skin affected by urticarial wheal.
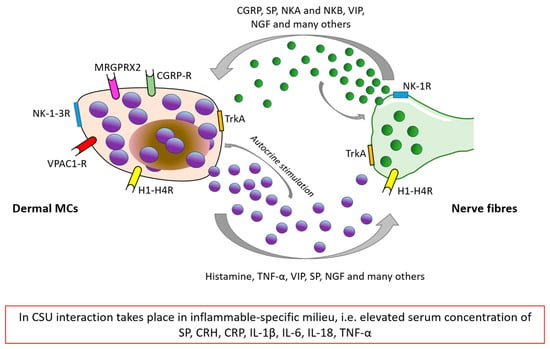
Figure 3.
The possible cross-talk between MCs and nerve fibers as a basis of skin neurogenic inflammation in CSU [5,74]. CGRP: Calcitonin gene-related peptide, NKA/NKB: neurokinin A and B, SP: substance P, CRH: corticoliberin-releasing hormone, CRP: C-reactive peptide, VIP: vasoactive intestinal peptide, NGF: nerve growth factor, TNF-α: tumor necrosis factor-α, NK-1-3R: Neurokinin type 1–3 receptor, TrkA: Tropomyosin receptor kinase A, H1R-H4R: Histamine receptor type 1–4, CGRP-R: Calcitonin gene-related peptide receptor, MRGPRX2/MrgX2: Mas-related G protein coupled X2 receptor, and VPAC-R: (vasoactive intestinal peptide receptor).
4. Recommended Psychological Intervention in Stress-Induced Skin Diseases
Based on the above discussions and taking into account the role of psychological factors in CSU, it is worth considering the inclusion of stress reduction and psychological care as one element in the treatment of CSU [75,76]. In clinical practice, it is of importance to educate the patient and implement simple methods of stress and tension relief to reduce its harmful effects on psychological functioning. These are so-called anti-stress training or relaxation techniques, which—when used regularly—can help reduce the level of perceived stress by the patient. Examples of anti-stress training that can be used in clinical practice and with patients are shown in Table 2.

Table 2.
Commonly used–anti stress training/relaxation techniques [77,78].
Authors should discuss the results and how they can be interpreted from the perspective of previous studies and of the working hypotheses. The findings and their implications should be discussed in the broadest context possible. Future research directions may also be highlighted.
The anti-stress training techniques listed in Table 2 are only some examples of the most popular techniques used in relaxation, tension reduction, and stress management support. It should be pointed out that not all anti-stress training is a substitute for professional therapeutic assistance, delivered by professional psychotherapists. Psychotherapy is effective as an adjunctive method for urticaria therapy and can be implemented when the patient is treated by an interdisciplinary team [79].
5. Conclusions
This manuscript highlights the complexity of stress and its association with MCs’ activity. Stress involves the HPA axis and SAM, but also the gut-brain axis, which provides bidirectional communication between the central and the enteric nervous system, linking the emotional and cognitive centers of the brain with peripheral intestinal functions. Mast cells are important effectors of those axes. Although FcεRI-dependent signaling is the main pathway in MC activation, FcεRI-independent mechanisms have also been suggested to play a role in CSU pathological cascade. Of special interest is the interaction between MCs and skin sensory nerve fibers as a source of many neuropeptides and neurotransmitters. Better knowledge of MCs’ stimulation via stress-related so-called neurohormonal receptors may be a key to the novel therapeutic approach to CSU. Recently, considerable progress has been made in developing new drugs that target MCs’ mediators or receptors. The possible inhibition of MRGPRX2 as a main molecule for substance P is being currently widely discussed. It is worth emphasizing that psychological stress in CSU not only acts as a pro-factor for the worsening of the symptoms of the disease but is also a consequence of the course of the disease. This leads to the emergence of a vicious circle, which is difficult to break. Therefore, the combination of psychological and traditional pharmacological therapeutic care as a permanent part of the treatment in CSU as well-done patient care executed within interdisciplinary teams to provide holistic care is highly expected.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zuberbier, T.; Abdul Latiff, A.H.; Abuzakouk, M.; Aquilina, S.; Asero, R.; Baker, D.; Ballmer-Weber, B.; Bangert, C.; Ben-Shoshan, M.; Bernstein, J.A.; et al. The International EAACI/GA2LEN/EuroGuiDerm/APAAACI Guideline for the Definition, Classification, Diagnosis, and Management of Urticaria. Allergy 2022, 77, 734–766. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Borges, M.; Ansotegui, I.J.; Baiardini, I.; Bernstein, J.; Canonica, G.W.; Ebisawa, M.; Gomez, M.; Gonzalez-Diaz, S.N.; Martin, B.; Morais-Almeida, M.; et al. The Challenges of Chronic Urticaria Part 1: Epidemiology, Immunopathogenesis, Comorbidities, Quality of Life, and Management. World Allergy Organ. J. 2021, 14, 100533. [Google Scholar] [CrossRef] [PubMed]
- Jain, S. Pathogenesis of chronic urticaria: An overview. Dermatol. Res. Pract. 2014, 2014, 674709. [Google Scholar] [CrossRef] [PubMed]
- Konstantinou, G.N.; Riedl, M.A.; Valent, P.; Podder, I.; Maurer, M. Urticaria and angioedema: Understanding complex pathomechanisms to facilitate patient communication, disease management, and future treatment. J. Allergy Clin. Immunol. Pract. 2023, 11, 94–106. [Google Scholar] [CrossRef] [PubMed]
- Church, M.K.; Kolkhir, P.; Metz, M.; Maurer, M. The role and relevance of mast cells in urticaria. Immunol. Rev. 2018, 282, 232–247. [Google Scholar] [CrossRef]
- Elieh Ali Komi, D.; Wöhrl, S.; Bielory, L. Mast cell biology at molecular level: A comprehensive review. Clin. Rev. Allergy Immunol. 2020, 58, 342–365. [Google Scholar] [CrossRef]
- Choi, J.E.; Di Nardo, A. Skin neurogenic inflammation. In Seminars in Immunopathology; Springer: Berlin/Heidelberg, Germany, 2018; pp. 249–259. [Google Scholar] [CrossRef]
- Xu, H.; Shi, X.; Li, X.; Zou, J.; Zhou, C.; Liu, W.; Shao, H.; Chen, H.; Shi, L. Neurotransmitter and neuropeptide regulation of mast cell function: A systematic review. J. Neuroinflammation 2020, 17, 356. [Google Scholar] [CrossRef]
- Kay, A.B.; Clark, P.; Maurer, M.; Ying, S. Elevations in T-helper-2-initiating cytokines (interleukin-33, interleukin-25 and thymic stromal lymphopoietin) in lesional skin from chronic spontaneous (‘idiopathic’) urticaria. Br. J. Dermatol. 2015, 172, 1294–1302. [Google Scholar] [CrossRef]
- Ferrer, M. Immunological events in chronic spontaneous urticaria. Clin. Transl. Allergy 2015, 5, 30. [Google Scholar] [CrossRef]
- Konstantinou, G.N.; Konstantinou, G.N. Psychological stress and chronic urticaria: A neuro-immuno-cutaneous crosstalk. A systematic review of the existing evidence. Clin. Ther. 2020, 42, 771–782. [Google Scholar] [CrossRef]
- Bansal, C.J.; Bansal, A.S. Stress, pseudoallergens, autoimmunity, infection and inflammation in chronic spontaneous urticaria. Allergy Asthma Clin. Immunol. 2019, 15, 56. [Google Scholar] [CrossRef] [PubMed]
- Kocatürk, E.; Salman, A.; Cherrez-Ojeda, I.; Criado, P.R.; Peter, J.; Comert-Ozer, E.; Abuzakouk, M.; Agondi, R.C.; Al-Ahmad, M.; Altrichter, S.; et al. The global impact of the COVID-19 pandemic on the management and course of chronic urticaria. Allergy 2020, 76, 816–830. [Google Scholar] [CrossRef] [PubMed]
- Beyaz, S.; Demir, S.; Oztop, N.; Karadag, P.; Coskun, R.; Colakoglu, B.; Buyukozturk, S.; Gelincik, A. Psychological burden of COVID-19 on mild and moderate chronic spontaneous urticaria. Allergy Asthma Proc. 2021, 42, e107–e115. [Google Scholar] [CrossRef]
- Ben-Shoshan, M.; Blinderman, I.; Raz, A. Psychosocial factors and chronic spontaneous urticaria: A systematic review. Allergy 2013, 68, 131–141. [Google Scholar] [CrossRef]
- Ferrer, M. Epidemiology, healthcare, resources, use and clinical features of different types of urticaria. J. Investig. Allergol. Clin. Immunol. 2009, 19 (Suppl. 2), 21–26. [Google Scholar] [PubMed]
- Delong, L.K.; Culler, S.D.; Saini, S.S.; Beck, L.A.; Chen, S.C. Annual direct and indirect health care costs of chronic idiopathic urticaria: A cost analysis of 50 nonimmunosuppressed patients. Arch. Dermatol. 2008, 144, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Consoli, S.G. Les facteurs psychologiques dans l’urticaire chronique [Psychological factors in chronic urticaria]. Ann. Dermatol. Venereol. 2003, 130, 1S73–1S77. [Google Scholar] [PubMed]
- Barbosa, F.; Freitas, J.; Barbosa, A. Chronic idiopathic urticaria and anxiety symptoms. J. Health Psychol. 2011, 16, 1038–1047. [Google Scholar] [CrossRef]
- Özkan, M.; Oflaz, S.B.; Kocaman, N.; Özşeker, F.; Gelincik, A.; Büyüköztürk, S.; Özkan, S.; Çolakoğlu, B. Psychiatric morbidity and quality of life in patients with chronic idiopathic urticaria. Ann. Allergy Asthma Immunol. 2007, 99, 29–33. [Google Scholar] [CrossRef]
- Malhotra, S.K.; Mehta, V. Role of stressful life events in induction or exacerbation of psoriasis and chronic urticaria. Indian J. Dermatol. Venereol. Leprol. 2008, 74, 594–599. [Google Scholar] [CrossRef]
- Tawil, S.; Irani, C.; Kfoury, R.; Abramian, S.; Salameh, P.; Weller, K.; Maurer, M.; Ezzedine, K. Association of Chronic Urticaria with Psychological Distress: A Multicentre Cross-sectional Study. Acta Derm. Venereol. 2022, 103, adv00865. [Google Scholar] [CrossRef] [PubMed]
- Kaaz, K.; Szepietowski, J.C.; Matusiak, Ł. Sleep quality among adult patients with chronic dermatoses. Postepy Dermatol Alergol. 2019, 36, 659–666. [Google Scholar] [CrossRef]
- Ates, H.; Firat, S.; Buhari, G.K.; Keren, M.; Cifci, B.; Erkekol, F.Ö. Relationships between quality of life, sleep problems, and sleep quality in patients with chronic idiopathic urticaria. J. Cosmet. Dermatol. 2022, 21, 4072–4079. [Google Scholar] [CrossRef] [PubMed]
- Alatas, E.T.; Unal, Y.; Demir Pektas, S.; Kutlu, G. Obstructive sleep apnea syndrome in patients with chronic idiopathic urticaria. Dermatol. Ther. 2020, 33, e14060. [Google Scholar] [CrossRef]
- Mikulska, J.; Juszczyk, G.; Gawrońska-Grzywacz, M.; Herbet, M. HPA Axis in the Pathomechanism of Depression and Schizophrenia: New Therapeutic Strategies Based on Its Participation. Brain Sci. 2021, 11, 1298. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, L. Hypothalamic–Pituitary–Adrenocortical Axis Regulation. Endocrinol. Metab. Clin. 2005, 34, 271–292. [Google Scholar] [CrossRef]
- Godoy, L.D.; Rossignoli, M.T.; Delfino-Pereira, P.; Garcia-Cairasco, N.; de Lima Umeoka, E.H. A Comprehensive Overview on Stress Neurobiology: Basic Concepts and Clinical Implications. Front. Behav. Neurosci. 2018, 12, 127. [Google Scholar] [CrossRef]
- Tank, A.W.; Wong, D.L. Peripheral and Central Effects of Circulating Catecholamines. Compr. Physiol. 2015, 5, 1–15. [Google Scholar] [CrossRef]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The Gut-Brain Axis: Interactions between Enteric Microbiota, Central and Enteric Nervous Systems. Ann. Gastroenterol. Q. Publ. Hell. Soc. Gastroenterol. 2015, 28, 78. [Google Scholar] [CrossRef]
- Farhadi, A.; Fields, J.Z.; Keshavarzian, A. Mucosal Mast Cells Are Pivotal Elements in Inflammatory Bowel Disease That Connect the Dots: Stress, Intestinal Hyperpermeability and Inflammation. World J. Gastroenterol. 2007, 13, 3027. [Google Scholar] [CrossRef]
- Wallon, C.; Yang, P.C.; Keita, Å.V.; Ericson, A.C.; McKay, D.M.; Sherman, P.M.; Perdue, M.H.; Söderholm, J.D. Corticotropin-Releasing Hormone (CRH) Regulates Macromolecular Permeability via Mast Cells in Normal Human Colonic Biopsies in Vitro. Gut 2008, 57, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Krišto, M.; Lugovi´c, L.; Mihi´c, L.-M.; Muñoz, M.; Rupnik, M.; Mahnic, A.; Ozreti´c, O.; Jaganjac, M.; Cesi´c, C.; Kuna, M. Gut Microbiome Composition in Patients with Chronic Urticaria: A Review of Current Evidence and Data. Life 2023, 13, 152. [Google Scholar] [CrossRef] [PubMed]
- Gubert, C.; Kong, G.; Renoir, T.; Hannan, A.J. Exercise, diet and stress as modulators of gut microbiota: Implications for neurodegenerative diseases. Neurobiol. Dis. 2020, 134, 104621. [Google Scholar] [CrossRef] [PubMed]
- Barrio, C.; Arias-Sanchez, S.; Martin-Monzon, I. The gut microbiota-brain axis, psychobiotics and its influence on brain and behaviour: A systematic review. Psychoneuroendocrinology 2022, 137, 105640. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, R.S. Psychological Stress and the Coping Process; McGraw-Hill: New York, NY, USA, 1966. [Google Scholar]
- Carver, C.S.; Scheier, M.F.; Weintraub, J.K. Assessing coping strategies: A theoretically based approach. J. Pers. Soc. Psychol. 1989, 56, 267–283. [Google Scholar] [CrossRef] [PubMed]
- Ogińska-Bulik, N. Zasoby osobiste jako wyznaczniki radzenia sobie ze stresem u dzieci. [Personal resources as determinants of coping with stress in children]. Folia Psychol. 2001, 5, 83–93. [Google Scholar]
- McEwen, B.S. Protection and Damage from Acute and Chronic Stress: Allostasis and Allostatic Overload and Relevance to the Pathophysiology of Psychiatric Disorders. Ann. N. Y. Acad. Sci. 2004, 1032, 1–7. [Google Scholar] [CrossRef]
- Bishop, G.D. Psychologia Zdrowia [Health Psychology]; Astrum: Wroclaw, Poland, 2007. [Google Scholar]
- Juczyński, Z.; Ogińska-Bulik, N. NPSR-Narzędzia Pomiaru Stresu i Radzenia Sobie ze Stresem [Stress and Coping Measurement Tools]; Polskie Towarzystwo Psychologiczne: Warsaw, Poland, 2009. [Google Scholar]
- Endler, N.S.; Parker, J.D. Coping Inventory for Stressful Situations (CISS): Manual; Multi-Health Systems: Toronto, ON, Canada, 1999. [Google Scholar]
- Zigmond, A.S.; Snaith, R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef]
- Spielberger, C.D.; Gorsuch, R.L.; Lushene, R.; Vagg, P.R.; Jacobs, G.A. Manual for the State-Trait Anxiety Inventory; Consulting Psychologists Press: Palo Alto, CA, USA, 1983. [Google Scholar]
- Dalgard, F.J.; Gieler, U.; Tomas-Aragones, L.; Lien, L.; Poot, F.; Jemec, G.B.; Misery, L.; Szabo, C.; Linder, D.; Sampogna, F.; et al. The psychological burden of skin diseases: A cross-sectional multicenter study among dermatological out-patients in 13 European countries. J. Investig. Dermatol. 2015, 135, 984–991. [Google Scholar] [CrossRef]
- Jerković, H.; Bešlić, I.; Ćesić, D.; Šitum, M. The psychosocial burden of urticaria. Rad Hrvat. Akad. Znan. I Umjetnosti. Med. Znan. 2021, 548, 98–103. [Google Scholar] [CrossRef]
- Tat, T.S. Higher levels of depression and anxiety in patients with chronic urticaria. Med. Sci. Monit. 2019, 25, 115. [Google Scholar] [CrossRef] [PubMed]
- Choi, G.S.; Nam, Y.H.; Park, C.S.; Kim, M.Y.; Jo, E.J.; Park, H.K.; Kim, H.K. Anxiety, depression, and stress in Korean patients with chronic urticaria. Korean J. Intern. Med. 2020, 35, 1507–1516. [Google Scholar] [CrossRef] [PubMed]
- Patella, V.; Zunno, R.; Florio, G.; Palmieri, M.; Palmieri, S.; Brancaccio, R. Omalizumab improves perceived stress, anxiety, and depression in chronic spontaneous urticaria. J. Allergy Clin. Immunol. Pract. 2021, 9, 1402–1404. [Google Scholar] [CrossRef] [PubMed]
- Pondeljak, N.; Lugović-Mihić, L. Stress-induced interaction of skin immune cells, hormones, and neurotransmitters. Clin. Ther. 2020, 42, 757–770. [Google Scholar] [CrossRef]
- Nedoszytko, B.; Sokołowska-Wojdyło, M.; Ruckemann-Dziurdzińska, K.; Roszkiewicz, J.; Nowicki, R. Chemokines and cytokines network in the pathogenesis of the inflammatory skin diseases: Atopic dermatitis, psoriasis and skin mastocytosis. Adv. Dermatol. Allergol. 2014, 31, 84–91. [Google Scholar] [CrossRef]
- Varghese, R.; Rajappa, M.; Chandrashekar, L.; Kattimani, S.; Archana, M.; Munisamy, M.; Revathy, G.; Thappa, D. Association among stress, hypocortisolism, systemic inflammation, and disease severity in chronic urticaria. Ann. Allergy Asthma Immunol. 2016, 116, 344–348. [Google Scholar] [CrossRef]
- Kolkhir, P.; Altrichter, S.; Hawro, T.; Maurer, M. C-reactive protein is linked to disease activity, impact, and response to treatment in patients with chronic spontaneous urticaria. Allergy. 2018, 73, 940–948. [Google Scholar] [CrossRef]
- Kim, J.E.; Cho, B.K.; Cho, D.H.; Park, H.J. Expression of hypothalamic–pituitary–adrenal axis in common skin diseases: Evidence of its association with stress-related disease activity. Acta Derm.-Venereol. 2013, 93, 387–393. [Google Scholar] [CrossRef]
- Papadopoulou, N.; Kalogeromitros, D.; Staurianeas, N.G.; Tiblalexi, D.; Theoharides, T.C. Corticotropin-releasing hormone receptor-1 and histidine decarboxylase expression in chronic urticaria. J. Investig. Dermatol. 2005, 125, 952–955. [Google Scholar] [CrossRef]
- Georgala, S.; Schulpis, K.H.; Papaconstantinou, E.; Varelzidis, A. Raised serum levels of β-endorphin in chronic urticaria. J. Eur. Acad. Dermatol. Venereol. 1994, 3, 27–30. [Google Scholar] [CrossRef]
- Vurgun, E.; Memet, B.; Kocaturk, E.; Guntas, G. Evaluation of serum 25-hydroxyvitamin D levels and cortisol/dehydroepiandrosterone sulfate ratio in chronic spontaneous urticaria. Turk. J. Biochem. 2020, 46, 191–196. [Google Scholar] [CrossRef]
- Huang, X.; Hussain, B.; Chang, J. Peripheral Inflammation and Blood–Brain Barrier Disruption: Effects and Mechanisms. CNS Neurosci. Ther. 2021, 27, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Brzoza, Z.; Kasperska-Zajac, A.; Rogala, B. Serum prolactin concentration and its relationship with dehydroepiandrosterone sulfate concentration in chronic urticaria patients with positive and negative response to autologous serum skin test. Allergy 2007, 62, 566–567. [Google Scholar] [CrossRef] [PubMed]
- Theoharides, T.C.; Singh, K.L.; Boucher, W.; Pang, X.; Letourneau, R.; Webster, E.; Chrousos, G. Corticotropin-Releasing Hormone Induces Skin Mast Cell Degranulation and Increased Vascular Permeability, a Possible Explanation for Its Proinflammatory Effects. Endocrinology 1998, 139, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Konstantinou, G.N.; Konstantinou, G.N.; Koulias, C.; Petalas, K.; Makris, M. Further understanding of Neuro-Immune Interactions in Allergy: Implications in Pathophysiology and Role in Disease Progression. J. Asthma Allergy 2022, 15, 1273–1291. [Google Scholar] [CrossRef] [PubMed]
- Dyke, S.M.; Carey, B.S.; Kaminski, E.R. Effect of Stress on Basophil Function in Chronic Idiopathic Urticaria. Clin. Exp. Allergy 2008, 38, 86–92. [Google Scholar] [CrossRef]
- Basak, P.Y.; Erturan, I.; Yuksel, O.; Kazanoglu, O.O.; Vural, H. Evaluation of serum neuropeptide levels in patients with chronic urticaria. Indian J. Dermatol. Venereol. Leprol. 2014, 80, 483. [Google Scholar] [CrossRef]
- Metz, M.; Krull, C.; Hawro, T.; Saluja, R.; Groffik, A. Substance P is upregulated in the serum of patients with chronic spontaneous urticaria. J. Investig. Dermatol. 2014, 134, 2833–2836. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, J.; Zhu, W.; Xu, C.; He, S. Upregulated expression of substance P in basophils of the patients with chronic spontaneous urticaria: Induction of histamine release and basophil accumulation by substance P. Cell Biol. Toxicol. 2016, 32, 217–228. [Google Scholar] [CrossRef]
- Borici-Mazi, R.; Kouridakis, S.; Kontou-Fili, K. Cutaneous responses to substance P and calcitonin gene-related peptide in chronic urticaria: The effect of cetirizine and dimethindene. Allergy 1999, 54, 46–56. [Google Scholar] [CrossRef]
- Chen, Y.; Lyga, J. Brain-Skin Connection: Stress, Inflammation and Skin Aging. Inflamm. Allergy-Drug Targets (Former. Curr. Drug Targets-Inflamm. Allergy)(Discontin.) 2014, 13, 177. [Google Scholar] [CrossRef] [PubMed]
- Voss, M.; Kotrba, J.; Gaffal, E.; Katsoulis-Dimitriou, K.; Dudeck, A. Mast Cells in the Skin: Defenders of Integrity or Offenders in Inflammation? Int. J. Mol. Sci. 2021, 22, 4589. [Google Scholar] [CrossRef] [PubMed]
- Asadi, S.; Alysandratos, K.D.; Angelidou, A.; Miniati, A.; Sismanopoulos, N.; Vasiadi, M.; Zhang, B.; Kalogeromitros, D.; Theoharides, T.C. Substance P (SP) Induces Expression of Functional Corticotropin-Releasing Hormone Receptor-1 (CRHR-1) in Human Mast Cells. J. Investig. Dermatol. 2012, 132, 324–329. [Google Scholar] [CrossRef]
- Fujisawa, D.; Kashiwakura, J.; Kita, H.; Kikukawa, Y.; Fujitani, Y.; Sasaki-Sakamoto, T.; Kuroda, K.; Nunomura, S.; Hayama, K.; Terui, T.; et al. Expression of Mas-related gene X2 on mast cells is upregulated in the skin of patients with severe chronic urticaria. J. Allergy Clin. Immunol. 2014, 134, 622–633. [Google Scholar] [CrossRef] [PubMed]
- Fok, J.S.; Kolkhir, P.; Church, M.K.; Maurer, M. Predictors of treatment response in chronic spontaneous urticaria. Allergy 2021, 76, 2965–2981. [Google Scholar] [CrossRef] [PubMed]
- Redegeld, F.A.; Yu, Y.; Kumari, S.; Charles, N.; Blank, U. Non-IgE mediated mast cell activation. Immunol. Rev. 2018, 282, 87–113. [Google Scholar] [CrossRef]
- Theoharides, T.C. Neuroendocrinology of mast cells: Challenges and controversies. Exp. Dermatol. 2017, 26, 751–759. [Google Scholar] [CrossRef]
- Zalewska-Janowska, A. (Red.) Psychodermatologia. In Ujęcie Interdyscyplinarne [Psychodermatology. Interdyscyplinary Approach]; PZWL Wydawnictwo lekarskie: Warsaw, Poland, 2022; ISBN 9788301223502. [Google Scholar]
- Tzur Bitan, D.; Berzin, D.; Cohen, A. The association of chronic spontaneous urticaria (CSU) with anxiety and depression: A nationwide cohort study. Arch. Dermatol. Res. 2021, 313, 33–39. [Google Scholar] [CrossRef]
- Berrino, A.M.; Voltolini, S.; Fiaschi, D.; Pellegrini, S.; Bignardi, D.; Minale, P.; Troise, C.; Maura, E. Chronic urticaria: Importance of a medical-psychological approach. Eur. Ann. Allergy Clin. Immunol. 2006, 38, 149–152. [Google Scholar]
- Kronenberger, M. Muzykoterapia. In Podstawy Teoretyczne do Zastosowania Muzykoterapii w Profilaktyce stresu [Music Therapy. Theoretical Basis for the Application of Music Therapy in Stress Prevention]; Mediatour: Szczecin, Poland, 2006. [Google Scholar]
- Błaszczak, A. Wpływ Treningu Redukcji Stresu opartego na uważności (MBSR) na zdrowie fizyczne [Effects of Mindfulness-Based Stress Reduction Training (MBSR) on physical health]. Ann. Univ. Mariae Curie-Skłodowska Sect. J. Paedagog.-Psychol. 2018, 31, 61–73. [Google Scholar] [CrossRef]
- Lindsay, K.; Goulding, J.; Solomon, M.; Broom, B. Treating chronic spontaneous urticaria using a brief 'whole person' treatment approach: A proof-of-concept study. Clin. Transl. Allergy 2015, 5, 40. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).