The Effects of Methylfolate on Cognitive Decline and Dementia: A Protocol for Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Protocol and Registration
2.2. Definitions
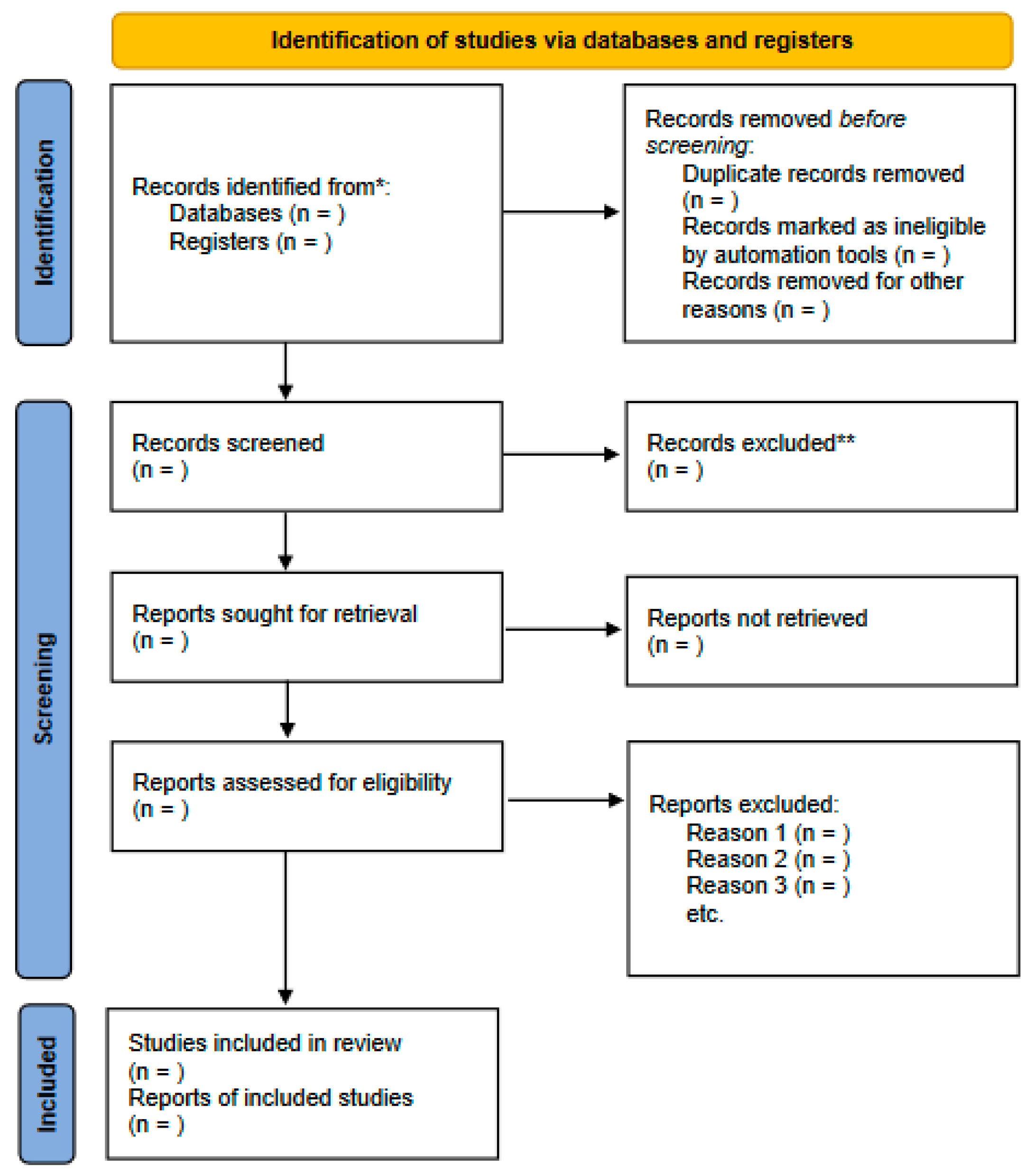
2.3. Research Strategy
2.4. Study Population
2.5. Criteria and Eligibility
2.5.1. Inclusion Criteria
- Older adults (aged ≥60 or ≥65 years, depending on the classification used where the research was conducted) with a diagnosis of MCI or some type of dementia.
- Randomized and controlled clinical trials based on methylfolate or folic-acid supplementation, regardless of the type of administration route, dose used, and intervention time.
- Representative population samples and a cohort of individuals in hospitals, health centers, outpatient clinics, or institutionalized settings, i.e., nursing homes.
- Studies published in English, Portuguese, or Spanish.
2.5.2. Exclusion Criteria
- Studies whose main data are not accessible, even upon author request.
- Duplicates: for studies published in more than one article, we will consider the most comprehensive, with the largest sample sizes.
- Studies carried out with indigenous individuals.
- Studies conducted on individuals diagnosed with liver disease, chronic kidney disease (on dialysis), cancers, malabsorptive diseases such as inflammatory bowel disease, celiac disease or other intestinal diseases that interfere with oral interventions, chronic infections, bipolar affective disorder, Parkinson’s disease, multiple sclerosis, motor neuron disease, developmental impairment, central-nervous-system inflammation, progressive malignancy, psychotic symptoms, schizophrenia, alcohol or other drug addiction, and any medical or psychological condition preventing them from completing the ratings.
- Studies with individuals who have used nutritional supplements known to interfere with nutritional status, folate metabolism, or cognitive function three months prior to recruitment.
2.6. Reviewer Training
Review Process
2.7. Data Extraction
2.8. Assessment of the Quality of the Articles and Risk of Bias
2.9. Synthesis of Evidence and Statistical Analyses
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nichols, E.; Szoeke, C.E.I.; Vollset, S.E.; Vollset, S.E.; Abbasi, N.; Abd-Allah, F.; Abdela, J.; Aichour, M.T.E.; Akinyemi, R.O.; Alahdab, F.; et al. Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 88–106. [Google Scholar] [CrossRef] [PubMed]
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C.; et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020, 396, 413–446. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, M.; Wang, J.; Davis, D.R.; Lane, M.J.; Cornman, C.B.; Fadden, M.K. Co-morbidity associated with dementia. Am. J. Alzheimer’s Dis. Other Dement. 2002, 17, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Zhou, X.; Li, Q.; Zhao, J.; Song, A.; An, P.; Du, Y.; Xu, W.; Huang, G. Effects of Folic Acid and Vitamin B12, Alone and in Combination on Cognitive Function and Inflammatory Factors in the Elderly with Mild Cognitive Impairment: A Single-blind Experimental Design. Curr. Alzheimer Res. 2019, 16, 622–632. [Google Scholar] [CrossRef]
- Patterson, C. World Alzeimer Report 2018; Alzheimer’s Disease International: London, UK, 2018. [Google Scholar]
- Malouf, R.; Evans, J.G. Folic acid with or without vitamin B12 for the prevention and treatment of healthy elderly and demented people. Cochrane Database Syst. Rev. 2008, 4, CD004514. [Google Scholar] [CrossRef]
- Dangour, A.D.; Whitehouse, P.J.; Rafferty, K.; Mitchell, S.A.; Smith, L.; Hawkesworth, S.; Vellas, B. B-vitamins and fatty acids in the prevention and treatment of Alzheimer’s disease and dementia: A systematic review. J. Alzheimer’s Dis. 2010, 22, 205–224. [Google Scholar] [CrossRef]
- Avitan, I.; Halperin, Y.; Saha, T.; Bloch, N.; Atrahimovich, D.; Polis, B.; Samson, A.O.; Braitbard, O. Towards a consensus on Alzheimer’s disease comorbidity? J. Clin. Med. 2021, 10, 4360. [Google Scholar] [CrossRef]
- Santiago, J.A.; Potashkin, J.A. The Impact of Disease Comorbidities in Alzheimer’s Disease. Front. Aging Neurosci. 2021, 13, 631770. [Google Scholar] [CrossRef]
- Haaksma, M.L.; Vilela, L.R.; Marengoni, A.; Calderon-Larrañaga, A.; Leoutsakos, J.M.S.; Rikkert, M.G.M.O.; Melis, R.J.F. Comorbidity and progression of late onset Alzheimer’s disease: A systematic review. PLoS ONE 2017, 12, e0177044. [Google Scholar] [CrossRef]
- Wu, Y.-M.; Kuo, H.-C.; Li, C.-C.; Wu, H.-L.; Chen, J.-T.; Cherng, Y.-G.; Chen, T.-J.; Dai, Y.-X.; Liu, H.-Y.; Tai, Y.-H. Preexisting dementia is associated with increased risks of mortality and morbidity following major surgery: A nationwide propensity score matching study. Int. J. Environ. Res. Public Health 2020, 17, 8431. [Google Scholar] [CrossRef]
- Yu, J.T.; Xu, W.; Tan, C.C.; Andrieu, S.; Suckling, J.; Evangelou, E.; Pan, A.; Zhang, C.; Jia, J.; Feng, L.; et al. Evidence-based prevention of Alzheimer’s disease: Systematic review and meta-analysis of 243 observational prospective studies and 153 randomised controlled trials. J. Neurol. Neurosurg. Psychiatry 2020, 91, 1201–1209. [Google Scholar] [CrossRef]
- Atlante, A.; Amadoro, G.; Bobba, A.; Latina, V. Functional Foods: An Approach to Modulate Molecular Mechanisms of Alzheimer’s Disease. Cells 2020, 9, 2347. [Google Scholar] [CrossRef]
- Zhang, D.-M.; Ye, J.-X.; Mu, J.-S.; Cui, X.-P. Efficacy of Vitamin B Supplementation on Cognition in Elderly Patients with Cognitive-Related Diseases: A Systematic Review and Meta-Analysis. J. Geriatr. Psychiatry Neurol. 2017, 30, 50–59. [Google Scholar] [CrossRef]
- Ma, F.; Wu, T.; Zhao, J.; Ji, L.; Song, A.; Zhang, M.; Huang, G. Plasma homocysteine and serum folate and vitamin B12 levels in mild cognitive impairment and Alzheimer’s disease: A case-control study. Nutrients 2017, 9, 725. [Google Scholar] [CrossRef]
- Price, B.R.; Wilcock, D.M.; Weekman, E.M. Hyperhomocysteinemia as a Risk Factor for Vascular Contributions to Cognitive Impairment and Dementia. Front. Aging Neurosci. 2018, 10, 350. [Google Scholar] [CrossRef]
- La Rue, A.; Koehler, K.M.; Wayne, S.J.; Chiulli, S.J.; Haaland, K.Y.; Garry, P.J. Nutritional status and cognitive functioning in a normally aging sample: A 6-y reassessment. Am. J. Clin. Nutr. 1997, 65, 20–29. [Google Scholar] [CrossRef]
- Agrawal, A.; Ilango, K.; Singh, P.K.; Karmakar, D.; Singh, G.; Kumari, R.; Dubey, G. Age dependent levels of plasma homocysteine and cognitive performance. Behav. Brain Res. 2015, 283, 139–144. [Google Scholar] [CrossRef]
- Choi, S.-W.; Mason, J.B. Recent Advances in Nutritional Sciences Folate and Carcinogenesis: An Integrated Scheme 1–3. J. Nutr. 2000, 130, 129–132. [Google Scholar] [CrossRef]
- Kruman, I.I.; Kumaravel, T.S.; Lohani, A.; Cutler, R.G.; Kruman, Y.; Haughey, N.; Lee, J.; Evans, M.; Mattson, M.P. Folic acid deficiency and homocysteine impair DNA repair in hippocampal neurons and sensitize them to amyloid toxicity in experimental models of Alzheimer’s disease. J. Neurosci. 2002, 22, 1752–1762. [Google Scholar] [CrossRef]
- Heppner, F.L.; Ransohoff, R.M.; Becher, B. Immune attack: The role of inflammation in Alzheimer disease. Nat. Rev. Neurosci. 2015, 16, 358–372. [Google Scholar] [CrossRef]
- Hardy, J.; Selokoe, D. The Amyloid Hypothesis of Alzheimer’s Disease. Amyloid Int. J. Exp. Clin. Investig. 2002, 297, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Connelly, P.J.; Prentice, N.P.; Cousland, G.; Bonham, J. A randomised double-blind placebo-controlled trial of folic acid supplementation of cholinesterase inhibitors in Alzheimer’s disease. Int. J. Geriatr. Psychiatry 2008, 23, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Wu, T.; Zhao, J.; Han, F.; Marseglia, A.; Liu, H.; Huang, G. Effects of 6-Month Folic Acid Supplementation on Cognitive Function and Blood Biomarkers in Mild Cognitive Impairment: A Randomized Controlled Trial in China. J. Gerontol.-Ser. A Biol. Sci. Med. Sci. 2016, 71, 1376–1383. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Liu, S.; Ji, L.; Wu, T.; Ji, Y.; Zhou, Y.; Zheng, M.; Zhang, M.; Xu, W.; Huang, G. Folic Acid Supplementation Mitigates Alzheimer’s Disease by Reducing Inflammation: A Randomized Controlled Trial. Mediat. Inflamm. 2016, 2016, 5912146. [Google Scholar] [CrossRef]
- Nilsson, K.; Gustafson, L.; Hultberg, B. Improvement of cognitive functions after cobalamin/folate supplementation in elderly patients with dementia and elevated plasma homocysteine. Int. J. Geriatr. Psychiatry 2001, 16, 609–614. [Google Scholar] [CrossRef]
- De Jager, C.A.; Oulhaj, A.; Jacoby, R.; Refsum, H.; Smith, A.D. Cognitive and clinical outcomes of homocysteine-lowering B-vitamin treatment in mild cognitive impairment: A randomized controlled trial. Int. J. Geriatr. Psychiatry 2012, 27, 592–600. [Google Scholar] [CrossRef]
- Wald, D.S.; Kasturiratne, A.; Simmonds, M. Effect of Folic Acid, with or without Other B Vitamins, on Cognitive Decline: Meta-Analysis of Randomized Trials. Am. J. Med. 2010, 123, 522–527.e2. [Google Scholar] [CrossRef]
- Hankey, G.J.; Ford, A.H.; Yi, Q.; Eikelboom, J.W.; Lees, K.R.; Chen, C.; Xavier, D.; Navarro, J.C.; Ranawaka, U.K.; Uddin, W.; et al. Effect of B vitamins and lowering homocysteine on cognitive impairment in patients with previous stroke or transient ischemic attack: A prespecified secondary analysis of a randomized, placebo-controlled trial and meta-analysis. Stroke 2013, 44, 2232–2239. [Google Scholar] [CrossRef]
- Rommer, P.S.; Fuchs, D.; Leblhuber, F.; Schroth, R.; Greilberger, M.; Tafeit, E.; Greilberger, J. Lowered levels of carbonyl proteins after Vitamin B supplementation in patients with mild cognitive impairment and Alzheimer’s disease. Neurodegener. Dis. 2016, 16, 284–289. [Google Scholar] [CrossRef]
- Mccleery, J.; Abraham, R.P.; Denton, D.A.; Rutjes, A.W.S.; Chong, L.-Y.; Al-Assaf, A.S.; Griffith, D.J.; Rafeeq, S.; Yaman, H.; Malik, M.A.; et al. Vitamin and mineral supplementation for preventing dementia or delaying cognitive decline in people with mild cognitive impairment. Cochrane Database Syst. Rev. 2018, 2018, CD011905. [Google Scholar] [CrossRef]
- Solfrizzi, V.; Agosti, P.; Lozupone, M.; Custodero, C.; Schilardi, A.; Valiani, V.; Santamato, A.; Sardone, R.; Dibello, V.; Di Lena, L.; et al. Nutritional interventions and cognitive-related outcomes in patients with late-life cognitive disorders: A systematic review. Neurosci. Biobehav. Rev. 2018, 95, 480–498. [Google Scholar] [CrossRef]
- Ford, A.H.; Almeida, O.P. Effect of Vitamin B Supplementation on Cognitive Function in the Elderly: A Systematic Review and Meta-Analysis. Drugs Aging 2019, 36, 419–434. [Google Scholar] [CrossRef]
- Rutjes, A.W.; Denton, D.A.; Di Nisio, M.; Chong, L.-Y.; Abraham, R.P.; Al-Assaf, A.S.; Anderson, J.L.; Malik, A.M.; Vernooij, R.; Martínez, G.; et al. Vitamin and mineral supplementation for maintaining cognitive function in cognitively healthy people in mid and late life. Cochrane Database Syst. Rev. 2018, 12, CD011906. [Google Scholar] [CrossRef]
- Hui, E.K.; Tischler, V.; Wong, G.H.Y.; Lau, W.Y.T.; Spector, A. Systematic review of the current psychosocial interventions for people with moderate to severe dementia. Int. J. Geriatr. Psychiatry 2021, 36, 1313–1329. [Google Scholar] [CrossRef]
- Samieri, C.; Yassine, H.N.; Melo van Lent, D.; Lefèvre-Arbogast, S.; van de Rest, O.; Bowman, G.L.; Scarmeas, N. Personalized nutrition for dementia prevention. Alzheimer’s Dement. 2022, 18, 1424–1437. [Google Scholar] [CrossRef]
- Nday, C.M.; Eleftheriadou, D.; Jackson, G. Shared pathological pathways of Alzheimer’s disease with specific comorbidities: Current perspectives and interventions. J. Neurochem. 2018, 144, 360–389. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Silveira, E.A.; de Sousa Romeiro, A.M.; Noll, M. Guide for scientific writing: How to avoid common mistakes in a scientific article. J. Hum. Growth Dev. 2022, 32, 341–352. [Google Scholar] [CrossRef]
- Eriksen, M.B.; Frandsen, T.F. The Impact of PICO as a Search Strategy Tool on Literature Search Quality: A Systematic Review. J. Med. Libr. Assoc. 2018, 106, 420–431. Available online: https://jmla.pitt.edu/ojs/jmla/article/view/345/726 (accessed on 25 January 2023). [CrossRef]
- Jia, L.; Du, Y.; Chu, L.; Zhang, Z.; Li, F.; Lyu, D.; Li, Y.; Zhu, M.; Jiao, H.; Song, Y.; et al. Prevalence, risk factors, and management of dementia and mild cognitive impairment in adults aged 60 years or older in China: A cross-sectional study. Lancet Public Health 2020, 5, e661–e671. [Google Scholar] [CrossRef]
- Albert, M.S.; DeKosky, S.T.; Dickson, D.; Dubois, B.; Feldman, H.H.; Fox, N.C.; Gamst, A.; Holtzman, D.M.; Jagust, W.J.; Petersen, R.C.; et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011, 7, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Langa, K.M.; Levine, D.A. The diagnosis and management of mild cognitive impairment: A clinical review. JAMA-J. Am. Med. Assoc. 2014, 312, 2551–2561. [Google Scholar] [CrossRef]
- Sommer, I.; Griebler, U.; Kien, C.; Auer, S.; Klerings, I.; Hammer, R.; Holzer, P.; Gartlehner, G. Vitamin D deficiency as a risk factor for dementia: A systematic review and meta-analysis. BMC Geriatr. 2017, 17, 16. [Google Scholar] [CrossRef] [PubMed]
- Folstein, M.; Folstein, S.; McHugh, P. Mini-mental satate. A Pratical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A Brief Screening Tool for Mild Cognitive Impairment. JAGS 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Pfeffer, R.I.; Kurosaki, T.T.; Harrah, C.H.; Chance, J.M.; Filos, R.S. Measurement of functional activities in older adults in the community. J. Gerontol. 1982, 37, 323–329. [Google Scholar] [CrossRef]
- Arvanitakis, Z.; Shah, R.C.; Bennett, D.A. Diagnosis and Management of Dementia: Review. JAMA-J. Am. Med. Assoc. 2019, 322, 1589–1599. [Google Scholar] [CrossRef]
- Chertkow, H.; Feldman, H.H.; Jacova, C.; Massoud, F. Definitions of dementia and predementia states in Alzheimer’s disease and vascular cognitive impairment: Consensus from the Canadian conference on diagnosis of dementia. Alzheimer’s Res. Ther. 2013, 5 (Suppl. 1), S2. [Google Scholar] [CrossRef]
- Burns, A.; Iliffe, S. Dementia. BMJ 2009, 338, b75. [Google Scholar] [CrossRef]
- McKhann, G.; Drachman, D.; Folstein, M.; Katzman, R.; Price, D.; Stadlan, E.M. Clinical diagnosis of Alzheimer’s disease. Neurology 1984, 34, 939. [Google Scholar] [CrossRef]
- McKhann, G.M.; Knopman, D.S.; Chertkow, H.; Hyman, B.T.; Jack, C.R., Jr.; Kawas, C.H.; Klunk, W.E.; Koroshetz, W.J.; Manly, J.J.; Mayeux, R.; et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011, 7, 263–269. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Stefani, L.; Galanti, G.; Padulo, J.; Bragazzi, N.L.; Maffulli, N. Sexual activity before sports competition: A systematic review. Front. Physiol. 2016, 7, 246. [Google Scholar] [CrossRef]
- Guyatt, G.; Oxman, A.D.; Akl, E.A.; Kunz, R.; Vist, G.; Brozek, J.; Norris, S.; Falck-Ytter, Y.; Glasziou, P.; DeBeer, H.; et al. GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables. J. Clin. Epidemiol. 2011, 64, 383–394. [Google Scholar] [CrossRef]
- Downs, S.H.; Black, N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J. Epidemiol. Community Health 1998, 52, 377–384. [Google Scholar] [CrossRef]
- Behrens, A.; Graessel, E.; Pendergrass, A.; Donath, C. Vitamin B-Can it prevent cognitive decline? A systematic review and meta-analysis. Syst. Rev. 2020, 9, 111. [Google Scholar] [CrossRef]
- Gofir, A.; Wibowo, S.; Hakimi, M.; Putera, D.D.; Satriotomo, I.; Mustofa, M. Folic Acid Treatment for Patients with Vascular Cognitive Impairment: A Systematic Review and Meta-Analysis. Int. J. Neuropsychopharmacol. 2022, 25, 136–143. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, J.; Chang, H.; Liu, X.; Zhu, R. Homocysteine and Folic Acid: Risk Factors for Alzheimer’s Disease—An Updated Meta-Analysis. Front. Aging Neurosci. 2021, 13, 665114. [Google Scholar] [CrossRef]
| 1. | Dementia OR Cognitive Impairments OR Cognitive disorders |
| 2. | Clinical Trial OR Randomized Controlled Trial OR Controlled Clinical Trial |
| 3. | Methylfolate OR Folic acid OR Vitamin B9 |
| 4. | 1 AND 2 AND 3 |
| PubMed | Results | |
|---|---|---|
| 1. | (((((((((((((((((((((((((((((dementia [MeSH Terms]) OR (“dementia, vascular” [MeSH Terms])) OR (“frontotemporal dementia” [MeSH Terms])) OR (“alzheimer disease” [MeSH Terms])) OR (“neurocognitive disorders” [MeSH Terms])) OR (“cognition disorders” [MeSH Terms])) OR (amnesia [MeSH Terms])) OR (“neurodegenerative diseases” [MeSH Terms])) OR (“cognitive dysfunction” [MeSH Terms])) OR (“Lewy body disease” [MeSH Terms])) OR (cognition [MeSH Terms])) OR (dementia [Title/Abstract])) OR (“dementia vascular” [Title/Abstract])) OR (“frontotemporal dementia” [Title/Abstract])) OR (“alzheimer disease” [Title/Abstract])) OR (“neurocognitive disorders” [Title/Abstract])) OR (“cognition disorders” [Title/Abstract])) OR (amnesia [Title/Abstract])) OR (“neurodegenerative diseases” [Title/Abstract])) OR (“neurodegenerative disease” [Title/Abstract])) OR (“cognitive dysfunction” [Title/Abstract])) OR (“cognitive impairments” [Title/Abstract])) OR (“alzheimer dementia” [Title/Abstract])) OR (“vascular dementia” [Title/Abstract])) OR (“Lewy body disease” [Title/Abstract])) OR (“Lewy body dementia” [Title/Abstract])) OR (“Cognitive function” [Title/Abstract])) OR (cognition [Title/Abstract])) OR (“Cognitive Symptoms” [Title/Abstract])) OR (“Cognitive Symptom” [Title/Abstract]) | 754,959 |
| 2. | (((((((((((“Double blind method” [MeSH Terms]) OR (“Cross-over studies” [MeSH Terms])) OR (“Clinical Trial” [Title/Abstract])) OR (“Randomized Controlled Trial” [Title/Abstract])) OR (“Controlled Clinical Trial” [Title/Abstract])) OR (“controlled trial” [Title/Abstract])) OR (trial [Title/Abstract])) OR (“double blind procedure” [Title/Abstract])) OR (“Double blind method” [Title/Abstract])) OR (“crossover procedure” [Title/Abstract])) OR (“Cross-over studies” [Title/Abstract])) OR (intervention [Title/Abstract]) | 1,351,025 |
| 3. | ((((((((((“folic acid” [MeSH Terms]) OR (“pteroylpolyglutamic acids” [MeSH Terms])) OR (Tetrahydrofolates [MeSH Terms])) OR (Formyltetrahydrofolates [MeSH Terms])) OR (“folic acid” [Title/Abstract])) OR (“pteroylpolyglutamic acids” [Title/Abstract])) OR (Tetrahydrofolate [Title/Abstract])) OR (“vitamin B9” [Title/Abstract])) OR (Formyltetrahydrofolate [Title/Abstract])) OR (Methylfolate [Title/Abstract])) OR (“L-methylfolate” [Title/Abstract]) | 50,208 |
| 4. | #1 AND #2 AND #3 | 249 |
| Embase | Results | |
|---|---|---|
| 1. | dementia:ti,ab,kw OR ‘multiinfarct dementia’:ti,ab,kw OR ‘frontotemporal dementia’:ti,ab,kw OR ‘alzheimer disease’:ti,ab,kw OR ‘disorders of higher cerebral function’:ti,ab,kw OR amnesia:ti,ab,kw OR ‘degenerative disease’:ti,ab,kw OR ‘cognitive defect’:ti,ab,kw OR ‘cognitive impairment no dementia’:ti,ab,kw OR ‘lewy body’:ti,ab,kw OR cognition:ti,ab,kw | 330,924 |
| 2. | ‘clinical trial’:ti,ab,kw OR ‘controlled study’:ti,ab,kw OR ‘randomized controlled trial’:ti,ab,kw OR ‘controlled clinical trial’:ti,ab,kw OR ‘double blind procedure’:ti,ab,kw OR ‘crossover procedure’:ti,ab,kw OR trial:ti,ab,kw OR intervention:ti,ab,kw | 1,856,599 |
| 3. | ‘folic acid’:ti,ab,kw OR pteroptin:ti,ab,kw OR ‘tetrahydrofolic acid derivative’:ti,ab,kw OR ‘vitamin b9’:ti,ab,kw OR methylfolate:ti,ab,kw | 30,177 |
| 4. | #1 AND #2 AND #3 | 192 |
| LILACS | Results | |
|---|---|---|
| 1. | (mh:(dementia)) OR (mh:(Alzheimer Dementia)) OR (mh:(Alzheimer Dementias)) OR (mh:(Alzheimer Type Dementia (ATD))) OR (mh:(Alzheimer Type Dementia Senile Dementia)) OR (mh:(Dementia, Frontotemporal)) OR (mh:(Dementia, Frontotemporal Lobe)) OR (mh:(Dementia, Lacunar)) OR (mh:(Dementia, Mixed)) OR (mh:(Dementia, Primary Senile Degenerative)) OR (mh:(Dementia, Senile)) OR (mh:(Dementia, Subcortical Vascular)) OR (mh:(Dementia, Vascular)) OR (mh:(Dementias, Frontotemporal)) OR (mh:(Dementias, Frontotemporal Lobe)) OR (mh:(Dementias, Lacunar)) OR (mh:(Dementias, Subcortical Vascular)) OR (mh:(Dementias, Vascular)) OR (Cognitive Impairments) OR (Dementia) OR (Alzheimer Dementia) OR (Alzheimer Dementias) OR (Dementia, Frontotemporal) OR (Dementias, Lacunar) OR (Impairments, Cognitive) OR (Cognitive Impairments, Mild) OR (Impairments, Mild Cognitive) OR (Mild Cognitive Impairments) OR (Cognitive Disorders) OR (Dementia, Cognitive Disordes) | 15.558 |
| 2. | (mh:(Clinical Trial)) OR (mh:(Double Blind Method)) OR (mh:(Cross-over Studies)) OR (Randomized Controlled Clinical Trial) OR (Randomized Clinical) | 9.686 |
| 3. | (mh:(Folic Acid)) OR (mh:(Pteroylpolyglutamic acids)) OR (mh:(Vitamin B9)) OR (Folic Acid) OR (Pteroylpolyglutamic acids) OR (Vitamin B9) | 947 |
| 4. | #1 AND #2 AND #3 | 151 |
| Scopus | Results | |
|---|---|---|
| 1. | (TITLE-ABS-KEY (dementia) OR TITLE-ABS-KEY (“dementia vascular”) OR TITLE-ABS-KEY (“frontotemporal dementia”) OR TITLE-ABS-KEY (“alzheimer disease”) OR TITLE-ABS-KEY (“neurocognitive disorders”) OR TITLE-ABS-KEY (“cognition disorders”) OR TITLE-ABS-KEY (amnesia) OR TITLE-ABS-KEY (“neurodegenerative diseases”) OR TITLE-ABS-KEY (“neurodegenerative disease”) OR TITLE-ABS-KEY (“cognitive dysfunction”) OR TITLE-ABS-KEY (“cognitive impairments”) OR TITLE-ABS-KEY (“alzheimer dementia”) OR TITLE-ABS-KEY (“alzheimer’s disease”) OR TITLE-ABS-KEY (“vascular dementia”) OR TITLE-ABS-KEY (“Lewy body disease”) OR TITLE-ABS-KEY (“Lewy body dementia”) OR TITLE-ABS-KEY (“Cognitive function”) OR TITLE-ABS-KEY (cognition) OR TITLE-ABS-KEY (“cognitive symptoms”) OR TITLE-ABS-KEY (“cognitive symptom”)) | 890,582 |
| 2. | (TITLE-ABS-KEY (“clinical trial”) OR TITLE-ABS-KEY (“randomized controlled trial”) OR TITLE-ABS-KEY (“Controlled Clinical Trial”) OR TITLE-ABS-KEY (“controlled trial”) OR TITLE-ABS-KEY (trial) OR TITLE-ABS-KEY (“double blind procedure”) OR TITLE-ABS-KEY (“double blind method”) OR TITLE-ABS-KEY (“crossover procedure”) OR TITLE-ABS-KEY (“Cross-over studies”) OR TITLE-ABS-KEY (intervention)) | 4,040,072 |
| 3. | (TITLE-ABS-KEY (“folic acid”) OR TITLE-ABS-KEY (“pteroylpolyglutamic acids”) OR TITLE-ABS-KEY (tetrahydrofolate) OR TITLE-ABS-KEY (“vitamin B9”) OR TITLE-ABS-KEY (formyltetrahydrofolate) OR TITLE-ABS-KEY (methylfolate) OR TITLE-ABS-KEY (“L-methylfolate”)) | 86,427 |
| 4. | #1 AND #2 AND #3 | 1221 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prado, L.I.d.A.; Junger, A.L.; Caixeta, L.F.; Noll, M.; Oliveira, C.d.; Silveira, É.A. The Effects of Methylfolate on Cognitive Decline and Dementia: A Protocol for Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 12, 3075. https://doi.org/10.3390/jcm12093075
Prado LIdA, Junger AL, Caixeta LF, Noll M, Oliveira Cd, Silveira ÉA. The Effects of Methylfolate on Cognitive Decline and Dementia: A Protocol for Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2023; 12(9):3075. https://doi.org/10.3390/jcm12093075
Chicago/Turabian StylePrado, Leícia Iris de Assunção, Ana Lúcia Junger, Leonardo Ferreira Caixeta, Matias Noll, Cesar de Oliveira, and Érika Aparecida Silveira. 2023. "The Effects of Methylfolate on Cognitive Decline and Dementia: A Protocol for Systematic Review and Meta-Analysis" Journal of Clinical Medicine 12, no. 9: 3075. https://doi.org/10.3390/jcm12093075
APA StylePrado, L. I. d. A., Junger, A. L., Caixeta, L. F., Noll, M., Oliveira, C. d., & Silveira, É. A. (2023). The Effects of Methylfolate on Cognitive Decline and Dementia: A Protocol for Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 12(9), 3075. https://doi.org/10.3390/jcm12093075








