Qualitative Assessment of Contrast-Enhanced Ultrasound in Differentiating Clear Cell Renal Cell Carcinoma and Oncocytoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Imaging Acquisition and Interpretation
2.3. Statistical Analysis
3. Results
3.1. Characteristics of the Included Patients
3.2. Renal Mass Characteristics on CUS and CEUS
3.3. Diagnostic Value of CEUS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hsieh, J.J.; Purdue, M.P.; Signoretti, S.; Swanton, C.; Albiges, L.; Schmidinger, M.; Heng, D.Y.; Larkin, J.; Ficarra, V. Renal cell carcinoma. Nat. Rev. Dis. Prim. 2017, 3, 17009. [Google Scholar] [CrossRef]
- van Oostenbrugge, T.J.; Fütterer, J.J.; Mulders, P.F.A. Diagnostic Imaging for Solid Renal Tumors: A Pictorial Review. Kidney Cancer 2018, 2, 79–93. [Google Scholar] [CrossRef]
- Tufano, A.; Drudi, F.M.; Angelini, F.; Polito, E.; Martino, M.; Granata, A.; Di Pierro, G.B.; Kutrolli, E.; Sampalmieri, M.; Canale, V.; et al. Contrast-Enhanced Ultrasound (CEUS) in the Evaluation of Renal Masses with Histopathological Validation-Results from a Prospective Single-Center Study. Diagnostics 2022, 12, 1209. [Google Scholar] [CrossRef]
- He, M.; Gao, Q.; Xiang, J.; Mao, Q.; Jiang, T. Diagnostic Value of Qualitative and Quantitative Contrast-Enhanced Ultrasound for Pathological Subtypes of Small Solid Renal Masses. J. Ultrasound Med. 2023; early view. [Google Scholar] [CrossRef]
- Siracusano, S.; Quaia, E.; Bertolotto, M.; Ciciliato, S.; Tiberio, A.; Belgrano, E. The application of ultrasound contrast agents in the characterization of renal tumors. World J. Urol. 2004, 22, 316–322. [Google Scholar] [CrossRef]
- Siracusano, S.; Bertolotto, M.; Ciciliato, S.; Valentino, M.; Liguori, G.; Visalli, F. The current role of contrast-enhanced ultrasound (CEUS) imaging in the evaluation of renal pathology. World J. Urol. 2011, 29, 633–638. [Google Scholar] [CrossRef]
- Sidhu, P.S.; Cantisani, V.; Dietrich, C.F.; Gilja, O.H.; Saftoiu, A.; Bartels, E.; Bertolotto, M.; Calliada, F.; Clevert, D.A.; Cosgrove, D.; et al. The EFSUMB Guidelines and Recommendations for the Clinical Practice of Contrast-Enhanced Ultrasound (CEUS) in Non-Hepatic Applications: Update 2017 (Long Version). Ultraschall Med. 2018, 39, e2–e44. (In English) [Google Scholar] [CrossRef]
- Vijay, V.; Vokshi, F.H.; Smigelski, M.; Nagpal, S.; Huang, W.C. Incidence of Benign Renal Masses in a Contemporary Cohort of Patients Receiving Partial Nephrectomy for Presumed Renal Cell Carcinoma. Clin. Genitourin. Cancer, 2022; Online ahead of print. [Google Scholar] [CrossRef]
- Kim, J.H.; Shim, S.R.; Lee, H.Y.; Park, J.J.; Khandwala, Y.S.; Jeong, I.G.; Chung, B.I. Prevalence of benign pathology after partial nephrectomy for suspected renal tumor: A systematic review and meta-analysis. Int. J. Surg. 2020, 84, 161–170. [Google Scholar] [CrossRef]
- Anderson, C.B.; Lipsky, M.; Nandula, S.V.; Freeman, C.E.; Matthews, T.; Walsh, C.E.; Li, G.; Szabolcs, M.; Mansukhani, M.M.; McKiernan, J.M.; et al. Cytogenetic analysis of 130 renal oncocytomas identify three distinct and mutually exclusive diagnostic classes of chromosome aberrations. Genes Chromosomes Cancer 2020, 59, 6–12. [Google Scholar] [CrossRef]
- Hagenkord, J.M.; Parwani, A.V.; Lyons-Weiler, M.A.; Alvarez, K.; Amato, R.; Gatalica, Z.; Gonzalez-Berjon, J.M.; Peterson, L.; Dhir, R.; Monzon, F.A. Virtual karyotyping with SNP microarrays reduces uncertainty in the diagnosis of renal epithelial tumors. Diagn. Pathol. 2008, 3, 44. [Google Scholar] [CrossRef]
- Kuroda, N.; Toi, M.; Hiroi, M.; Shuin, T.; Enzan, H. Review of renal oncocytoma with focus on clinical and pathobiological aspects. Histol. Histopathol. 2003, 18, 935–942. [Google Scholar] [CrossRef]
- Wobker, S.E.; Williamson, S.R. Modern Pathologic Diagnosis of Renal Oncocytoma. J. Kidney Cancer VHL 2017, 4, 1–12. [Google Scholar] [CrossRef]
- Morra, M.N.; Das, S. Renal oncocytoma: A review of histogenesis, histopathology, diagnosis and treatment. J. Urol. 1993, 150 Pt 1, 295–302. [Google Scholar] [CrossRef]
- Montironi, R.; Cheng, L.; Scarpelli, M.; Lopez-Beltran, A. Pathology and Genetics: Tumours of the Urinary System and Male Genital System: Clinical Implications of the 4th Edition of the WHO Classification and Beyond. Eur. Urol. 2016, 70, 120–123. [Google Scholar] [CrossRef]
- Schieda, N.; Lim, R.S.; McInnes, M.D.F.; Thomassin, I.; Renard-Penna, R.; Tavolaro, S.; Cornelis, F.H. Characterization of small (<4 cm) solid renal masses by computed tomography and magnetic resonance imaging: Current evidence and further development. Diagn. Interv. Imaging 2018, 99, 443–455. [Google Scholar] [CrossRef]
- Choudhary, S.; Rajesh, A.; Mayer, N.J.; Mulcahy, K.A.; Haroon, A. Renal oncocytoma: CT features cannot reliably distinguish oncocytoma from other renal neoplasms. Clin. Radiol. 2009, 64, 517–522. [Google Scholar] [CrossRef]
- Wei, S.P.; Xu, C.L.; Zhang, Q.; Zhang, Q.R.; Zhao, Y.E.; Huang, P.F.; Xie, Y.D.; Zhou, C.S.; Tian, F.L.; Yang, B. Contrast-enhanced ultrasound for differentiating benign from malignant solid small renal masses: Comparison with contrast-enhanced CT. Abdom. Radiol. 2017, 42, 2135–21455. [Google Scholar] [CrossRef]
- Tufano, A.; Flammia, R.S.; Antonelli, L.; Minelli, R.; Franco, G.; Leonardo, C.; Cantisani, V. The Value of Contrast-Enhanced Ultrasound (CEUS) in Differentiating Testicular Masses: A Systematic Review and Meta-Analysis. Appl. Sci. 2021, 11, 8990. [Google Scholar] [CrossRef]
- Xue, L.Y.; Lu, Q.; Huang, B.J.; Li, C.X.; Yan, L.X.; Wang, W.P. Differentiation of subtypes of renal cell carcinoma with contrast-enhanced ultrasonography. Clin. Hemorheol. Microcirc. 2016, 63, 361–371. [Google Scholar] [CrossRef]
- Rennert, J.; Georgieva, M.; Schreyer, A.G.; Jung, W.; Ross, C.; Stroszczynski, C.; Jung, E.M. Image fusion of contrast enhanced ultrasound (CEUS) with computed tomography (CT) or magnetic resonance imaging (MRI) using volume navigation for detection, characterization and planning of therapeutic interventions of liver tumors. Clin. Hemorheol. Microcirc. 2011, 49, 67–81. [Google Scholar] [CrossRef] [PubMed]
- MacLennan, S.; Imamura, M.; Lapitan, M.C.; Omar, M.I.; Lam, T.B.; Hilvano-Cabungcal, A.M.; Royle, P.; Stewart, F.; MacLennan, G.; MacLennan, S.J.; et al. Systematic review of perioperative and quality-of-life outcomes following surgical management of localised renal cancer. Eur. Urol. 2012, 62, 1097–1117. [Google Scholar] [CrossRef] [PubMed]
- Tufano, A.; Antonelli, L.; Di Pierro, G.B.; Flammia, R.S.; Minelli, R.; Anceschi, U.; Leonardo, C.; Franco, G.; Drudi, F.M.; Cantisani, V. Diagnostic Performance of Contrast-Enhanced Ultrasound in the Evaluation of Small Renal Masses: A Systematic Review and Meta-Analysis. Diagnostics 2022, 12, 2310. [Google Scholar] [CrossRef] [PubMed]
- Gulati, M.; King, K.G.; Gill, I.S.; Pham, V.; Grant, E.; Duddalwar, V.A. Contrast-enhanced ultrasound (CEUS) of cystic and solid renal lesions: A review. Abdom. Imaging 2015, 40, 1982–1996. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.F.; Xu, H.X.; Xie, X.Y.; Liu, G.J.; Zheng, Y.L.; Liang, J.Y.; Lu, M.D. Renal cell carcinoma: Real-time contrast-enhanced ultrasound findings. Abdom. Imaging 2009, 35, 750–756. [Google Scholar] [CrossRef]
- Liu, H.; Cao, H.; Chen, L.; Fang, L.; Liu, Y.; Zhan, J.; Diao, X.; Chen, Y. The quantitative evaluation of contrast-enhanced ultrasound in the differentiation of small renal cell carcinoma subtypes and angiomyolipoma. Quant. Imaging Med. Surg. 2022, 12, 106–118. [Google Scholar] [CrossRef]
- Ignee, A.; Straub, B.; Schuessler, G.; Dietrich, C.F. Contrast enhanced ultrasound of renal masses. World J. Radiol. 2010, 2, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Barr, R.G. Use of lumason/sonovue in contrast-enhanced ultrasound of the kidney for characterization of renal masses—A meta-analysis. Abdom. Radiol. 2022, 47, 272–287. [Google Scholar] [CrossRef]
- Schwarze, V.; Marschner, C.; Negrão de Figueiredo, G.; Knösel, T.; Rübenthaler, J.; Clevert, D.A. Single-center study: The diagnostic performance of contrast-enhanced ultrasound (CEUS) for assessing renal oncocytoma. Scand. J. Urol. 2020, 54, 135–140. [Google Scholar] [CrossRef]
- Wei, S.; Fu, N.; Yao, C.; Liu, P.; Yang, B. Two- and three-dimensional contrast-enhanced sonography for assessment of renal tumor vasculature: Preliminary observations. J. Ultrasound Med. 2013, 32, 429–437. [Google Scholar] [CrossRef]
- Dai, W.B.; Yu, B.; Diao, X.H.; Cao, H.; Chen, L.; Chen, Y.; Zhan, J. Renal Masses: Evaluation with Contrast-Enhanced Ultrasound, with a Special Focus on the Pseudocapsule Sign. Ultrasound Med. Biol. 2019, 45, 1924–1932. [Google Scholar] [CrossRef]
- Chen, L.; Wang, L.; Diao, X.; Qian, W.; Fang, L.; Pang, Y.; Zhan, J.; Chen, Y. The diagnostic value of contrast-enhanced ultrasound in differentiating small renal carcinoma and angiomyolipoma. Biosci. Trends 2015, 9, 252–258. [Google Scholar] [CrossRef]
- Ascenti, G.; Gaeta, M.; Magno, C.; Mazziotti, S.; Blandino, A.; Melloni, D.; Zimbaro, G. Contrast-enhanced second-harmonic sonography in the detection of pseudocapsule in renal cell carcinoma. AJR Am. J. Roentgenol. 2004, 182, 1525–1530. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.Q.; Shen, Y.; Xu, L.W.; Xu, C.M.; Bi, W.; Wang, X.M. Contrast-enhanced ultrasound for detection and diagnosis of renal clear cell carcinoma. Chin. Med. J. 2009, 122, 1179–1183. [Google Scholar] [PubMed]
- Firouzabadi, F.D.; Gopal, N.; Homayounieh, F.; Anari, P.Y.; Li, X.; Ball, M.W.; Jones, E.C.; Samimi, S.; Turkbey, E.; Malayeri, A.A. CT radiomics for differentiating oncocytoma from renal cell carcinomas: Systematic review and meta-analysis. Clin. Imaging 2022, 94, 9–17. [Google Scholar] [CrossRef] [PubMed]
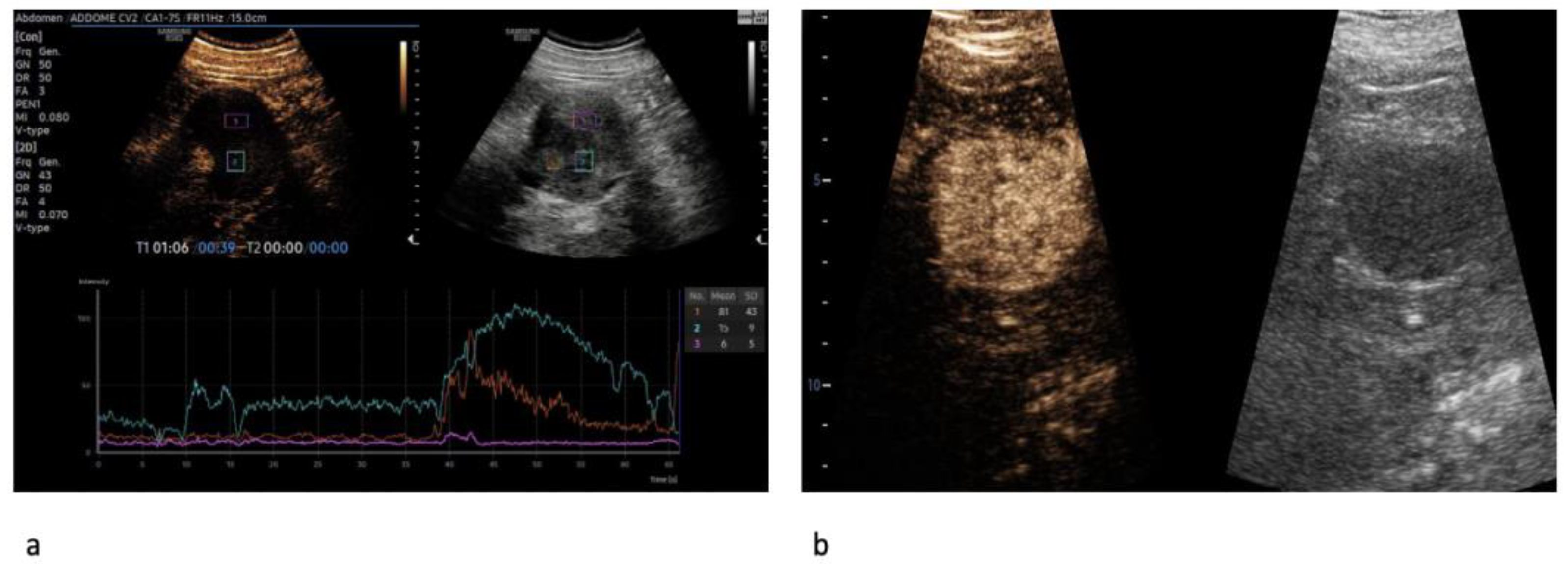
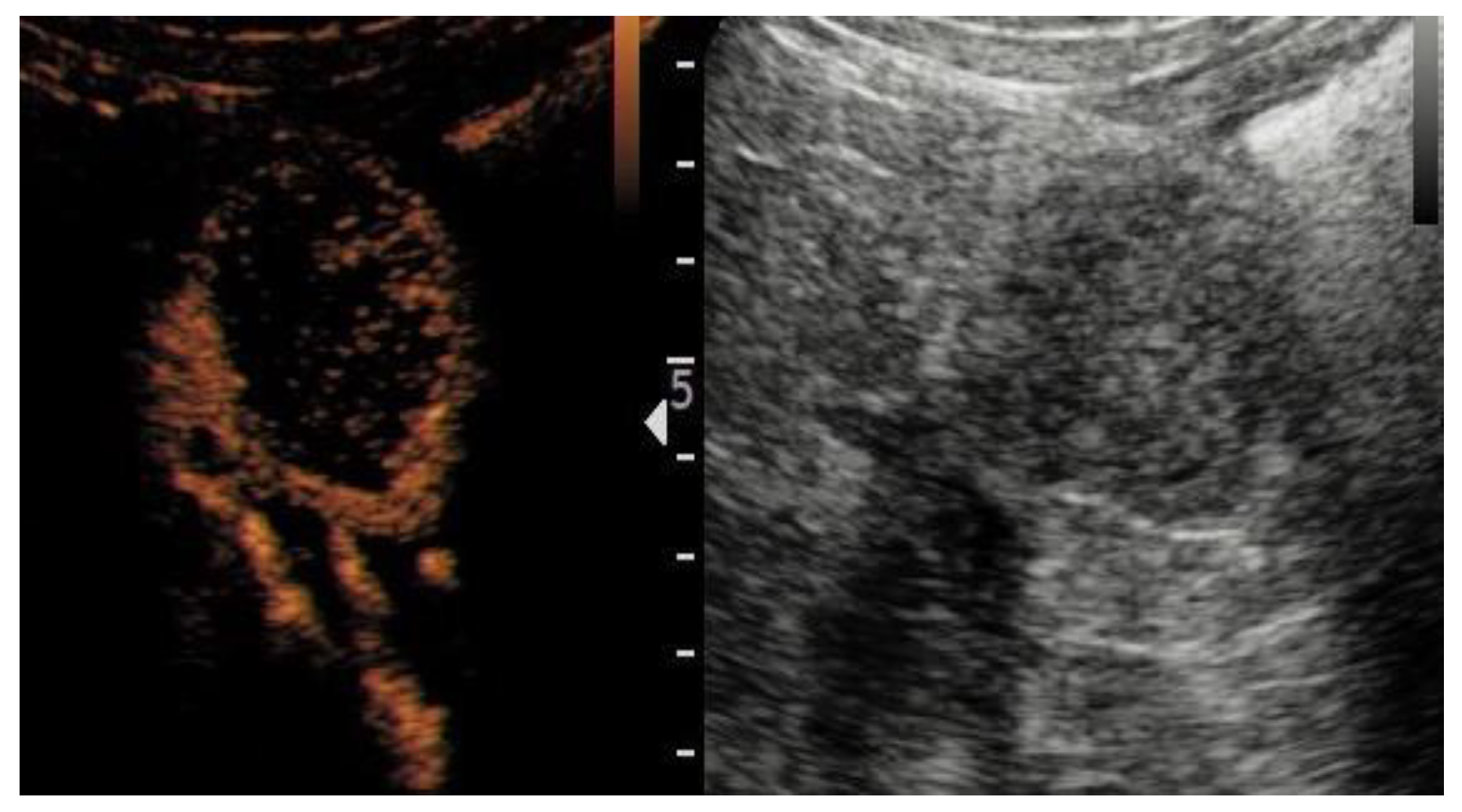
| Variable | Overall n = 126 | ccRCC n = 103 | RO n = 23 | p Value |
|---|---|---|---|---|
| Gender, n (%) | ||||
| Male | 76 (69.1%) | 60 (65.2%) | 16 (88.9%) | 0.047 |
| Female | 34 (30.9%) | 32 (34.8%) | 2 (11.1%) | |
| Age, years, median (IQR) | 64 (54–71) | 64 (54–71) | 64 (55–74) | 0.848 |
| Laterality, n (%) | ||||
| Left | 50 (48.1%) | 40 (45.5%) | 10 (62.5%) | 0.209 |
| Right | 54 (51.9%) | 48 (54.5%) | 6 (37.5%) | |
| Location (%) | ||||
| Superior | 50 (39.7%) | 39 (37.9%) | 11 (47.8%) | 0.521 |
| Middle | 43 (34.1%) | 35 (34.0%) | 8 (34.8%) | |
| Inferior | 33 (26.2%) | 29 (28.1%) | 4 (17.4%) | |
| Exophytic rate (%) | ||||
| >50% Exophytic | 83 (65.9%) | 67 (65.0%) | 16 (69.6%) | 0.68 |
| >50% Endophytic | 43 (34.1%) | 36 (35.0%) | 7 (30.4%) | |
| Size, mm (IQR) | 31 (20–50) | 32 (20–48) | 30 (20–59) | 0.924 |
| Variable | Overall n = 126 | ccRCC n = 103 | RO n = 23 | p Value |
|---|---|---|---|---|
| Margins | ||||
| Regular | 97 (77%) | 76 (73.8%) | 21 (91.3%) | 0.071 |
| Irregular | 29 (23%) | 27 (26.2%) | 2 (8.7%) | |
| Echogenicity | ||||
| Hypo | 69 (54.8%) | 62 (60.2%) | 7 (30.4%) | <0.001 |
| Iso | 36 (28.6%) | 22 (21.4%) | 14 (60.9%) | |
| Hyper | 21 (16.7%) | 19 (18.4%) | 2 (8.7%) | |
| CDFI | ||||
| Perilesional | 42 (33.3%) | 32 (31.1%) | 10 (43.5%) | 0.254 |
| Mixed | 84 (66.7%) | 71 (68.9%) | 13 (56.5%) | |
| Wash-in | ||||
| Fast | 95 (75.4%) | 83 (80.6%) | 12 (52.2%) | 0.004 |
| Synchronous/Slow | 31 (24.6%) | 20 (19.4%) | 11 (47.8%) | |
| Enhancement | ||||
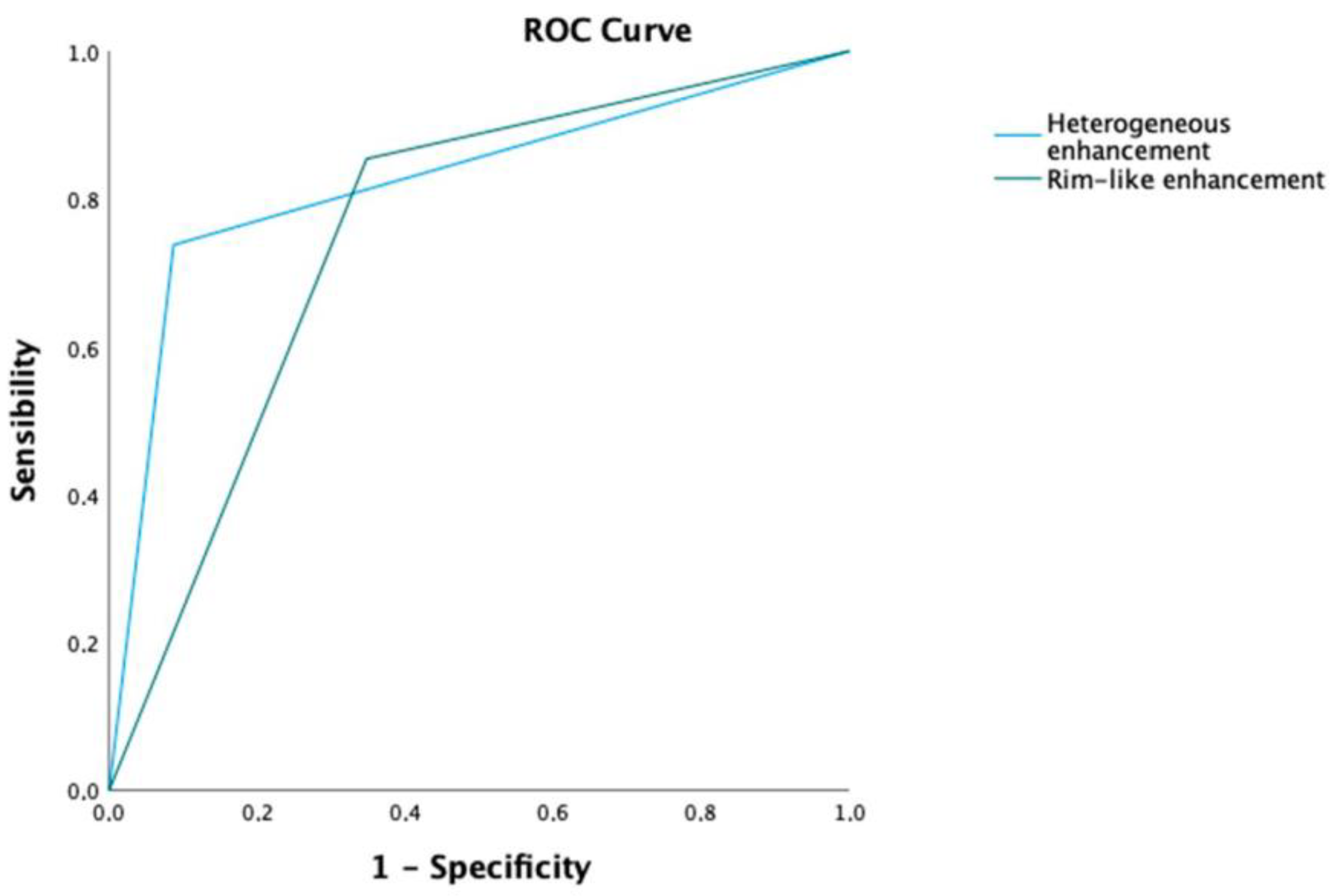
| Homogeneous | 48 (38.1%) | 27 (26.2%) | 21 (91.3%) | <0.001 |
| Heterogeneous | 78 (61.9%) | 76 (73.8%) | 2 (8.7%) | |
| Wash-out | ||||
| Fast | 55 (43.7%) | 52 (50.5%) | 3 (13.0%) | 0.001 |
| Synchronous/Slow | 71 (56.3%) | 51 (49.5%) | 20 (87.0%) | |
| Enhancement intensity | ||||
| Hyper | 86 (68.3%) | 68 (66%) | 18 (78.3%) | |
| Iso/Hypo | 40 (31.7%) | 35 (34%) | 5 (21.7%) | 0.254 |
| Rim-like enhancement | ||||
| No | 30 (23.8%) | 15 (14.6%) | 15 (65.2%) | |
| Yes | 96 (76.2%) | 88 (85.4%) | 8 (34.8%) | <0.001 |
| Univariable Analysis | Multivariable Analysis | |||||
|---|---|---|---|---|---|---|
| Variable | O.R. | 95% CI | p Value | O.R. | 95% CI | p Value |
| Wash-in (Fast vs. Synchronous/Slow) | 0.26 | 0.10–0.68 | 0.006 | 2.67 | 0.63–11.36 | 0.184 |
| Enhancement (Heterogeneous vs. Homogeneous) | 29.56 | 6.49–134.52 | 0.001 | 19.37 | 3.37–111.45 | <0.001 |
| Wash-out (Fast vs. Synchronous/Slow) | 6.79 | 1.90–24.29 | 0.003 | 1.25 | 0.22–7.11 | 0.802 |
| Enhancement intensity (Hyper vs. Iso/Hypo) | 0.54 | 0.18–1.58 | 0.259 | - | - | - |
| Rim-like enhancement | 11.00 | 3.97–30.44 | <0.001 | 3.73 | 1.01–13.00 | 0.049 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tufano, A.; Leonardo, C.; Di Bella, C.; Lucarelli, G.; Dolcetti, V.; Dipinto, P.; Proietti, F.; Flammia, R.S.; Anceschi, U.; Perdonà, S.; et al. Qualitative Assessment of Contrast-Enhanced Ultrasound in Differentiating Clear Cell Renal Cell Carcinoma and Oncocytoma. J. Clin. Med. 2023, 12, 3070. https://doi.org/10.3390/jcm12093070
Tufano A, Leonardo C, Di Bella C, Lucarelli G, Dolcetti V, Dipinto P, Proietti F, Flammia RS, Anceschi U, Perdonà S, et al. Qualitative Assessment of Contrast-Enhanced Ultrasound in Differentiating Clear Cell Renal Cell Carcinoma and Oncocytoma. Journal of Clinical Medicine. 2023; 12(9):3070. https://doi.org/10.3390/jcm12093070
Chicago/Turabian StyleTufano, Antonio, Costantino Leonardo, Chiara Di Bella, Giuseppe Lucarelli, Vincenzo Dolcetti, Piervito Dipinto, Flavia Proietti, Rocco Simone Flammia, Umberto Anceschi, Sisto Perdonà, and et al. 2023. "Qualitative Assessment of Contrast-Enhanced Ultrasound in Differentiating Clear Cell Renal Cell Carcinoma and Oncocytoma" Journal of Clinical Medicine 12, no. 9: 3070. https://doi.org/10.3390/jcm12093070
APA StyleTufano, A., Leonardo, C., Di Bella, C., Lucarelli, G., Dolcetti, V., Dipinto, P., Proietti, F., Flammia, R. S., Anceschi, U., Perdonà, S., Franco, G., Sciarra, A., Di Pierro, G. B., & Cantisani, V. (2023). Qualitative Assessment of Contrast-Enhanced Ultrasound in Differentiating Clear Cell Renal Cell Carcinoma and Oncocytoma. Journal of Clinical Medicine, 12(9), 3070. https://doi.org/10.3390/jcm12093070







