Strain Analysis in Patients with Frequent Premature Ventricular Complexes and Preserved Left Ventricular Function Undergoing Ablation
Abstract
1. Background
2. Methods
2.1. Inclusion and Exclusion Criteria
2.2. Holter Recordings
2.3. Mapping and Ablation
2.4. Echocardiography
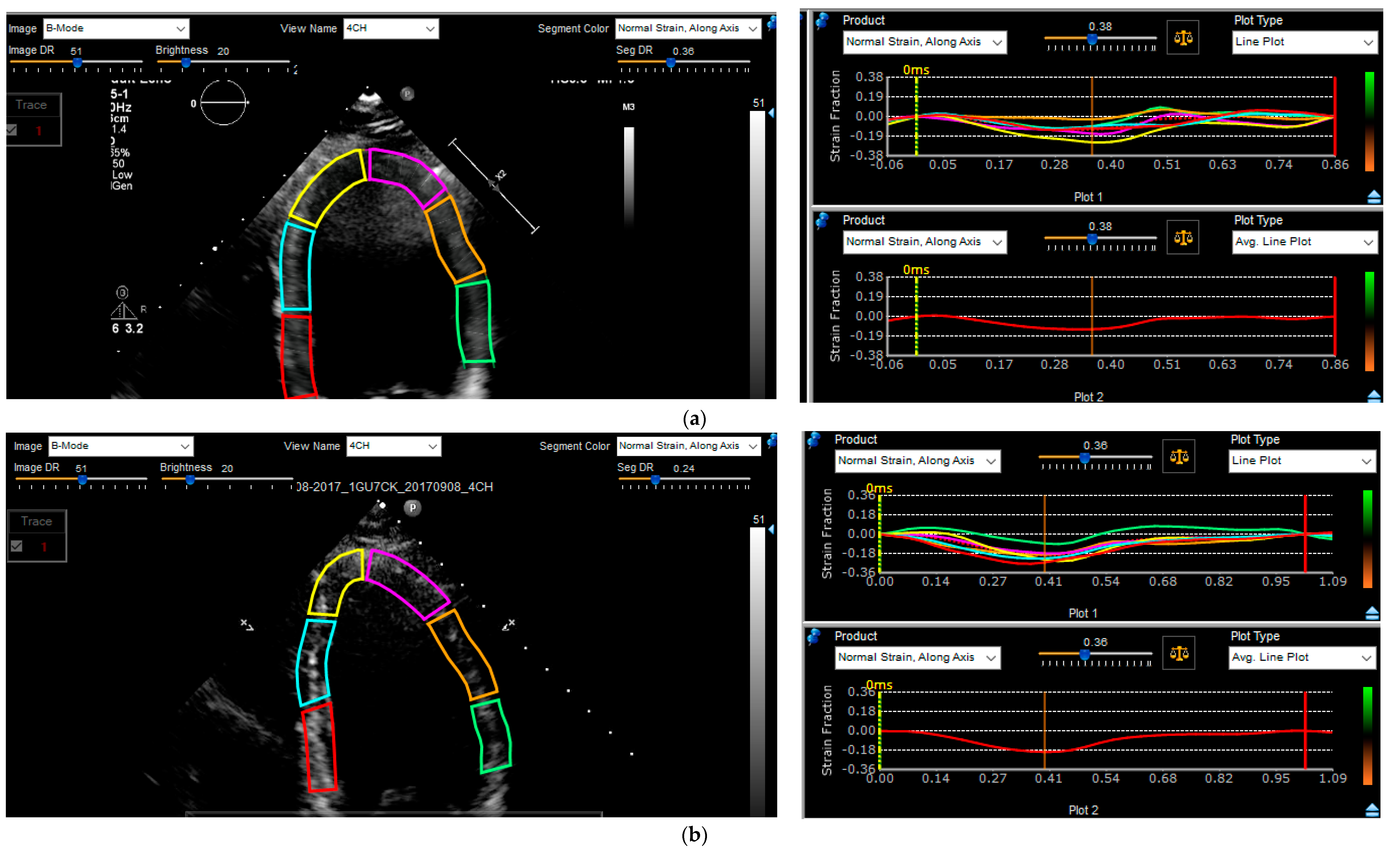
2.5. Strain Analysis
2.6. Follow-Up
2.7. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Ablation Details
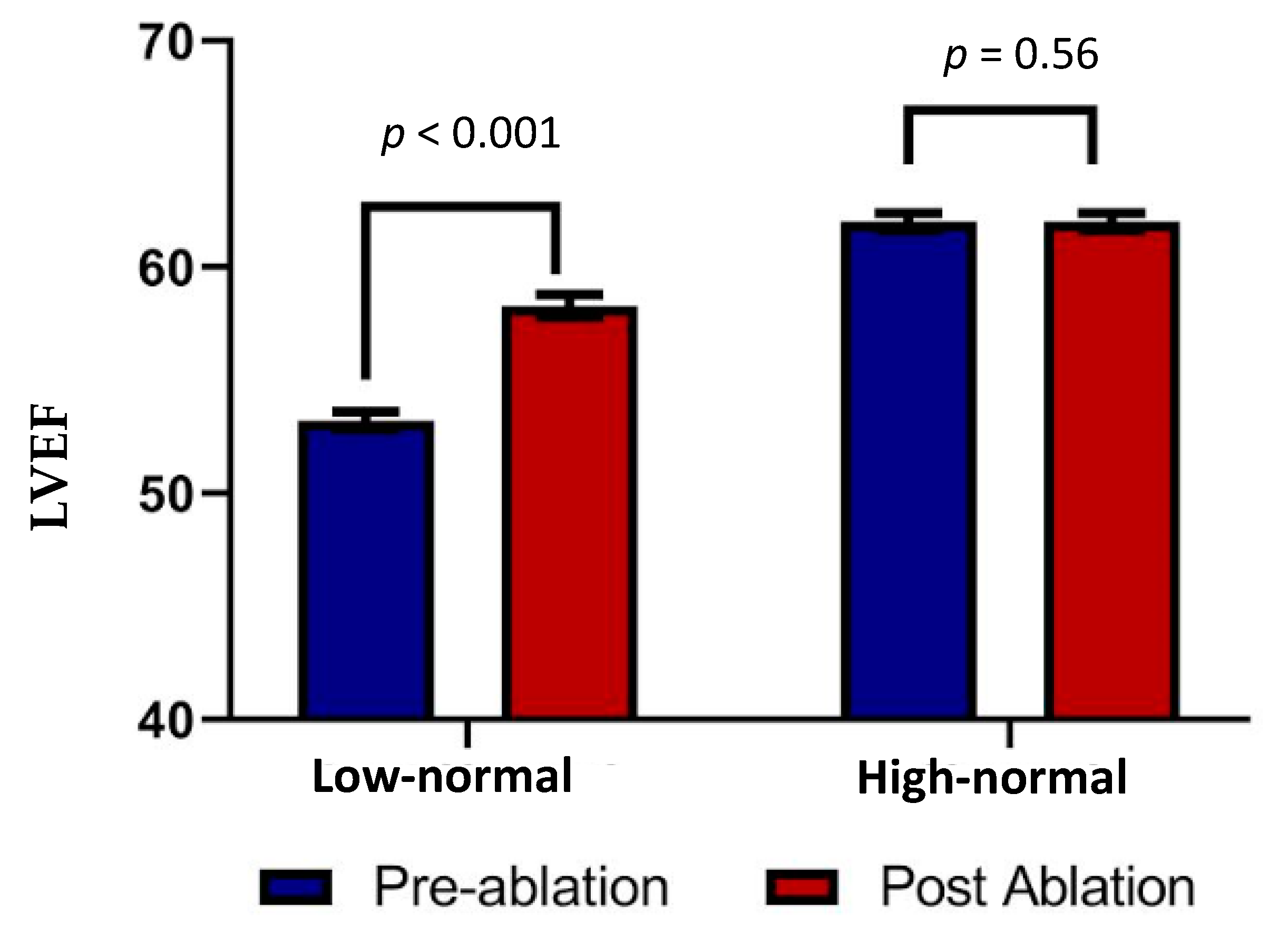
3.3. Echocardiographic Analysis
3.4. Follow-Up
4. Discussion
4.1. Main Findings
4.2. Left Ventricular Function and Frequent PVCs
4.3. Strain Analysis
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baman, T.S.; Lange, D.C.; Ilg, K.J.; Gupta, S.K.; Liu, T.Y.; Alguire, C.; Armstrong, W.; Good, E.; Chugh, A.; Jongnarangsin, K.; et al. Relationship between burden of premature ventricular complexes and left ventricular function. Heart Rhythm 2010, 7, 865–869. [Google Scholar] [CrossRef] [PubMed]
- Yokokawa, M.; Kim, H.M.; Good, E.; Chugh, A.; Pelosi, F., Jr.; Alguire, C.; Armstrong, W.; Crawford, T.; Jongnarangsin, K.; Oral, H.; et al. Relation of symptoms and symptom duration to premature ventricular complex-induced cardiomyopathy. Heart Rhythm 2012, 9, 92–95. [Google Scholar] [CrossRef] [PubMed]
- Yokokawa, M.; Kim, H.M.; Good, E.; Crawford, T.; Chugh, A.; Pelosi, F., Jr.; Jongnarangsin, K.; Latchamsetty, R.; Armstrong, W.; Alguire, C.; et al. Impact of QRS duration of frequent premature ventricular complexes on the development of cardiomyopathy. Heart Rhythm 2012, 9, 1460–1464. [Google Scholar] [CrossRef] [PubMed]
- Olgun, H.; Yokokawa, M.; Baman, T.; Kim, H.M.; Armstrong, W.; Good, E.; Chugh, A.; Pelosi, F., Jr.; Crawford, T.; Oral, H.; et al. The role of interpolation in PVC-induced cardiomyopathy. Heart Rhythm 2011, 8, 1046–1049. [Google Scholar] [CrossRef] [PubMed]
- Ghannam, M.; Yokokawa, M.; Liang, J.J.; Cochet, H.; Jais, P.; Dabagh, G.S.; Latchamsetty, R.; Jongnarangsin, K.; Morady, F.; Bogun, F. Clinical significance of myocardial scar in patients with frequent premature ventricular complexes undergoing catheter ablation. Heart Rhythm 2021, 18, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Deyell, M.W.; Park, K.M.; Han, Y.; Frankel, D.S.; Dixit, S.; Cooper, J.M.; Hutchinson, M.D.; Lin, D.; Garcia, F.; Bala, R.; et al. Predictors of recovery of left ventricular dysfunction after ablation of frequent ventricular premature depolarizations. Heart Rhythm 2012, 9, 1465–1472. [Google Scholar] [CrossRef] [PubMed]
- Cronin, E.M.; Bogun, F.M.; Maury, P.; Peichl, P.; Chen, M.; Namboodiri, N.; Aguinaga, L.; Leite, L.R.; Al-Khatib, S.M.; Anter, E.; et al. 2019 HRS/EHRA/APHRS/LAHRS expert consensus statement on catheter ablation of ventricular arrhythmias: Executive summary. Heart Rhythm 2020, 17, e155–e205. [Google Scholar] [CrossRef]
- Stoodley, P.W.; Richards, D.A.; Hui, R.; Boyd, A.; Harnett, P.R.; Meikle, S.R.; Clarke, J.; Thomas, L. Two-dimensional myocardial strain imaging detects changes in left ventricular systolic function immediately after anthracycline chemotherapy. Eur. J. Echocardiogr. 2011, 12, 945–952. [Google Scholar] [CrossRef]
- Fallah-Rad, N.; Walker, J.R.; Wassef, A.; Lytwyn, M.; Bohonis, S.; Fang, T.; Tian, G.; Kirkpatrick, I.D.; Singal, P.K.; Krahn, M.; et al. The utility of cardiac biomarkers, tissue velocity and strain imaging, and cardiac magnetic resonance imaging in predicting early left ventricular dysfunction in patients with human epidermal growth factor receptor II-positive breast cancer treated with adjuvant trastuzumab therapy. J. Am. Coll. Cardiol. 2011, 57, 2263–2270. [Google Scholar] [PubMed]
- Poterucha, J.T.; Kutty, S.; Lindquist, R.K.; Li, L.; Eidem, B.W. Changes in left ventricular longitudinal strain with anthracycline chemotherapy in adolescents precede subsequent decreased left ventricular ejection fraction. J. Am. Soc. Echocardiogr. 2012, 25, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Wijnmaalen, A.P.; Delgado, V.; Schalij, M.J.; van Huls van Taxis, C.F.; Holman, E.R.; Bax, J.J.; Zeppenfeld, K. Beneficial effects of catheter ablation on left ventricular and right ventricular function in patients with frequent premature ventricular contractions and preserved ejection fraction. Heart 2010, 96, 1275–1280. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Bierig, M.; Devereux, R.B.; Flachskampf, F.A.; Foster, E.; Pellikka, P.A.; Picard, M.H.; Roman, M.J.; Seward, J.; Shanewise, J.S.; et al. Recommendations for chamber quantification: A report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J. Am. Soc. Echocardiogr. 2005, 18, 1440–1463. [Google Scholar] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef] [PubMed]
- Farsalinos, K.E.; Daraban, A.M.; Unlu, S.; Thomas, J.D.; Badano, L.P.; Voigt, J.U. Head-to-Head Comparison of Global Longitudinal Strain Measurements among Nine Different Vendors: The EACVI/ASE Inter-Vendor Comparison Study. J Am Soc Echocardiogr 2015, 28, 1171–1181.e1172. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, M.; Parreira, L.; Farinha, J.M.; Marinheiro, R.; Esteves, A.; Goncalves, S.; Caria, R. Premature ventricular contractions of the right ventricular outflow tract: Is there an incipient underlying disease? New insights from a speckle tracking echocardiography study. Indian Pacing Electrophysiol. J. 2021, 21, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Bansal, N.; Mercadante, A.; Rochelson, E.; Mahgerefteh, J.; Clark, B.C. Speckle Tracking Echocardiography in Pediatric Patients with Premature Ventricular Contractions. Pediatr. Cardiol. 2020, 41, 1587–1593. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Larson, M.G.; McCabe, E.L.; Osypiuk, E.; Lehman, B.T.; Stanchev, P.; Aragam, J.; Benjamin, E.J.; Solomon, S.D.; Vasan, R.S. Reproducibility of speckle-tracking-based strain measures of left ventricular function in a community-based study. J. Am. Soc. Echocardiogr. 2013, 26, 1258–1266.e1252. [Google Scholar] [CrossRef] [PubMed]
| Variable | Patients with EF 50–<55% | Patients with EF ≥ 55% | p-Value |
|---|---|---|---|
| Patients (n) | 35 | 35 | |
| Age | 53.9 | 58.7 | 0.17 |
| Sex (Male %) | 18 (51.4) | 18 (51.4) | 1 |
| EF (%) | 53.3 | 61.4 | <0.001 |
| PVC Burden (%) | 21.7 | 21.7 | 0.82 |
| Comorbidities | |||
| DM (%) | 6 (17.1) | 6 (17.1) | 1 |
| HTN (%) | 14 (40.0) | 17 (48.6) | 0.47 |
| AF (%) | 2 (5.7) | 2 (5.7) | 1 |
| COPD (%) | 1 (2.9) | 3 (8.6) | 0.31 |
| HLP (%) | 12 (34.3) | 18 (51.4) | 0.15 |
| Renal Insufficiency (Cr >1.5) | 1 (2.9) | 3 (8.6) | 0.31 |
| OSA (%) | 7 (20.0) | 10 (28.6) | 0.41 |
| CVA (%) | 2 (5.7) | 2 (5.7) | 1 |
| Medical Therapy | |||
| Beta-Blocker | 25 (71.4) | 23 (65.7) | 0.61 |
| Verapamil | 8 (22.6) | 3 (8.6) | 0.10 |
| Diltiazem | 2 (6.0) | 5 (14.3) | 0.24 |
| Digoxin | 0 | 0 | 1 |
| ACEI/ARB | 10 (28.6) | 11 (31.4) | 0.80 |
| AAD | 11 (31.4) | 9 (26.0) | 0.60 |
| Prior Ablation Procedure | 6 (17) | 4 (11) | 0.50 |
| Variables | Before Ablation | Post-Ablation | p-Value |
|---|---|---|---|
| EF (%) | |||
| 53.2 ± 0.4 | 58.3 ± 0.5 | <0.001 |
| 53 ± 0.6 | 55.6 ± 0.7 | >0.05 |
| 62 ± 0.4 | 62 ± 0.4 | 0.56 |
| 61.7 ± 0.4 | 62.7 ± 0.4 | >0.05 |
| PVC Burden (%) | |||
| 21.8 ± 12 | 3.2 ± 6.3 | <0.001 |
| 22.4 ± 11 | 3.8 ± 7.9 | <0.001 |
| Longitudinal Strain | |||
| −15.2 ± 3.3 | −16.6 ± 3 | 0.007 |
| −15.8 ± 4 | −16 ± 3 | 0.8 |
| −17.4 ± 3 | −17.4 ± 3 | 0.63 |
| −19.1 ± 0 | −19.0 ± 0 | 0.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jamé, S.; Liu, Z.; Kolias, T.; Liang, J.; Labounty, T.; Ghannam, M.; Latchamsetty, R.; Jongnarangsin, K.; Morady, F.; Bogun, F. Strain Analysis in Patients with Frequent Premature Ventricular Complexes and Preserved Left Ventricular Function Undergoing Ablation. J. Clin. Med. 2023, 12, 3017. https://doi.org/10.3390/jcm12083017
Jamé S, Liu Z, Kolias T, Liang J, Labounty T, Ghannam M, Latchamsetty R, Jongnarangsin K, Morady F, Bogun F. Strain Analysis in Patients with Frequent Premature Ventricular Complexes and Preserved Left Ventricular Function Undergoing Ablation. Journal of Clinical Medicine. 2023; 12(8):3017. https://doi.org/10.3390/jcm12083017
Chicago/Turabian StyleJamé, Sina, Zhigang Liu, Theodore Kolias, Jackson Liang, Troy Labounty, Michael Ghannam, Rakesh Latchamsetty, Krit Jongnarangsin, Fred Morady, and Frank Bogun. 2023. "Strain Analysis in Patients with Frequent Premature Ventricular Complexes and Preserved Left Ventricular Function Undergoing Ablation" Journal of Clinical Medicine 12, no. 8: 3017. https://doi.org/10.3390/jcm12083017
APA StyleJamé, S., Liu, Z., Kolias, T., Liang, J., Labounty, T., Ghannam, M., Latchamsetty, R., Jongnarangsin, K., Morady, F., & Bogun, F. (2023). Strain Analysis in Patients with Frequent Premature Ventricular Complexes and Preserved Left Ventricular Function Undergoing Ablation. Journal of Clinical Medicine, 12(8), 3017. https://doi.org/10.3390/jcm12083017






