Effect of Hemolysis Regarding the Characterization and Prognostic Relevance of Neuron Specific Enolase (NSE) after Cardiopulmonary Resuscitation with Extracorporeal Circulation (eCPR)
Abstract
1. Introduction
Aim of This Study
2. Methods
2.1. Patients
2.2. Primary Endpoint
2.3. VA-ECMO
2.4. Neuron-Specific Enolase and Serum-Free Hemoglobin
2.5. Patient Data
2.6. Statistical Analyses
3. Results
3.1. Patients and eCPR Associated Data
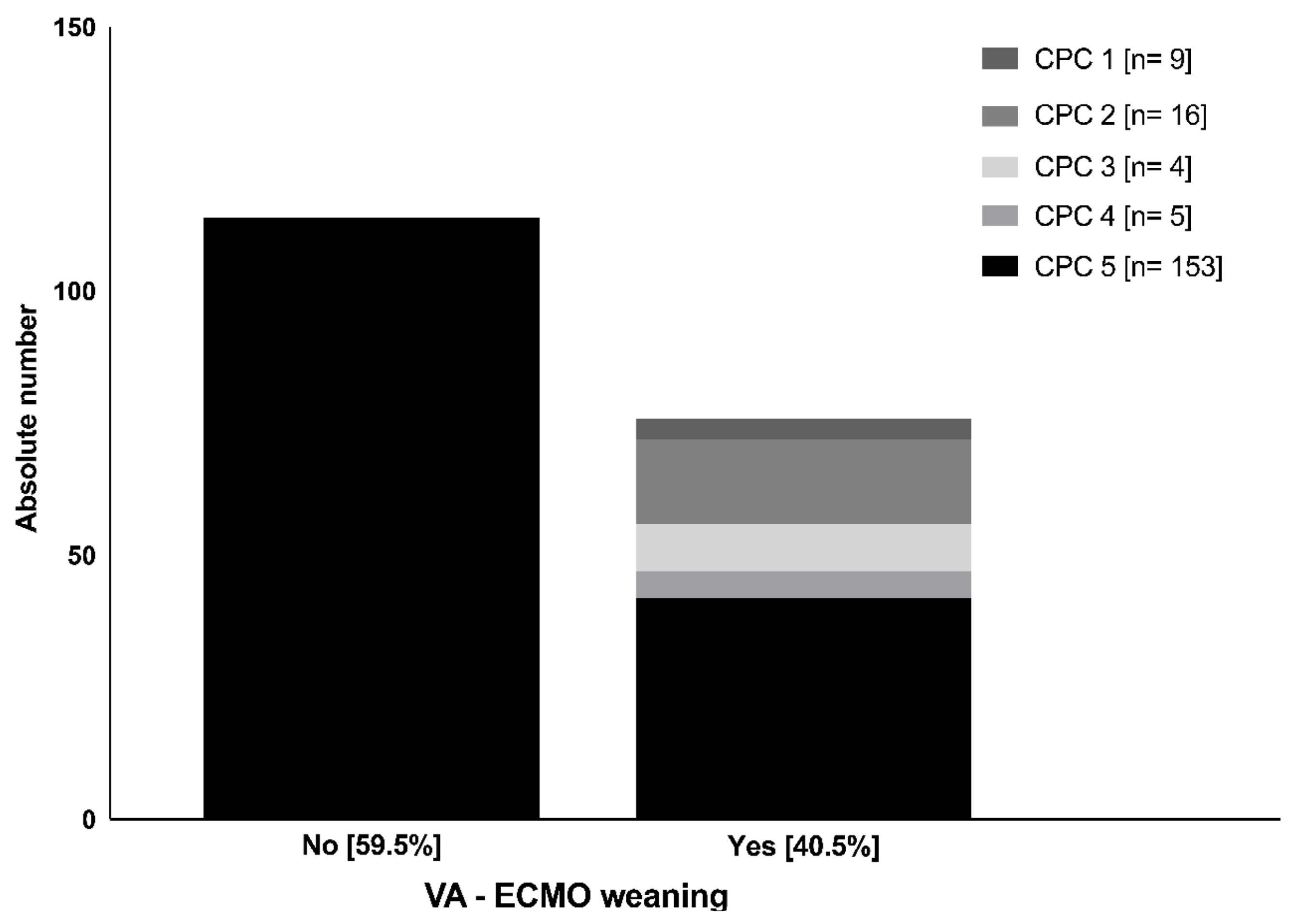
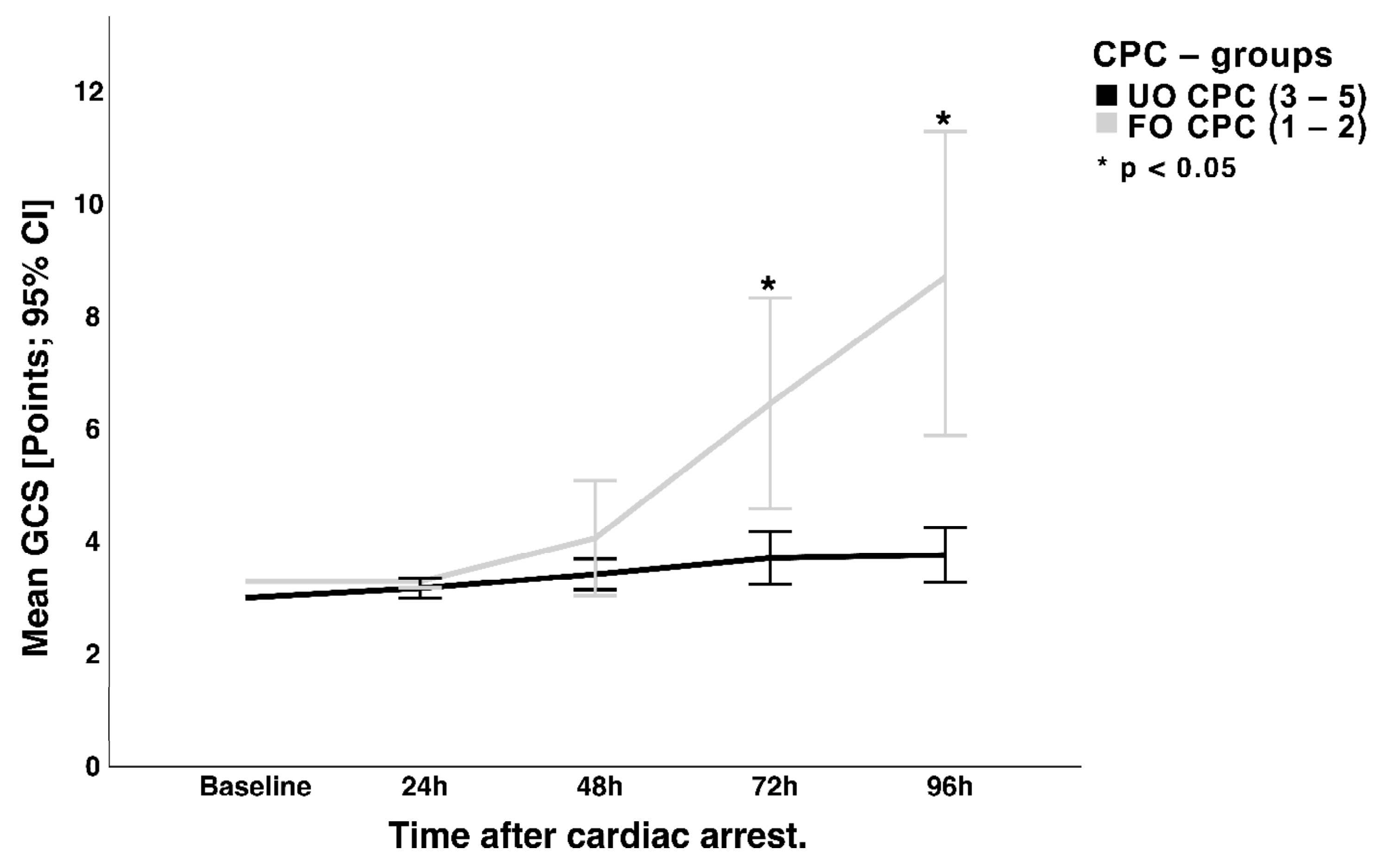
3.2. Wake-Up Attempt and Neurological Status
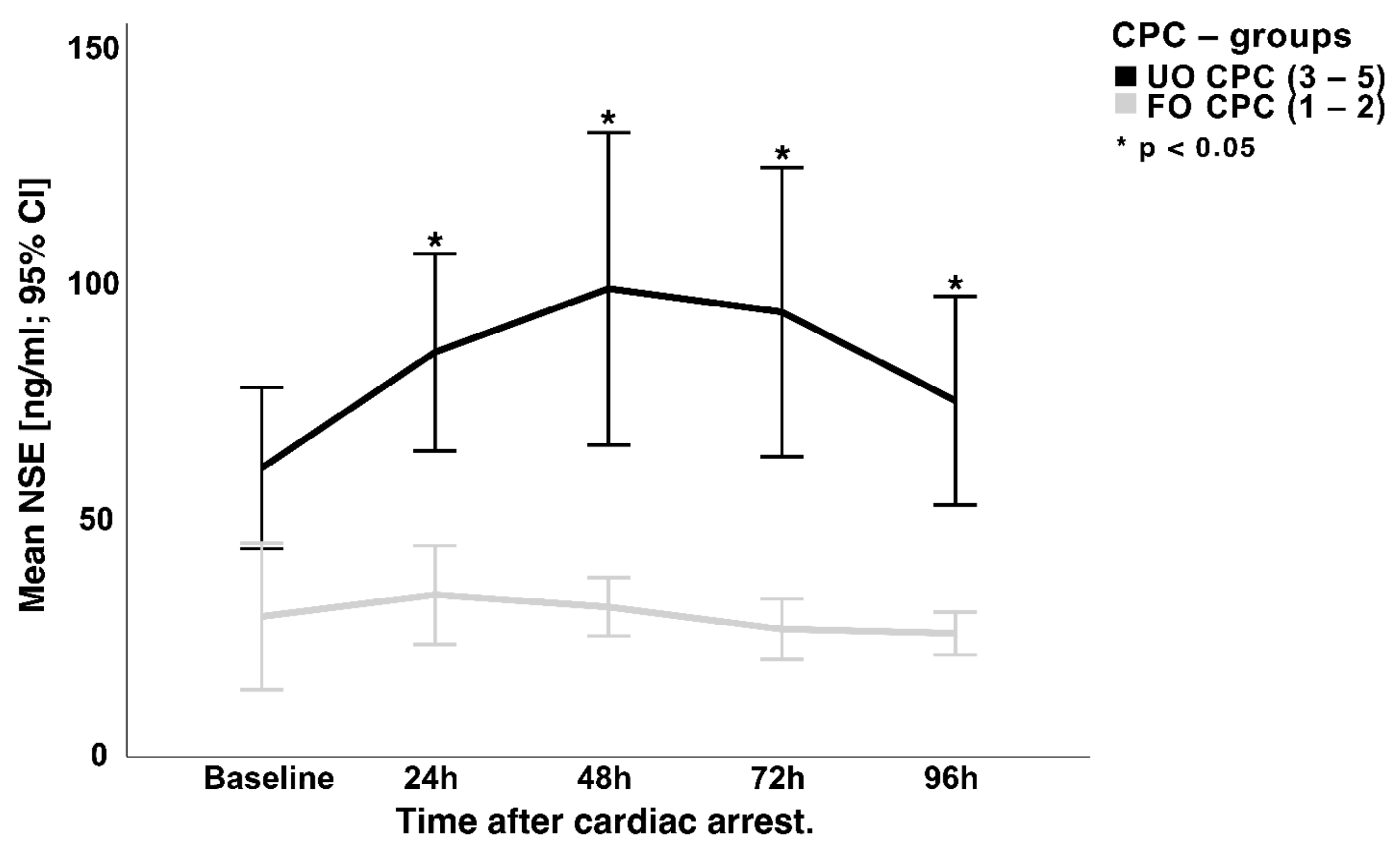
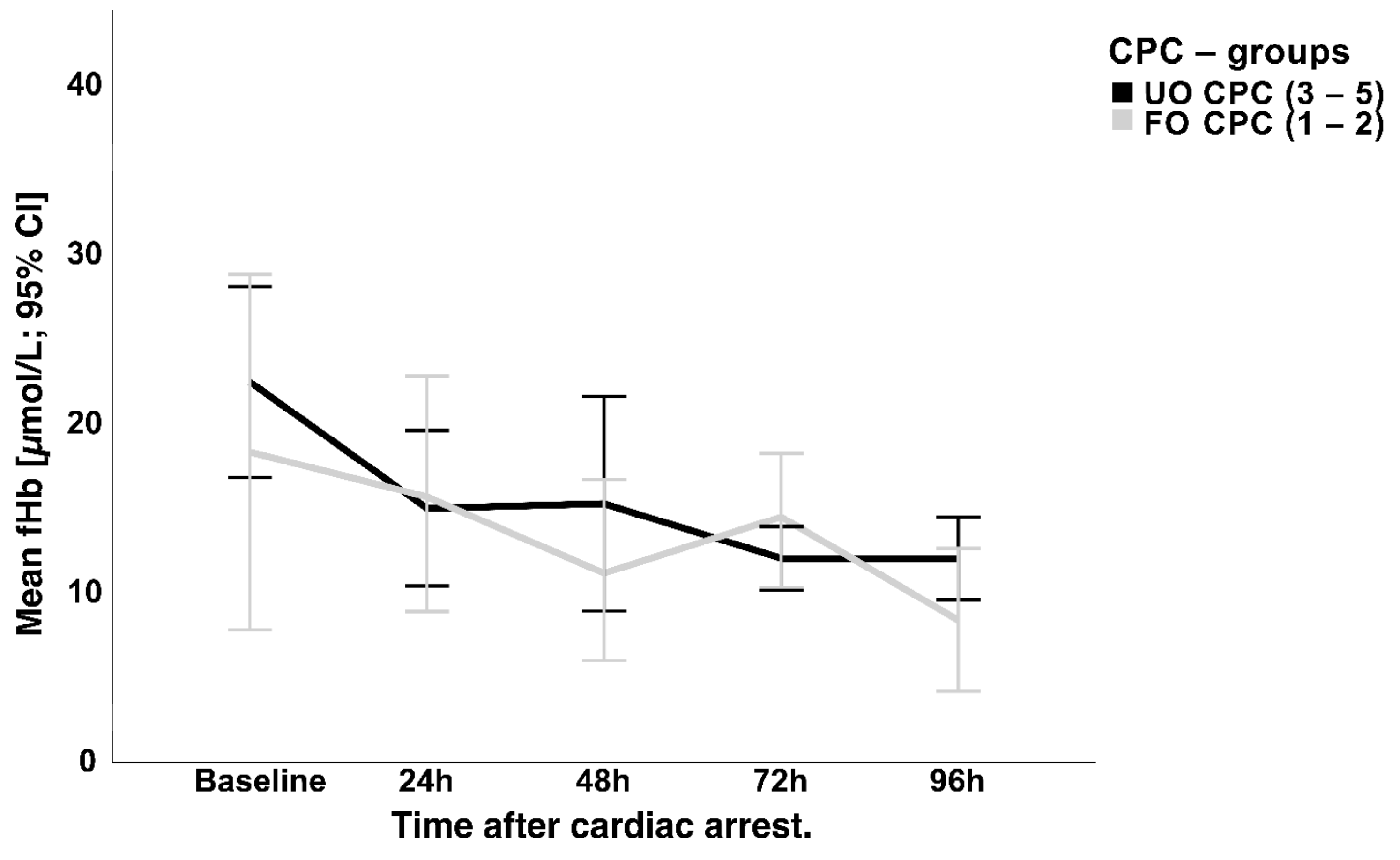
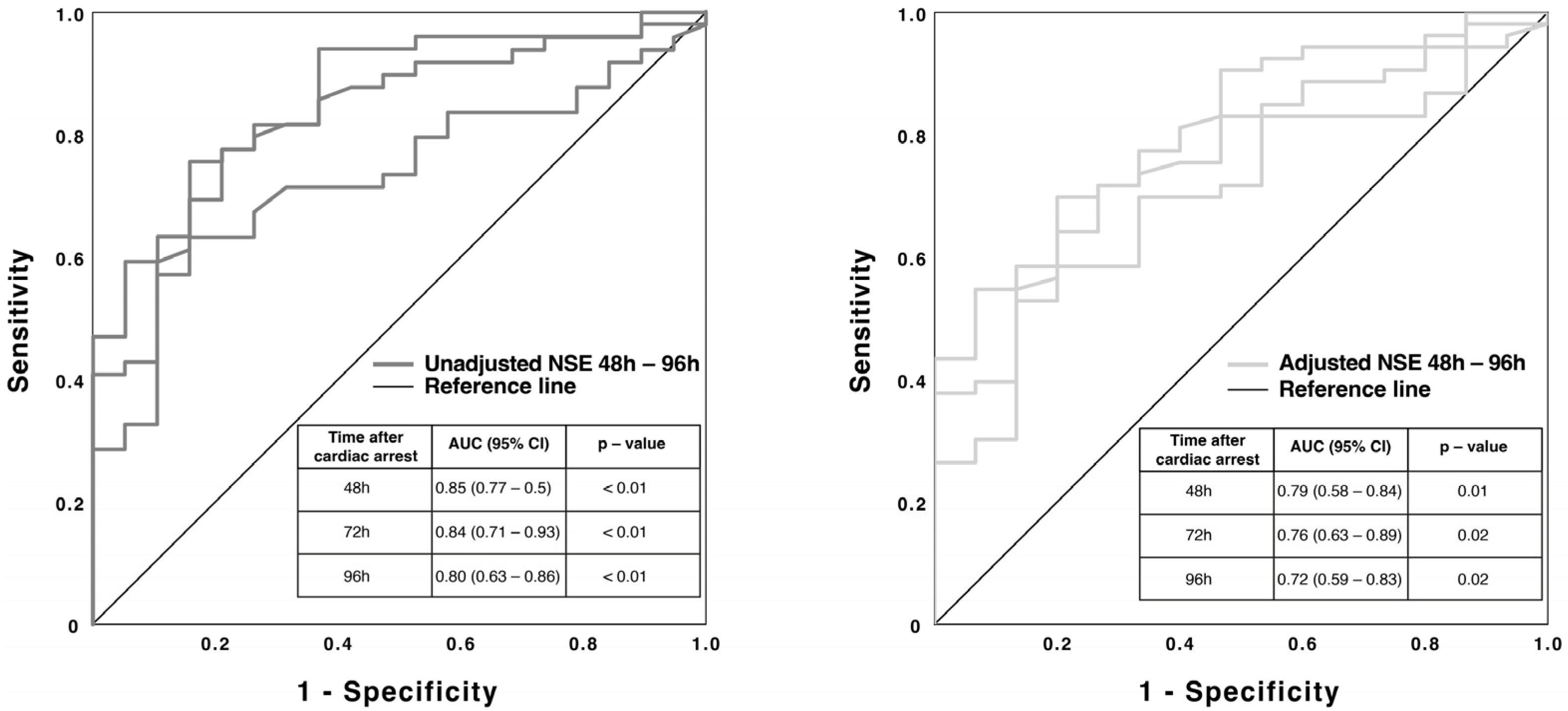
3.3. NSE Serum Concentration and Hemolysis
4. Discussion
4.1. NSE and Neurological Outcome
4.2. NSE and Hemolysis
4.3. Calculation of a New Cut-Off Value
4.4. Strengths and Limitations of This Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nolan, J.P.; Sandroni, C.; Böttiger, B.W.; Cariou, A.; Cronberg, T.; Friberg, H.; Genbrugge, C.; Haywood, K.; Lilja, G.; Moulaert, V.R.M.; et al. European Resuscitation Council and European Society of Intensive Care Medicine guidelines 2021: Post-resuscitation care. Intensiv. Care Med. 2021, 47, 369–421. [Google Scholar] [CrossRef] [PubMed]
- Schmechel, D.; Marangos, P.J.; Zis, A.P.; Brightman, M.; Goodwin, F.K. Brain endolases as specific markers of neuronal and glial cells. Science 1978, 199, 313–315. [Google Scholar] [CrossRef] [PubMed]
- Marangos, P.J.; Schmechel, D.E. Neuron specific enolase, a clinically useful marker for neurons and neuroendocrine cells. Annu. Rev. Neurosci. 1987, 10, 269–295. [Google Scholar] [CrossRef]
- Fogel, W.; Krieger, D.; Veith, M.; Adams, H.-P.; Hund, E.; Storch-Hagenlocher, B.; Buggle, F.; Mathias, D.; Hacke, W. Serum neuron-specific enolase as early predictor of outcome after cardiac arrest. Crit. Care Med. 1997, 25, 1133–1138. [Google Scholar] [CrossRef] [PubMed]
- Johnsson, P.; Blomquist, S.; Lührs, C.; Malmkvist, G.; Alling, C.; Solem, J.-O.; Ståhl, E. Neuron-specific enolase increases in plasma during and immediately after extracorporeal circulation. Ann. Thorac. Surg. 2000, 69, 750–754. [Google Scholar] [CrossRef]
- Oddo, M.; Rossetti, A. Predicting neurological outcome after cardiac arrest. Curr. Opin. Crit. Care 2011, 17, 254–259. [Google Scholar] [CrossRef]
- Ben-Hamouda, N.; Ltaief, Z.; Kirsch, M.; Novy, J.; Liaudet, L.; Oddo, M.; Rossetti, A.O. Neuroprognostication Under ECMO After Cardiac Arrest: Are Classical Tools Still Performant? Neurocrit. Care 2022, 37, 293–301. [Google Scholar] [CrossRef]
- Pfeifer, R.; Börner, A.; Krack, A.; Sigusch, H.H.; Surber, R.; Figulla, H.R. Outcome after cardiac arrest: Predictive values and limitations of the neuroproteins neuron-specific enolase and protein S-100 and the Glasgow Coma Scale. Resuscitation 2005, 65, 49–55. [Google Scholar] [CrossRef]
- Rech, T.H.; Vieira, S.R.R.; Nagel, F.; Brauner, J.S.; Scalco, R. Serum neuron-specific enolase as early predictor of outcome after in-hospital cardiac arrest: A cohort study. Crit. Care 2006, 10, R133. [Google Scholar] [CrossRef]
- Reuter, J.; Peoc’H, K.; Bouadma, L.; Ruckly, S.; Chicha-Cattoir, V.; Faille, D.; Bourrienne, M.-C.; Dupuis, C.; Magalhaes, E.; Tanaka, S.; et al. Neuron-Specific Enolase Levels in Adults Under Venoarterial Extracorporeal Membrane Oxygenation. Crit. Care Explor. 2020, 2, e0239. [Google Scholar] [CrossRef]
- Rundgren, M.; Karlsson, T.; Nielsen, N.; Cronberg, T.; Johnsson, P.; Friberg, H. Neuron specific enolase and S-100B as predictors of outcome after cardiac arrest and induced hypothermia. Resuscitation 2009, 80, 784–789. [Google Scholar] [CrossRef] [PubMed]
- Shinozaki, K.; Oda, S.; Sadahiro, T.; Nakamura, M.; Abe, R.; Nakada, T.-A.; Nomura, F.; Nakanishi, K.; Kitamura, N.; Hirasawa, H. Serum S-100B is superior to neuron-specific enolase as an early prognostic biomarker for neurological outcome following cardiopulmonary resuscitation. Resuscitation 2009, 80, 870–875. [Google Scholar] [CrossRef]
- Tiainen, M.; Roine, R.O.; Pettila, V.; Takkunen, O. Serum Neuron-Specific Enolase and S-100B Protein in Cardiac Arrest Patients Treated with Hypothermia. Stroke 2003, 34, 2881–2886. [Google Scholar] [CrossRef]
- Wennervirta, J.E.; Ermes, M.J.; Tiainen, S.M.; Salmi, T.K.; Hynninen, M.S.; Särkelä, M.O.K.; Hynynen, M.J.; Stenman, U.-H.; Viertiö-Oja, H.E.; Saastamoinen, K.-P.; et al. Hypothermia-treated cardiac arrest patients with good neurological outcome differ early in quantitative variables of EEG suppression and epileptiform activity*. Crit. Care Med. 2009, 37, 2427–2435. [Google Scholar] [CrossRef]
- Schrage, B.; Rübsamen, N.; Becher, P.M.; Roedl, K.; Söffker, G.; Schwarzl, M.; Dreher, A.; Schewel, J.; Ghanem, A.; Grahn, H.; et al. Neuron-specific-enolase as a predictor of the neurologic outcome after cardiopulmonary resuscitation in patients on ECMO. Resuscitation 2019, 136, 14–20. [Google Scholar] [CrossRef]
- Grossestreuer, A.V.; Abella, B.S.; Sheak, K.R.; Cinousis, M.J.; Perman, S.M.; Leary, M.; Wiebe, D.J.; Gaieski, D.F. Inter-rater reliability of post-arrest cerebral performance category (CPC) scores. Resuscitation 2016, 109, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Reisinger, J.; Höllinger, K.; Lang, W.; Steiner, C.; Winter, T.; Zeindlhofer, E.; Mori, M.; Schiller, A.; Lindorfer, A.; Wiesinger, K.; et al. Prediction of neurological outcome after cardiopulmonary resuscitation by serial determination of serum neuron-specific enolase. Eur. Heart J. 2006, 28, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Wijdicks, E.F.M.; Hijdra, A.; Young, G.B.; Bassetti, C.L.; Wiebe, S. Practice Parameter: Prediction of outcome in comatose survivors after cardiopulmonary resuscitation (an evidence-based review): Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2006, 67, 203–210. [Google Scholar] [CrossRef]
- Meißner, S.; Nuding, S.; Schröder, J.; Werdan, K.; Ebelt, H. Relationship between body temperature, neuron-specific enolase, and clinical course in patients after out-of-hospital cardiac arrest. Med. Klin. Intensivmed. Notf. 2020, 115, 43–51. [Google Scholar] [CrossRef]
- Wanek, F.; Meißner, S.; Nuding, S.; Hoberück, S.; Werdan, K.; Noutsias, M.; Ebelt, H. Influence of therapeutic temperature management on the clinical course in patients after in-hospital cardiac arrest: A retrospective analysis. Med. Klin. Intensivmed. Notf. 2022, 117, 297–304. [Google Scholar] [CrossRef]
- Floerchinger, B.; Philipp, A.; Camboni, D.; Foltan, M.; Lunz, D.; Lubnow, M.; Zuasig, Y.; Schmid, C. NSE serum levels in extracorporeal life support patients-Relevance for neurological outcome? Resuscitation 2017, 121, 166–171. [Google Scholar] [CrossRef]
- Geisen, U.; Benk, C.; Beyersdorf, F.; Klemm, R.; Trummer, G.; Özbek, B.; Kern, F.; Heilmann, C. Neuron-specific enolase correlates to laboratory markers of haemolysis in patients on long-term circulatory support. Eur. J. Cardio Thorac. Surg. 2015, 48, 416–420. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.; Bissmann, B.; Becker, C.; Beck, K.; Loretz, N.; Gross, S.; Amacher, S.A.; Bohren, C.; Pargger, H.; Tisljar, K.; et al. Neuron-Specific Enolase (NSE) Predicts Long-Term Mortality in Adult Patients after Cardiac Arrest: Results from a Prospective Trial. Medicines 2021, 8, 72. [Google Scholar] [CrossRef] [PubMed]
- Stammet, P.; Collignon, O.; Hassager, C.; Wise, M.P.; Hovdenes, J.; Åneman, A.; Horn, J.; Devaux, Y.; Erlinge, D.; Kjaergaard, J.; et al. Neuron-Specific Enolase as a Predictor of Death or Poor Neurological Outcome After Out-of-Hospital Cardiac Arrest and Targeted Temperature Management at 33 degrees C and 36 degrees C. J. Am. Coll. Cardiol. 2015, 65, 2104–2114. [Google Scholar] [CrossRef] [PubMed]
- Daubin, C.; Quentin, C.; Allouche, S.; Etard, O.; Gaillard, C.; Seguin, A.; Valette, X.; Parienti, J.-J.; Prevost, F.; Ramakers, M.; et al. Serum neuron-specific enolase as predictor of outcome in comatose cardiac-arrest survivors: A prospective cohort study. BMC Cardiovasc. Disord. 2011, 11, 48. [Google Scholar] [CrossRef]
| Study Population | CPC 1–2 | CPC 3–5 | p-Value * | |
|---|---|---|---|---|
| (n = 190) | (n = 25) | (n = 165) | ||
| Biometrics | ||||
| Age (years–mean ± SD) | 60.1 ± 15.6 | 61.2 ± 20.2 | 60 ± 15.1 | 0.72 |
| Male–(n (%)) | 141 (74) | 16 (64) | 125 (75.8) | 0.42 |
| Female–(n (%)) | 49 (26%) | 9 (36) | 40 (24.2) | 0.42 |
| BMI (kg/m2-mean ± SD) | 28.5 ± 6.1 | 27.2 ± 7.2 | 28.6 ± 5.9 | 0.46 |
| Number of comorbidities (mean ± SD) | 2.2 ± 1.1 | 2.3 ± 1.5 | 2.2 ± 1.1 | 0.89 |
| Left ventricular ejection fraction (%–mean ± Std) | 25.1 ± 15.5 | 35.3 ± 15.3 | 24.7 ± 15.4 | 0.002 |
| CPR data | ||||
| Duration of CPR (minutes–mean ± SD) | 53.5 ± 38.1 | 31.9 ± 24.4 | 55.2 ± 38.6 | 0.004 |
| Primary rhythm | ||||
| VT/VF (n (%)) | 92 (48) | 15 (60) | 77 (46.7) | 0.06 |
| PEA/Asystole (n (%)) | 98 (52) | 10 (40) | 88 (53.3) | 0.54 |
| Cumulative dose of adrenaline (mg-mean ± SD) | 5.4 ± 5.2 | 2.5 ± 2.4 | 5.5 ± 5.4 | 0.007 |
| Number of defibrillations (n (%)) | 3.6 ± 8.4 | 1.9 ± 4.3 | 3.6 ± 8.6 | 0.33 |
| TTM (n (%)) | 148 (77.9) | 20 (80) | 128 (77.6) | 0.34 |
| Bystander CPR (n (%)) | 91 (47.8) | 11 (44) | 80 (48) | 0.25 |
| In-hospital CPR (n (%)) | 133 (70) | 17 (68) | 116 (70.3) | 0.58 |
| Major etiology of CPR | ||||
| STEMI (n (%)) | 83 (43.6) | 13 (52) | 70 (42.4) | 0.56 |
| VA-ECMO data | ||||
| Intervall collapse-implantation [(minutes–mean ± SD)] | 52.4 ± 42.6 | 32.6 ± 31.9 | 67.3 ± 43.3 | <0.001 |
| Pulsatile flow after ECMO implantation (n (%)) | 144 (76.3) | 25 (100) | 119 (72.1) | <0.001 |
| Duration of VA-ECMO support (hours ± SD) | 103.4 ± 112.1 | 121.9 ± 131.9 | 103.2 ± 108.3 | 0.44 |
| Initial liters/minute (mean ± SD) | 4.2 ± 0.9 | 3.8 ± 1.1 | 4.3 ± 0.9 | 0.013 |
| Initial pump flow index (mL/minute/kg-mean ± SD) | 51.02 ± 14.4 | 48.8 ± 17.6 | 51.3 ± 14.1 | 0.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haertel, F.; Babst, J.; Bruening, C.; Bogoviku, J.; Otto, S.; Fritzenwanger, M.; Gecks, T.; Ebelt, H.; Moebius-Winkler, S.; Schulze, P.C.; et al. Effect of Hemolysis Regarding the Characterization and Prognostic Relevance of Neuron Specific Enolase (NSE) after Cardiopulmonary Resuscitation with Extracorporeal Circulation (eCPR). J. Clin. Med. 2023, 12, 3015. https://doi.org/10.3390/jcm12083015
Haertel F, Babst J, Bruening C, Bogoviku J, Otto S, Fritzenwanger M, Gecks T, Ebelt H, Moebius-Winkler S, Schulze PC, et al. Effect of Hemolysis Regarding the Characterization and Prognostic Relevance of Neuron Specific Enolase (NSE) after Cardiopulmonary Resuscitation with Extracorporeal Circulation (eCPR). Journal of Clinical Medicine. 2023; 12(8):3015. https://doi.org/10.3390/jcm12083015
Chicago/Turabian StyleHaertel, Franz, Josephine Babst, Christiane Bruening, Jurgen Bogoviku, Sylvia Otto, Michael Fritzenwanger, Thomas Gecks, Henning Ebelt, Sven Moebius-Winkler, P. Christian Schulze, and et al. 2023. "Effect of Hemolysis Regarding the Characterization and Prognostic Relevance of Neuron Specific Enolase (NSE) after Cardiopulmonary Resuscitation with Extracorporeal Circulation (eCPR)" Journal of Clinical Medicine 12, no. 8: 3015. https://doi.org/10.3390/jcm12083015
APA StyleHaertel, F., Babst, J., Bruening, C., Bogoviku, J., Otto, S., Fritzenwanger, M., Gecks, T., Ebelt, H., Moebius-Winkler, S., Schulze, P. C., & Pfeifer, R. (2023). Effect of Hemolysis Regarding the Characterization and Prognostic Relevance of Neuron Specific Enolase (NSE) after Cardiopulmonary Resuscitation with Extracorporeal Circulation (eCPR). Journal of Clinical Medicine, 12(8), 3015. https://doi.org/10.3390/jcm12083015






