Effect of Exercise on Liver Function and Insulin Resistance Markers in Patients with Non-Alcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Data Extraction
2.4. Study Quality
2.5. Statistical Analysis
3. Results
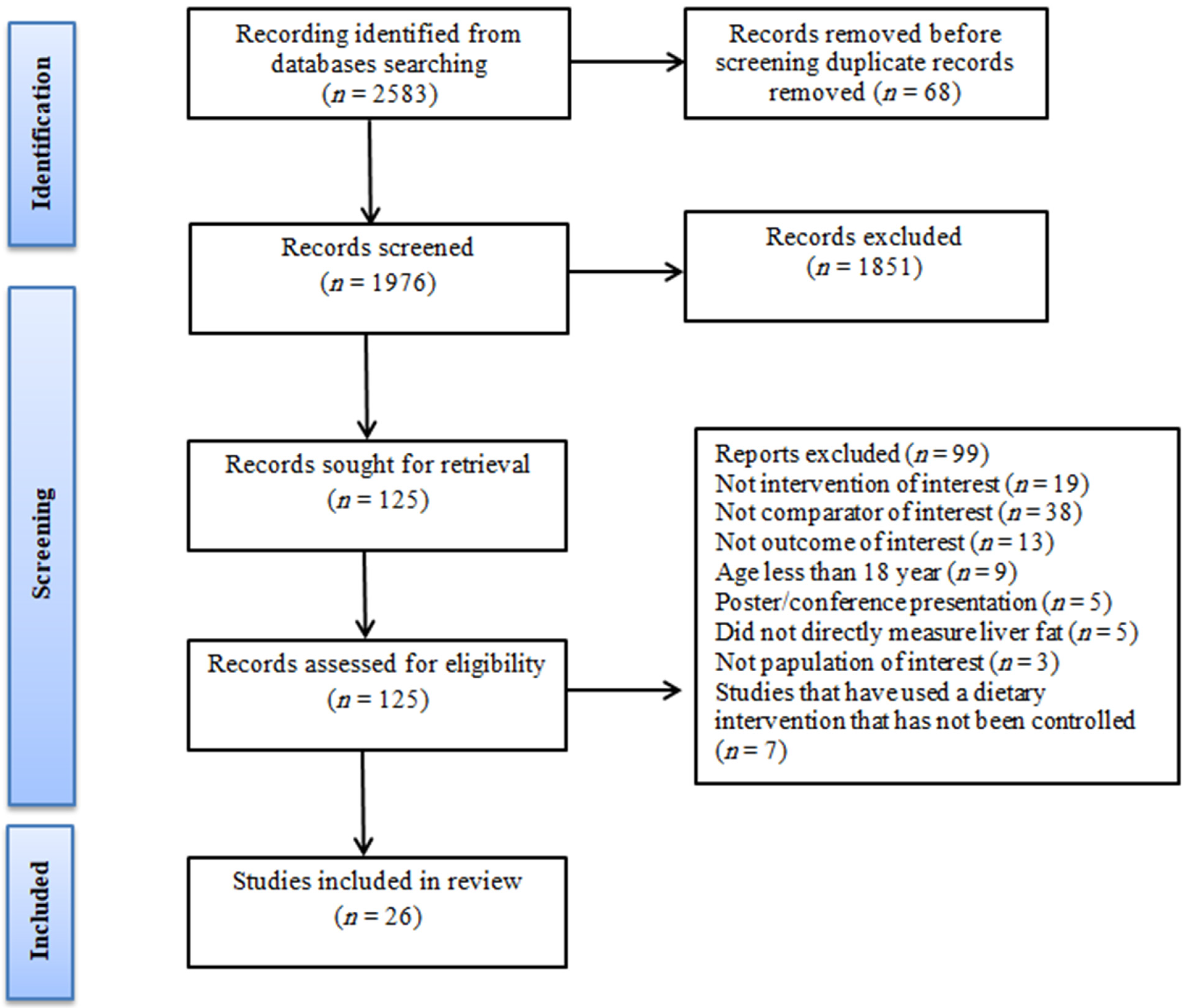
3.1. Study Selection
3.2. Study and Participant Characteristics
| Participants | |||||||
|---|---|---|---|---|---|---|---|
| Author, Year | Country | Disease | Ex (CON) | BMI | Gender (M/F) | Age | Outcome Assumed |
| Abdelbasset et al., 2019 [26] | Egypt | NAFLD | 16 (16) | 36.1 | M F | 54.8 | ALT, HBA1c, FBG, HOMA-IR |
| Cuthbertson et al., 2016 [42] | UK | NAFLD | 30 (20) | 30.15 | M F | 51 | ALT, AST, FBG, HOMA-IR, Insulin |
| Eckard et al., 2013 [37] | USA | NAFLD | 9 (11) | 33.3 | M | 51.5 | ALT, AST, FBG, HOMA-IR, Insulin |
| George et al., 2008 [36] | Australia | NAFLD | 109 (34) | 31.7 | M | 48.12 | ALT, AST, FBG, HOMA-IR, Insulin |
| Hallsworth et al., 2011 [43] | UK | NAFLD | 11 (8) | 32.3 | M F | 57 | ALT, FBG, HOMA-IR, Insulin |
| Hatami et al., 2016 [35] | Iran | NAFLD | 16 (16) | 28 | M | 32.93 | ALT, AST |
| Hoseini et al., 2020 [38] | Iran | NAFLD | 10 (10) | 34.11 | F | 62.3 | ALT, AST, FBG, HOMA-IR, Insulin |
| Houghton et al., 2017 [44] | Australia | NAFLD | 12 (12) | 33.00 | M F | 52.00 | ALT, AST, FBG, HOMA-IR |
| Javanmardi Fard et al., 2015 [45] | Iran | NAFLD | 30 (30) | 28.65 | M F | 19 | ALT, AST |
| Keating et al., 2015 [46] | Australia | NAFLD | 36 (12) | 33.42 | M F | 43.6 | ALT, AST, FBG, Insulin |
| Khaoshbaten et al., 2013 [47] | Iran | NAFLD | 45 (45) | 29.2 | M F | 37.6 | ALT, AST, FBG |
| Mohammadi et al., 2019 [34] | Iran | NAFLD | 10 (10) | 32.58 | M | 37.2 | ALT, AST |
| Moradi Kelardeh et al., 2017 [33] | Iran | NAFLD | 12 (12) | 29.27 | M | 38.24 | ALT, AST |
| Moradi Kelardeh et al., 2020 [39] | Iran | NAFLD | 12 (11) | 27.36 | F | 65.27 | ALT, AST |
| Nourian et al., 2020 [48] | Iran | NAFLD | 36 (33) | 32.19 | M F | 48.84 | ALT, AST |
| Pugh et al., 2014 [49] | UK | NAFLD | 34 (20) | 30.5 | M F | 47.5 | ALT, AST, FBG, HOMA-IR, Insulin |
| Rezende et al., 2016 [40] | Brazil | NAFLD | 19 (21) | 33.05 | F | 55.3 | ALT, AST, FBG, HOMA-IR, Insulin |
| Sadeghi et al., 2019 [32] | Iran | NAFLD | 11 (11) | 26.96 | M | 40.8 | ALT, AST |
| Shamsoddini et al., 2015 [31] | Iran | NAFLD | 20 (10) | 28.96 | M | 43.8 | ALT, AST, HOMA-IR |
| Shojaee-Moradie et al., 2016 [30] | UK | NAFLD | 15 (12) | 31.65 | M | 52.6 | ALT, AST, FBG, HOMA-IR, Insulin |
| Sreenivasa Baba et al., 2006 [29] | India | NAFLD | 44 (15) | 27.1 | M | 38.7 | ALT, AST |
| Sullivan et al., 2012 [50] | USA | NAFLD | 12 (6) | 38.1 | M F | 48.05 | ALT |
| Takahashi et al., 2015 [51] | Japan | NAFLD | 31 (22) | 28.35 | M F | 53.45 | ALT, AST, FBG, HOMA-IR, Insulin |
| Valizadeh et al., 2011 [28] | Iran | NAFLD | 12 (12) | NR | M | 32.5 | ALT, AST |
| Yao et al., 2018 [52] | China | NAFLD | 60 (31) | 26.26 | M F | 58.38 | ALT, FBG, Insulin |
| Zhang et al., 2016 [41] | China | NAFLD | 146 (74) | 28.0 | F | 53.9 | ALT, AST, FBG |
3.3. Intervention Characteristics
3.4. Effect of Exercise on Hepatic Enzyme Parameters
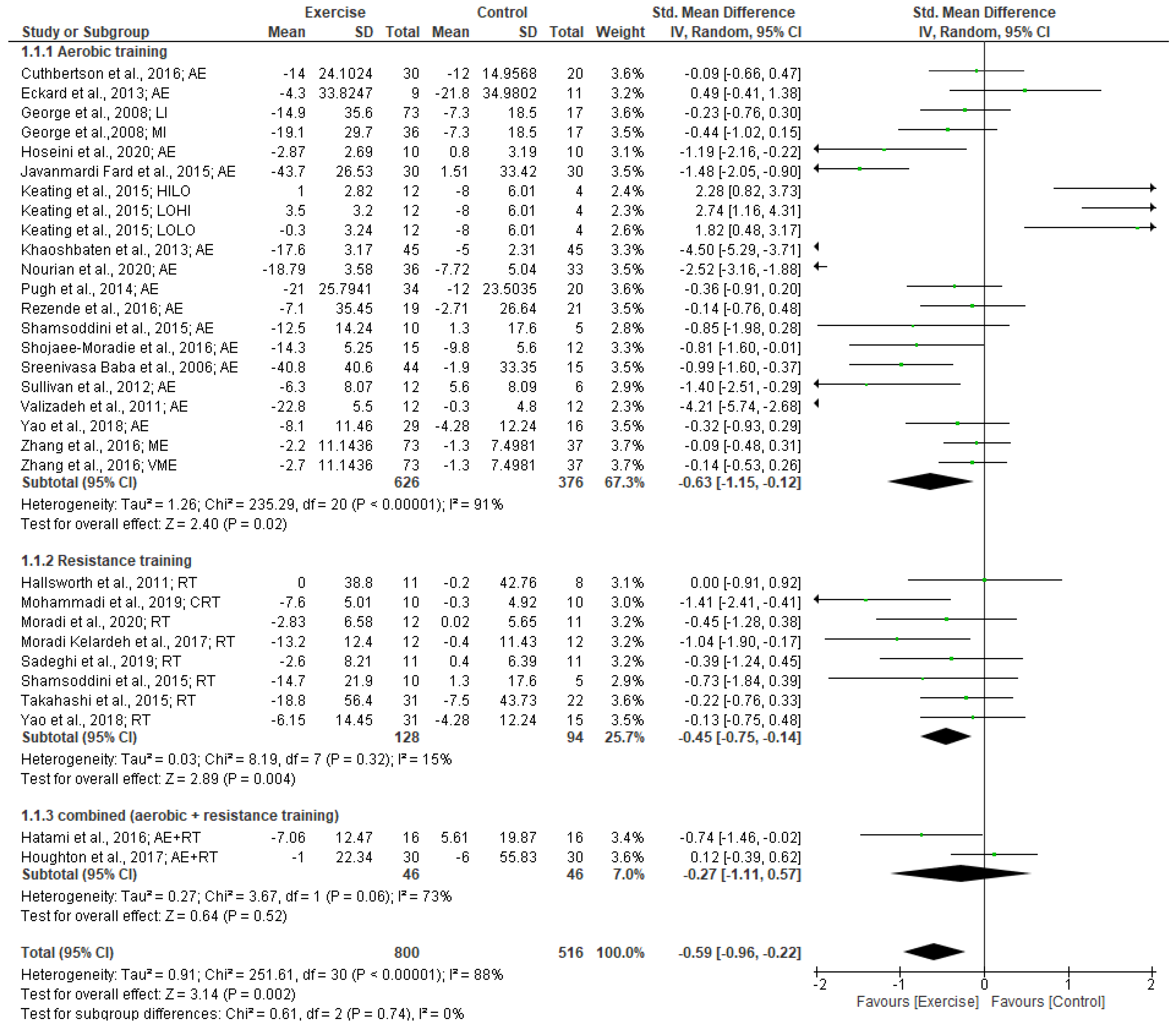
3.4.1. ALT Levels
3.4.2. AST Levels
3.5. Effect of Physical Activity on Glucose Metabolism Parameters
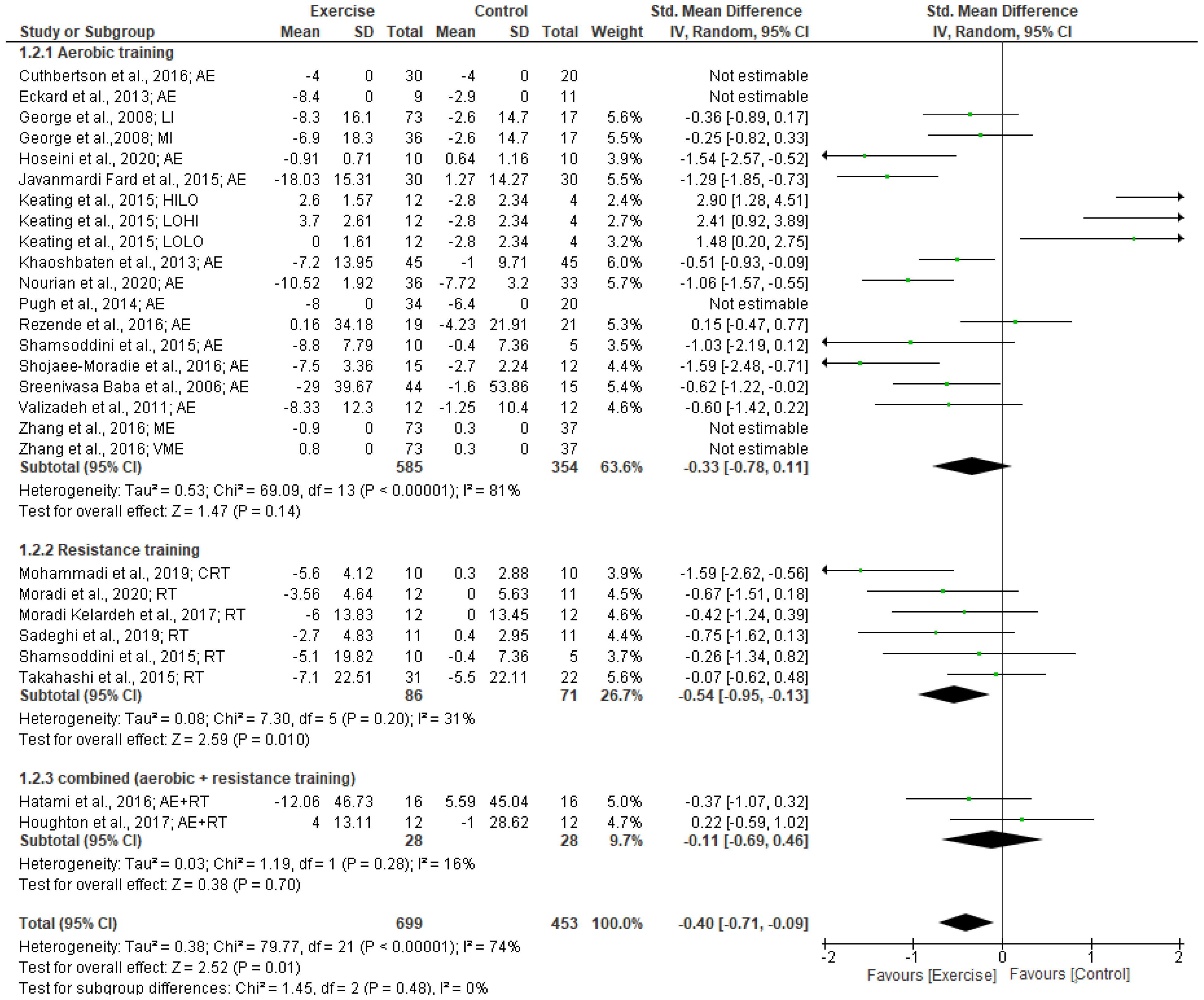
3.5.1. FBG Levels
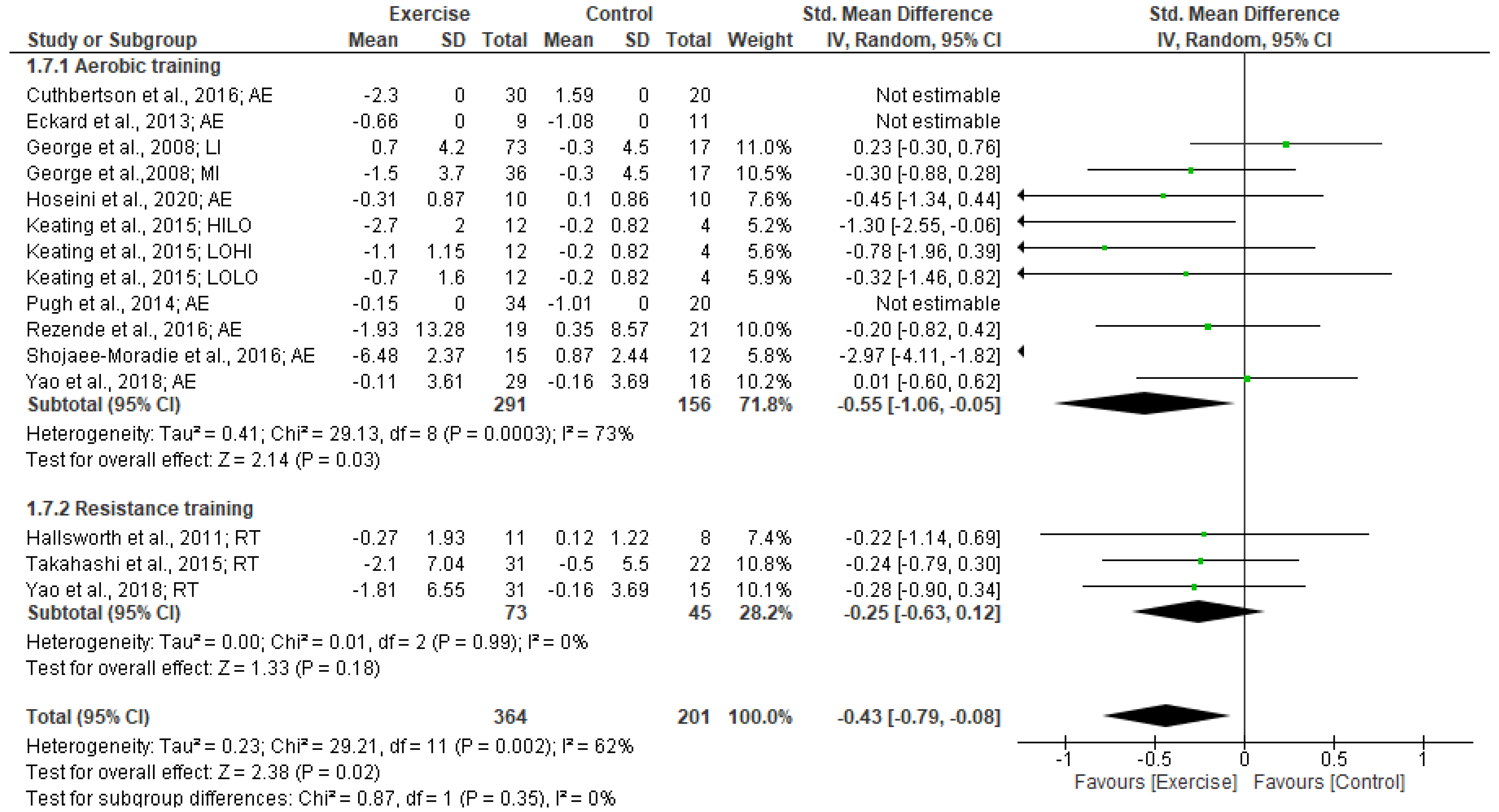
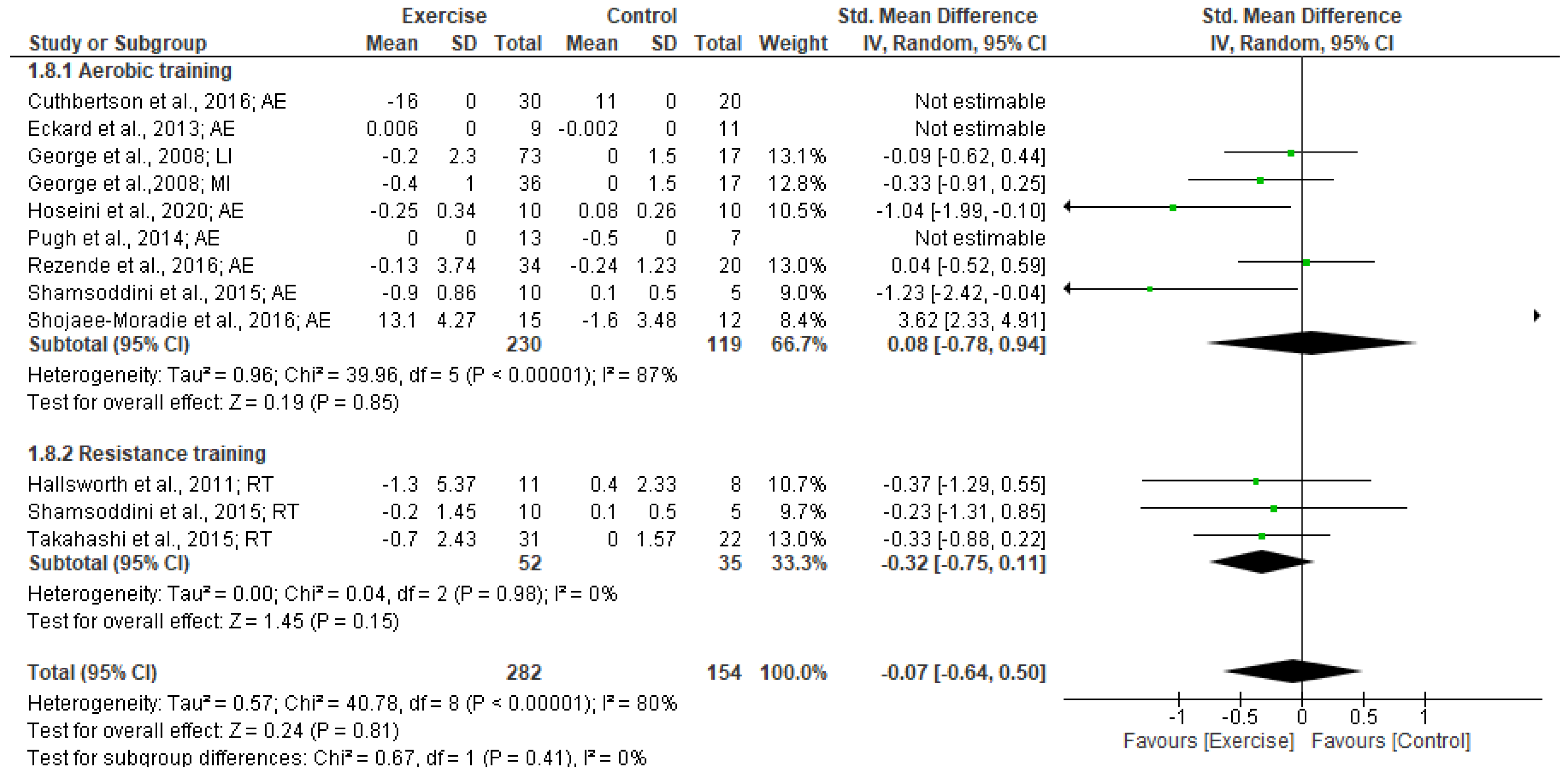
3.5.2. Insulin levels
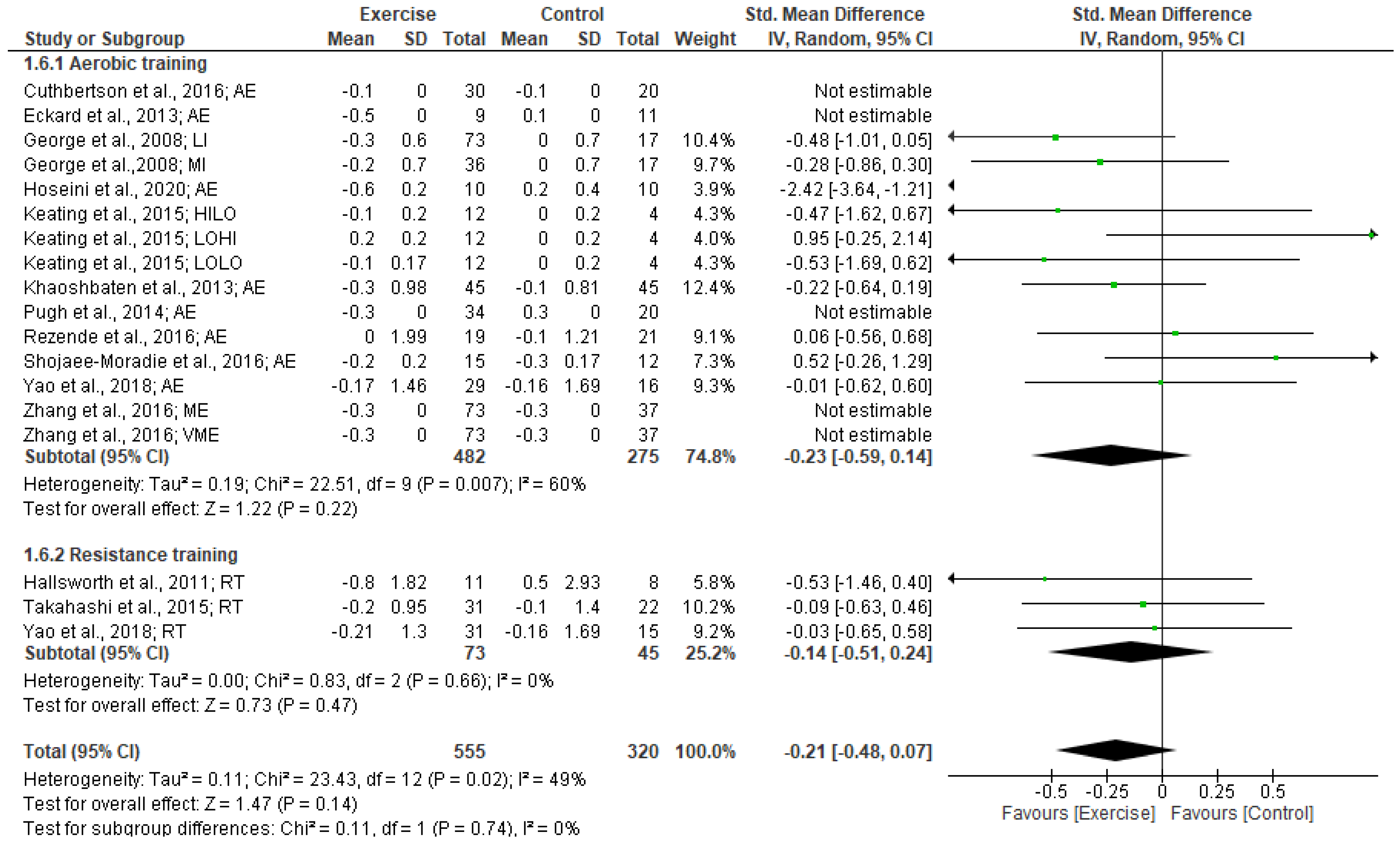
3.5.3. HOMA-IR
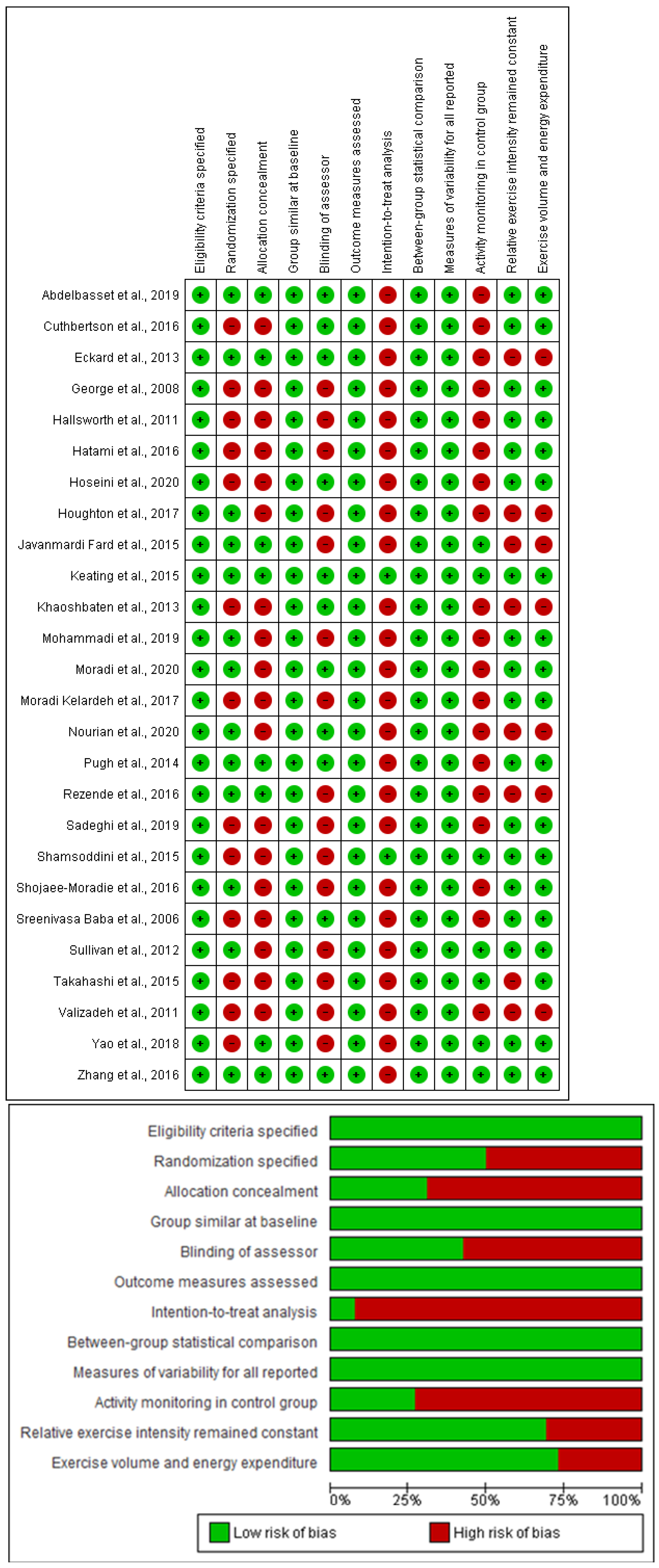
3.6. Study Quality
3.7. Heterogeneity and Publication Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kneeman, J.M.; Misdraji, J.; Corey, K.E. Secondary causes of nonalcoholic fatty liver disease. Ther. Adv. Gastroenterol. 2012, 5, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Muthiah, M.D.; Cheng Han, N.; Sanyal, A.J. A clinical overview of non-alcoholic fatty liver disease: A guide to diagnosis, the clinical features, and complications—What the non-specialist needs to know. Diabetes Obes. Metab. 2022, 24, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.; Valero-Breton, M.; Huerta-Salgado, C.; Achiardi, O.; Simon, F.; Cabello-Verrugio, C. Impact of exercise training on the sarcopenia criteria in non-alcoholic fatty liver disease: A systematic review and meta-analysis. Eur. J. Transl. Myol. 2021, 31, 9630. [Google Scholar]
- Nseir, W.; Hellou, E.; Assy, N. Role of diet and lifestyle changes in nonalcoholic fatty liver disease. World J. Gastroenterol. WJG 2014, 20, 9338. [Google Scholar] [PubMed]
- Krasnoff, J.B.; Painter, P.L.; Wallace, J.P.; Bass, N.M.; Merriman, R.B. Health-related fitness and physical activity in patients with nonalcoholic fatty liver disease. Hepatology 2008, 47, 1158–1166. [Google Scholar] [CrossRef] [PubMed]
- George, A.; Bauman, A.; Johnston, A.; Farrell, G.; Chey, T.; George, J. Independent effects of physical activity in patients with nonalcoholic fatty liver disease. Hepatology 2009, 50, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.W.; Adams, L.A. Non-alcoholic fatty liver disease. Crit. Rev. Clin. Lab. Sci. 2011, 48, 97–113. [Google Scholar] [CrossRef]
- Younossi, Z.; Anstee, Q.M.; Marietti, M.; Hardy, T.; Henry, L.; Eslam, M.; George, J.; Bugianesi, E. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 11–20. [Google Scholar] [CrossRef]
- Schindhelm, R.K.; Diamant, M.; Dekker, J.M.; Tushuizen, M.E.; Teerlink, T.; Heine, R.J. Alanine aminotransferase as a marker of non-alcoholic fatty liver disease in relation to type 2 diabetes mellitus and cardiovascular disease. Diabetes/Metab. Res. Rev. 2006, 22, 437–443. [Google Scholar] [CrossRef]
- Stefan, N.; Cusi, K. A global view of the interplay between non-alcoholic fatty liver disease and diabetes. Lancet Diabetes Endocrinol. 2022, 10, 284–296. [Google Scholar] [CrossRef]
- Leoni, S.; Tovoli, F.; Napoli, L.; Serio, I.; Ferri, S.; Bolondi, L. Current guidelines for the management of non-alcoholic fatty liver disease: A systematic review with comparative analysis. World J. Gastroenterol. 2018, 24, 3361. [Google Scholar] [CrossRef] [PubMed]
- Babu, A.F.; Csader, S.; Lok, J.; Gómez-Gallego, C.; Hanhineva, K.; El-Nezami, H.; Schwab, U. Positive Effects of Exercise Intervention without Weight Loss and Dietary Changes in NAFLD-Related Clinical Parameters: A Systematic Review and Meta-Analysis. Nutrients 2021, 13, 3135. [Google Scholar] [CrossRef] [PubMed]
- Thoma, C.; Day, C.P.; Trenell, M.I. Lifestyle interventions for the treatment of non-alcoholic fatty liver disease in adults: A systematic review. J. Hepatol. 2012, 56, 255–266. [Google Scholar] [CrossRef]
- Qiu, S.; Cai, X.; Sun, Z.; Li, L.; Zügel, M.; Steinacker, J.M.; Schumann, U. Association between physical activity and risk of nonalcoholic fatty liver disease: A meta-analysis. Ther. Adv. Gastroenterol. 2017, 10, 701–713. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of The Liver; European Association for the Study of Diabetes. EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016, 64, 1388–1402. [Google Scholar] [CrossRef] [PubMed]
- Keating, S.E.; Hackett, D.A.; George, J.; Johnson, N.A. Exercise and non-alcoholic fatty liver disease: A systematic review and meta-analysis. J. Hepatol. 2012, 57, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Smart, N.; King, N.; McFarlane, J.; Graham, P.; Dieberg, G. Effect of exercise training on liver function in adults who are overweight or exhibit fatty liver disease: A systematic review and meta-analysis. Br. J. Sport. Med. 2018, 52, 834–843. [Google Scholar] [CrossRef]
- Xiong, Y.; Peng, Q.; Cao, C.; Xu, Z.; Zhang, B. Effect of different exercise methods on non-alcoholic fatty liver disease: A meta-analysis and meta-regression. Int. J. Environ. Res. Public Health 2021, 18, 3242. [Google Scholar] [CrossRef]
- Zhou, B.; Huang, G.; Wang, W.; Zhu, L.; Deng, Y.; He, Y.; Ma, F. Intervention effects of four exercise modalities on nonalcoholic fatty liver disease: A systematic review and Bayesian network meta-analysis. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 7687–7697. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Wojtyniak, J.G.; Britz, H.; Selzer, D.; Schwab, M.; Lehr, T. Data digitizing: Accurate and precise data extraction for quantitative systems pharmacology and physiologically-based pharmacokinetic modeling. CPT Pharmacomet. Syst. Pharmacol. 2020, 9, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Smart, N.A.; Waldron, M.; Ismail, H.; Giallauria, F.; Vigorito, C.; Cornelissen, V.; Dieberg, G. Validation of a new tool for the assessment of study quality and reporting in exercise training studies: TESTEX. Int. J. Evid.-Based Healthc. 2015, 13, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.; Rothstein, H.R. Introduction to Meta-Analysis; John Wiley & Sons: Hoboken, NJ, USA, 2021. [Google Scholar]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Routledge: London, UK, 2013. [Google Scholar]
- Abdelbasset, W.K.; Tantawy, S.A.; Kamel, D.M.; Alqahtani, B.A.; Soliman, G.S. A randomized controlled trial on the effectiveness of 8-week high-intensity interval exercise on intrahepatic triglycerides, visceral lipids, and health-related quality of life in diabetic obese patients with nonalcoholic fatty liver disease. Medicine 2019, 98, e14918. [Google Scholar] [CrossRef]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Valizadeh, R.; Nikbakht, M.; Davodi, M.; Khodadoost, M. The effect of eight weeks elected aerobic exercise on the levels of (AST, ALT) enzymes of men patients with have fat liver. Procedia-Soc. Behav. Sci. 2011, 15, 3362–3365. [Google Scholar] [CrossRef]
- Sreenivasa Baba, C.; Alexander, G.; Kalyani, B.; Pandey, R.; Rastogi, S.; Pandey, A.; Choudhuri, G. Effect of exercise and dietary modification on serum aminotransferase levels in patients with nonalcoholic steatohepatitis. J. Gastroenterol. Hepatol. 2006, 21, 191–198. [Google Scholar] [CrossRef]
- Shojaee-Moradie, F.; Cuthbertson, D.; Barrett, M.; Jackson, N.; Herring, R.; Thomas, E.; Bell, J.; Kemp, G.; Wright, J.; Umpleby, A. Exercise training reduces liver fat and increases rates of VLDL clearance but not VLDL production in NAFLD. J. Clin. Endocrinol. Metab. 2016, 101, 4219–4228. [Google Scholar] [CrossRef]
- Shamsoddini, A.; Sobhani, V.; Chehreh, M.E.G.; Alavian, S.M.; Zaree, A. Effect of aerobic and resistance exercise training on liver enzymes and hepatic fat in Iranian men with nonalcoholic fatty liver disease. Hepat. Mon. 2015, 15, e31434. [Google Scholar] [CrossRef]
- Sadeghi, A.; Pourrazi, H.; Yazdi, H.-R. The Effect of Eight-Week Total Body Resistance Exercise on Liver Functional Parameters in Patients with Non-Alcoholic Fatty Liver Disease. Hormozgan Med. J. 2019, 23, e97644. [Google Scholar] [CrossRef]
- Moradi Kelardeh, B.; Keshavarz, S.; Karimi, M. Effects of Nonlinear Resistance Training with Curcumin Supplement on Liver Enzymes in Men with Non-Alcoholic Fatty Liver Disease. Rep. Health Care 2017, 3, 1–9. [Google Scholar]
- Mohammadi, F.; Ghalavand, A.; Delaramnasab, M. Effect of Circuit Resistance Training and L-Carnitine Supplementation on Body Composition and Liver Function in Men with Non-Alcoholic Fatty Liver Disease. Jundishapur J. Chronic Dis. Care 2019, 8, e90213. [Google Scholar] [CrossRef]
- Hatami, M.T.; Eftekhari, E. The Effect of Combined Aerobic and Resistance Training on Hepatic Enzymes in Males with Nonalcoholic Fatty Liver. Biotechnol. Health Sci. 2016, 3, 25–29. [Google Scholar] [CrossRef]
- George, A.; Bauman, A.; Johnston, A.; Farrell, G.; Chey, T.; George, J. Effect of a lifestyle intervention in patients with abnormal liver enzymes and metabolic risk factors. J. Gastroenterol. Hepatol. 2008, 24, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Eckard, C.; Cole, R.; Lockwood, J.; Torres, D.M.; Williams, C.D.; Shaw, J.C.; Harrison, S.A. Prospective histopathologic evaluation of lifestyle modification in nonalcoholic fatty liver disease: A randomized trial. Ther. Adv. Gastroenterol. 2013, 6, 249–259. [Google Scholar] [CrossRef]
- Hoseini, Z.; Behpour, N.; Hoseini, R. Co-treatment with vitamin D supplementation and aerobic training in elderly women with Vit D deficiency and NAFLD: A single-blind controlled trial. Hepat. Mon. 2020, 20, e96437. [Google Scholar] [CrossRef]
- Moradi, B.; Rahmati-Ahmadabad, S.; Farzanegi, P.; Helalizadeh, M.; Azarbayjani, M.-A. Effects of non-linear resistance training and curcumin supplementation on the liver biochemical markers levels and structure in older women with non-alcoholic fatty liver disease. J. Bodyw. Mov. Ther. 2020, 24, 154–160. [Google Scholar] [CrossRef]
- Rezende, R.E.; Duarte, S.; Stefano, J.T.; Roschel, H.; Gualano, B.; de Sá Pinto, A.L.; Vezozzo, D.C.; Carrilho, F.J.; Oliveira, C.P. Randomized clinical trial: Benefits of aerobic physical activity for 24 weeks in postmenopausal women with nonalcoholic fatty liver disease. Menopause 2016, 23, 876–883. [Google Scholar] [CrossRef]
- Zhang, H.-J.; He, J.; Pan, L.-L.; Ma, Z.-M.; Han, C.-K.; Chen, C.-S.; Chen, Z.; Han, H.-W.; Chen, S.; Sun, Q. Effects of moderate and vigorous exercise on nonalcoholic fatty liver disease: A randomized clinical trial. JAMA Intern. Med. 2016, 176, 1074–1082. [Google Scholar] [CrossRef]
- Cuthbertson, D.J.; Shojaee-Moradie, F.; Sprung, V.S.; Jones, H.; Pugh, C.J.; Richardson, P.; Kemp, G.J.; Barrett, M.; Jackson, N.C.; Thomas, E.L. Dissociation between exercise-induced reduction in liver fat and changes in hepatic and peripheral glucose homoeostasis in obese patients with non-alcoholic fatty liver disease. Clin. Sci. 2016, 130, 93–104. [Google Scholar] [CrossRef]
- Hallsworth, K.; Fattakhova, G.; Hollingsworth, K.G.; Thoma, C.; Moore, S.; Taylor, R.; Day, C.P.; Trenell, M.I. Resistance exercise reduces liver fat and its mediators in non-alcoholic fatty liver disease independent of weight loss. Gut 2011, 60, 1278–1283. [Google Scholar] [CrossRef] [PubMed]
- Houghton, D.; Thoma, C.; Hallsworth, K.; Cassidy, S.; Hardy, T.; Burt, A.D.; Tiniakos, D.; Hollingsworth, K.G.; Taylor, R.; Day, C.P. Exercise reduces liver lipids and visceral adiposity in patients with nonalcoholic steatohepatitis in a randomized controlled trial. Clin. Gastroenterol. Hepatol. 2017, 15, 96–102.e103. [Google Scholar] [CrossRef] [PubMed]
- Javanmardi Fard, S.; Ghodsbin, F.; Kaviani, M.J.; Jahanbin, I.; Bagheri, Z. The effect of follow up (telenursing) on liver enzymes in patients with nonalcoholic fatty liver disease: A randomized controlled clinical trial. Int. J. Community Based Nurs. Midwifery 2015, 4, 239. [Google Scholar]
- Keating, S.E.; Hackett, D.A.; Parker, H.M.; O’Connor, H.T.; Gerofi, J.A.; Sainsbury, A.; Baker, M.K.; Chuter, V.H.; Caterson, I.D.; George, J. Effect of aerobic exercise training dose on liver fat and visceral adiposity. J. Hepatol. 2015, 63, 174–182. [Google Scholar] [CrossRef]
- Khaoshbaten, M.; Gholami, N.; Sokhtehzari, S.; Monazami, A.H.; Nejad, M.R. The effect of an aerobic exercise on serum level of liver enzymes and liver echogenicity in patients with non alcoholic fatty liver disease. Gastroenterol. Hepatol. Bed Bench 2013, 6, S112. [Google Scholar]
- Nourian, M.; Askari, G.; Golshiri, P.; Miraghajani, M.; Shokri, S.; Arab, A. Effect of lifestyle modification education based on health belief model in overweight/obese patients with non-alcoholic fatty liver disease: A parallel randomized controlled clinical trial. Clin. Nutr. ESPEN 2020, 38, 236–241. [Google Scholar] [CrossRef]
- Pugh, C.J.; Sprung, V.S.; Kemp, G.J.; Richardson, P.; Shojaee-Moradie, F.; Umpleby, A.M.; Green, D.J.; Cable, N.T.; Jones, H.; Cuthbertson, D.J. Exercise training reverses endothelial dysfunction in nonalcoholic fatty liver disease. Am. J. Physiol.-Heart Circ. Physiol. 2014, 307, H1298–H1306. [Google Scholar] [CrossRef]
- Sullivan, S.; Kirk, E.P.; Mittendorfer, B.; Patterson, B.W.; Klein, S. Randomized trial of exercise effect on intrahepatic triglyceride content and lipid kinetics in nonalcoholic fatty liver disease. Hepatology 2012, 55, 1738–1745. [Google Scholar] [CrossRef]
- Takahashi, A.; Abe, K.; Usami, K.; Imaizumi, H.; Hayashi, M.; Okai, K.; Kanno, Y.; Tanji, N.; Watanabe, H.; Ohira, H. Simple resistance exercise helps patients with non-alcoholic fatty liver disease. Int. J. Sport. Med. 2015, 94, 848–852. [Google Scholar] [CrossRef]
- Yao, J.; Meng, M.; Yang, S.; Li, F.; Anderson, R.M.; Liu, C.; Liu, L.; Yuan, X.; Fang, Z.; Lou, Q. Effect of aerobic and resistance exercise on liver enzyme and blood lipids in Chinese patients with nonalcoholic fatty liver disease: A randomized controlled trial. Int. J. Clin. Exp. Med. 2018, 11, 4867–4874. [Google Scholar]
- Pugh, C.J.; Cuthbertson, D.J.; Sprung, V.S.; Kemp, G.J.; Richardson, P.; Margot Umpleby, A.; Green, D.J.; Timothy Cable, N.; Jones, H. Exercise training improves cutaneous microvascular function in nonalcoholic fatty liver disease. Am. J. Physiol.-Endocrinol. Metab. 2013, 305, E50–E58. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, F.; Chen, K.; Xia, Y.; Lu, J.; Zhou, Y.; Guo, C. Effects of physical activity on liver function in patients with non-alcoholic fatty liver disease: A meta-analysis. So. J. Immunol. 2015, 3, 1–6. [Google Scholar]
- Wang, S.-T.; Zheng, J.; Peng, H.-W.; Cai, X.-L.; Pan, X.-T.; Li, H.-Q.; Hong, Q.-Z.; Peng, X.-E. Physical activity intervention for non-diabetic patients with non-alcoholic fatty liver disease: A meta-analysis of randomized controlled trials. BMC Gastroenterol. 2020, 20, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Brea, A.; Puzo, J. Non-alcoholic fatty liver disease and cardiovascular risk. Int. J. Cardiol. 2013, 167, 1109–1117. [Google Scholar] [CrossRef]
- Katsagoni, C.N.; Georgoulis, M.; Papatheodoridis, G.V.; Panagiotakos, D.B.; Kontogianni, M.D. Effects of lifestyle interventions on clinical characteristics of patients with non-alcoholic fatty liver disease: A meta-analysis. Metabolism 2017, 68, 119–132. [Google Scholar] [CrossRef]
- Nikroo, H.; Attarzade Hosseini, S.; Sima, H.; Nematy, M. Effects of diets with or without aerobic exercise program on anthropometric indices and cardiorespiratory fitness in patients with non-alcoholic steatohepatitis. J. North Khorasan Univ. Med. Sci. 2011, 3, 91–99. [Google Scholar]
- Mohammad Rahimi, G.R.; Attarzadeh Hosseini, S.R. Effect of Aerobic Exercise Alone or in Conjunction With Diet on Liver Function, Insulin Resistance and Lipids in Non-Alcoholic Fatty Liver Disease. Biol. Res. Nurs. 2022, 24, 259–276. [Google Scholar] [CrossRef]
- Rice, B.; Janssen, I.; Hudson, R.; Ross, R. Effects of aerobic or resistance exercise and/or diet on glucose tolerance and plasma insulin levels in obese men. Diabetes Care 1999, 22, 684–691. [Google Scholar] [CrossRef]
- Kanaley, J.A.; Colberg, S.R.; Corcoran, M.H.; Malin, S.K.; Rodriguez, N.R.; Crespo, C.J.; Kirwan, J.P.; Zierath, J.R. Exercise/physical activity in individuals with type 2 diabetes: A consensus statement from the American College of Sports Medicine. Med. Sci. Sport. Exerc. 2022, 54, 353–368. [Google Scholar] [CrossRef]
- Boulé, N.G.; Haddad, E.; Kenny, G.P.; Wells, G.A.; Sigal, R.J. Effects of exercise on glycemic control and body mass in type 2 diabetes mellitus: A meta-analysis of controlled clinical trials. JAMA 2001, 286, 1218–1227. [Google Scholar] [CrossRef]
- Chudyk, A.; Petrella, R.J. Effects of exercise on cardiovascular risk factors in type 2 diabetes: A meta-analysis. Diabetes Care 2011, 34, 1228–1237. [Google Scholar] [CrossRef] [PubMed]
- Phielix, E.; Meex, R.; Moonen-Kornips, E.; Hesselink, M.; Schrauwen, P. Exercise training increases mitochondrial content and ex vivo mitochondrial function similarly in patients with type 2 diabetes and in control individuals. Diabetologia 2010, 53, 1714–1721. [Google Scholar] [CrossRef] [PubMed]
- Winnick, J.J.; Sherman, W.M.; Habash, D.L.; Stout, M.B.; Failla, M.L.; Belury, M.A.; Schuster, D.P. Short-term aerobic exercise training in obese humans with type 2 diabetes mellitus improves whole-body insulin sensitivity through gains in peripheral, not hepatic insulin sensitivity. J. Clin. Endocrinol. Metab. 2008, 93, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Kirwan, J.P.; Solomon, T.P.; Wojta, D.M.; Staten, M.A.; Holloszy, J.O. Effects of 7 days of exercise training on insulin sensitivity and responsiveness in type 2 diabetes mellitus. Am. J. Physiol.-Endocrinol. Metab. 2009, 297, E151–E156. [Google Scholar] [CrossRef] [PubMed]
- Melsen, W.; Bootsma, M.; Rovers, M.; Bonten, M. The effects of clinical and statistical heterogeneity on the predictive values of results from meta-analyses. Clin. Microbiol. Infect. 2014, 20, 123–129. [Google Scholar] [CrossRef]
- Pickering, C.; Kiely, J. Do non-responders to exercise exist—And if so, what should we do about them? Sport. Med. 2019, 49, 1–7. [Google Scholar] [CrossRef]
- Sparks, L.M. Exercise training response heterogeneity: Physiological and molecular insights. Diabetologia 2017, 60, 2329–2336. [Google Scholar] [CrossRef]
- Sabag, A.; Barr, L.; Armour, M.; Armstrong, A.; Baker, C.J.; Twigg, S.M.; Chang, D.; Hackett, D.A.; Keating, S.E.; George, J.; et al. The Effect of High-intensity Interval Training vs Moderate-intensity Continuous Training on Liver Fat: A Systematic Review and Meta-Analysis. J. Clin. Endocrinol. Metab. 2022, 107, 862–881. [Google Scholar] [CrossRef]
| Intervention | ||||||
|---|---|---|---|---|---|---|
| Study | Groups | Mode | Frequency (Days) | Intervention Duration (Weeks) | Duration of Each Session (Min) | Intensity |
| Abdelbasset et al., 2019 [26] | HIIT CON | Exercise program was performed on a cycle ergometer | 3/7 | 8 | 40 min | HIIT group: 80–85% VO2max CON group: No intervention |
| Cuthbertson et al., 2016 [42] | AE CON | Aerobic exercise (treadmill, cross-trainer, bike ergometer, rower) | 3–5/7 | 12 | 30–45 min | AE group: 30–60% HRmax CON group: No intervention |
| Eckard et al., 2013 [37] | AE CON | Participants followed an 18-step program, including warm up, exercise bicycle, walking on a treadmill, various arm and leg strength exercises, and cool down, with gradual ramp up over the 6 months | 4–7/7 | 24 | 20–60 min | AE group: Moderate exercise CON group: No intervention |
| George et al., 2008 [36] | LI MI CON | Participants were encouraged to increase both planned and incidental moderate-intensity PA to achieve at least 150 min/week for general health and to target more than 200 min/week for weight loss | 3/7 6/7 | 4 10 | 45–60 min | LI group: 35–40% VO2max MI group: 70–75% VO2max CON group: No intervention |
| Hallsworth et al., 2011 [43] | RT CON | The program consisted of eight exercises: biceps curl; calf raise; triceps press; chest press; seated hamstrings curl; shoulder press; leg extension; and lateral pull down | 3/7 | 8 | 45–60 min | RT group: 50–70% 1RM CON group: No intervention |
| Hatami et al., 2016 [35] | AE + RT CON | The aerobic training section consisted of rhythmic movements and the resistance training section consisted of biceps, triceps, pectoralis, quadriceps, and hamstring muscles in the position of dumbbell biceps (seated concentration curl), dumbbell triceps (triceps kickback), bench press, knee extension, and knee flexion | 3/7 | 8 | 40 min | AE + RT group: 3 sets/8–12 reps at 60–80% 1RM 65–85% HRmax CON group: No intervention |
| Hoseini et al., 2020 [38] | AE CON | Walking and jogging/running | 3/7 | 8 | 20–40 min | AE group: 60–75%, HRmax CON group: No intervention |
| Houghton et al., 2017 [44] | AE + RT CON | The exercise program consisted of aerobic (cycling) and resistance training | 3/7 | 12 | 60 min | AE + RT group: Moderate vigorous Activity CON group: No intervention |
| Javanmardi Fard et al., 2015 [45] | AE CON | The physical activities included jogging, cycling, aerobic exercise or any other activity that is of a similar intensity | 4–5/7 | 12 | 30 min | AE group: Moderate physical activities CON group: No intervention |
| Keating et al., 2015 [46] | HILO LOHI LOLO CON | Performed continuous cycling on the ergometer/brisk walk | 3–4/7 | 8 | 45–60 min | LO:HI group: 50% VO2peak HI:LO group: 70% VO2peak LO:LO group: 50% VO2peak CON group: No intervention |
| Khaoshbaten et al., 2013 [47] | AE CON | Walking/running | 3/7 | 12 | 30 min | AE group: Maximal heart rate CON group: No intervention |
| Mohammadi et al., 2019 [34] | CRT CON | The resistance training included three circles with nine stations per circle | 3/7 | 12 | 30–45 min | CRT group: 3 sets/10–20 reps at 40–80% 1RM CON group: No intervention |
| Moradi Kelardeh et al., 2017 [33] | RT CON | Knee extension, bench press, incline bench press, seated row, dead lift, pulley crunches, lat pull-downs, calf raise, hamstring curl, press behind neck, upright row, arm curl | 3/7 | 12 | 45–60 min | RT group: 1–3 sets/2–20 reps at 40–95% 1RM CON group: No intervention |
| Moradi Kelardeh et al., 2020 [39] | RT CON | Knee extension, bench press, incline bench press, seated row, dead lift, pulley crunches, lat pull-downs, calf raise, hamstring curl, press behind neck, upright row, arm curl | 3/7 | 12 | 60–70 min | RT group: 1–4 sets/2–20 reps at 40–95% 1RM CON group: No intervention |
| Nourian et al., 2020 [48] | AE CON | The educational content of each session was presented to participants by using a short lecture, slide show, pamphlet, and discussion. | 5/7 | 8 | 30–60 min | AE group: Lifestyle modification education training CON group: No intervention |
| Pugh et al., 2014 [49] | AE CON | Exercise training comprised a combination of treadmill and cycle ergometer-based exercise, which progressively increased in both intensity and duration throughout the course of the intervention | 3–5/7 | 12 | 30–45 min | AE group: 30–60 HRmax CON group: No intervention |
| Rezende et al., 2016 [40] | AE CON | Aerobic exercise on treadmill | 2/7 | 24 | 120 min | AE group: Increased from VAT up to 10% below RCP CON group: No intervention |
| Sadeghi et al., 2019 [32] | RT CON | These exercises include TRX plank on elbows, TRX T deltoid fly, TRX chest press, TRX high row, TRX triceps press, TRX biceps curl, TRX squat, and TRX hip press | 3/7 | 8 | 60 min | RT group: 3 sets/8 reps at 70–100% 1RM CON group: No intervention |
| Shamsoddini et al., 2015 [31] | AE RT CON | Running on treadmill/seven exercises with machine: triceps press, biceps curl, calf raise, leg press, leg extension, lat pull down, and sit-ups | 3/7 | 8 | 45 min | AE group: 60–75% HRmax RT group: 50–70% 1RM CON group: No intervention |
| Shojaee-Moradie et al., 2016 [30] | AE CON | Types of activities were either gym-based aerobic plus resistance exercise or outdoor aerobic activities and resistance exercise | 4–5/7 | 16 | 20–60 min | AE group: 40–60% HRmax CON group: No intervention |
| Sreenivasa Baba et al., 2006 [29] | AE CON | Brisk walking/jogging or rhythmic aerobic exercises set to beat music | 5/7 | 12 | 20–45 min | AE group: 60–70% HRmax CON group: No intervention |
| Sullivan et al., 2012 [50] | AE CON | Brisk walk/walking on a treadmill | 5/7 | 16 | 30–60 min | AE group: 45–55% VO2peak CON group: No intervention |
| Takahashi et al., 2015 [51] | RT CON | Resistance exercise comprising push-ups and squats at the beginning of the study. | 3/7 | 12 | 20–30 min | RT group: 3 sets/10 reps CON group: No intervention |
| Valizadeh et al., 2011 [28] | AE CON | Walking/running | 5/7 | 8 | 45 min | AE group: 50–70% VO2max CON group: No intervention |
| Yao et al., 2018 [52] | AE RT CON | Walking/running Elastic band was used as the load instrument | 3/7 | 22 | 40–60 min | AE group: 60–70% HRmax RT group: 3 sets/10 reps; 60–70% 1RM CON group: No intervention |
| Zhang et al., 2016 [41] | ME VME CON | Jogged on a treadmill and gradually increased exercise intensity | 5/7 | 48 | 30 min | ME group: 65–80% HRmax CON group: No intervention |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hejazi, K.; Hackett, D. Effect of Exercise on Liver Function and Insulin Resistance Markers in Patients with Non-Alcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Clin. Med. 2023, 12, 3011. https://doi.org/10.3390/jcm12083011
Hejazi K, Hackett D. Effect of Exercise on Liver Function and Insulin Resistance Markers in Patients with Non-Alcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Journal of Clinical Medicine. 2023; 12(8):3011. https://doi.org/10.3390/jcm12083011
Chicago/Turabian StyleHejazi, Keyvan, and Daniel Hackett. 2023. "Effect of Exercise on Liver Function and Insulin Resistance Markers in Patients with Non-Alcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials" Journal of Clinical Medicine 12, no. 8: 3011. https://doi.org/10.3390/jcm12083011
APA StyleHejazi, K., & Hackett, D. (2023). Effect of Exercise on Liver Function and Insulin Resistance Markers in Patients with Non-Alcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Journal of Clinical Medicine, 12(8), 3011. https://doi.org/10.3390/jcm12083011






