How Can We Best Measure Frailty in Cardiosurgical Patients?
Abstract
1. Introduction
2. Materials and Methods
2.1. Comparison of Frailty Tests
2.2. Laboratory Analysis
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Frailty Measurements
3.2.1. Frailty Group (Frail)
3.2.2. Comparison of Frailty Tests and Risk Scores
- 1.
- Mortality: Patients who died during their hospital stay had higher STS scores for mortality, stroke, morbidity or mortality, and long length of stay: Dead vs. survivors in STS mortality: 2.05 ± 1.47 vs. 1.22 ± 1.18, p = 0.006. STS stroke: 1.77 ± 1.42 vs. 1.05 ± 0.64, p = 0.033. STS morbidity or mortality: 12.59 ± 6.84 vs. 7.84 ± 4.91, p = 0.005. STS long length of stay: 7.92 ± 6.17 vs. 4.28 ± 6.85 p = 0.004. Regarding 1-year mortality, a longer distance in 6 MW predicted lower mortality (317.92 ± 94.17 m vs. 387.08 ± 93.43 m, p = 0.006). Additionally, the MMS scale (25.72 ± 4.36 vs. 27.71 ± 1.9, p = 0.048) and clinical frail scale (3.65 ± 1.32 vs. 2.82 ± 0.86 p = 0.005) showed differences in 1-year mortality. Unsurprisingly, the EuroSCORE (6.75 ± 9.18 vs. 2.19 ± 1.84, p = 0.003) as well as STS scores differed (STS mortality: 2.61 ± 2.09 vs. 1.15 ± 1, p < 0.001, STS stroke: 1.69 ± 1.18 vs. 1.03 ± 0.58, p = 0.001, STS morbidity and mortality: 13.71 ± 6.87 vs. 7.65 ± 4.66, p < 0.001, STS long length of stay: 8.02 ± 5.6 vs. 4.25 ± 7.16, p < 0.001).
- 2.
- Complication rates: Patients with a wound healing disorder had higher STS scores regarding stroke (1.33 ± 0.84 vs. 1.02 ± 0.61, p = 0.031) and STS scores regarding long length of stay (4.59 ± 3.6 vs. 4.36 ± 7.13 p = 0.038). Patients who suffered from a stroke had a higher EuroSCORE (3.45 ± 2.04 vs. 2.42 ± 2.62, p = 0.039) and STS score regarding stroke (1.32 ± 0.47 vs. 1.05 ± 0.65, p = 0.048). Additionally, patients who post-operatively presented new episodes of atrial fibrillation had higher STS scores regarding stroke (1.13 ± 0.56 vs. 1.03 ± 0.68, p = 0.04). Patients undergoing re-thoracotomy due to bleeding had a higher STS score calculated risk for mortality (1.8 ± 1.36 vs. 1.19 ± 1.13, p = 0.028).
- 3.
- Length of treatment: In-hospital stay correlated with TUG (TAU: 0.094, p = 0.037), Barthel index (TAU-0.114, p = 0.032), hand grip strength (TAU-0.173, p < 0.001), EuroSCORE II (TAU 0.119, p = 0.008), STS mortality score (TAU 0.13 p = 0.003), STS stroke score (TAU 0.135, p = 0.003), STS morbidity or mortality score (TAU 0.106, p = 0.017), and STS long length of stay score (TAU 0.098, p = 0.027). The duration of intensive care treatment correlated with TUG (TAU 0.196, p < 0.001), 6 MW (TAU-0.133, p = 0.008), MMS (TAU-0.106, p = 0.045), EuroSCORE II (TAU 0.181, p < 0.001), STS mortality score (TAU 0.243, p <0.001), STS stroke score (TAU 0.171, p < 0.001), STS morbidity and mortality score (TAU 0.246, p < 0.001), and STS long length of stay score (TAU 0.252, p = <0.001). We added an analysis of intensive care treatment including intermediate care treatment. Correlations were found between TUG (TAU 0.186, p < 0.001), 6 MW (TAU-0.149, p = 0.002), hand grip strength (TAU-0.22, p < 0.001), EuroSCORE II (TAU 0.209, p < 0.001), STS mortality score (TAU 0.247, p < 0.001), STS stroke score (TAU 0.205, p < 0.001), STS morbidity and mortality score (TAU 0.21, p < 0.001), and STS long length of stay score (TAU 0.232, p < 0.001).
- 4.
- Activities of daily living and AGE-Reader showed no significant correlation with outcome. We had additionally asked for information regarding falls and the number of hospital stays in the last year, which also showed no significant correlation with the outcome of this group.
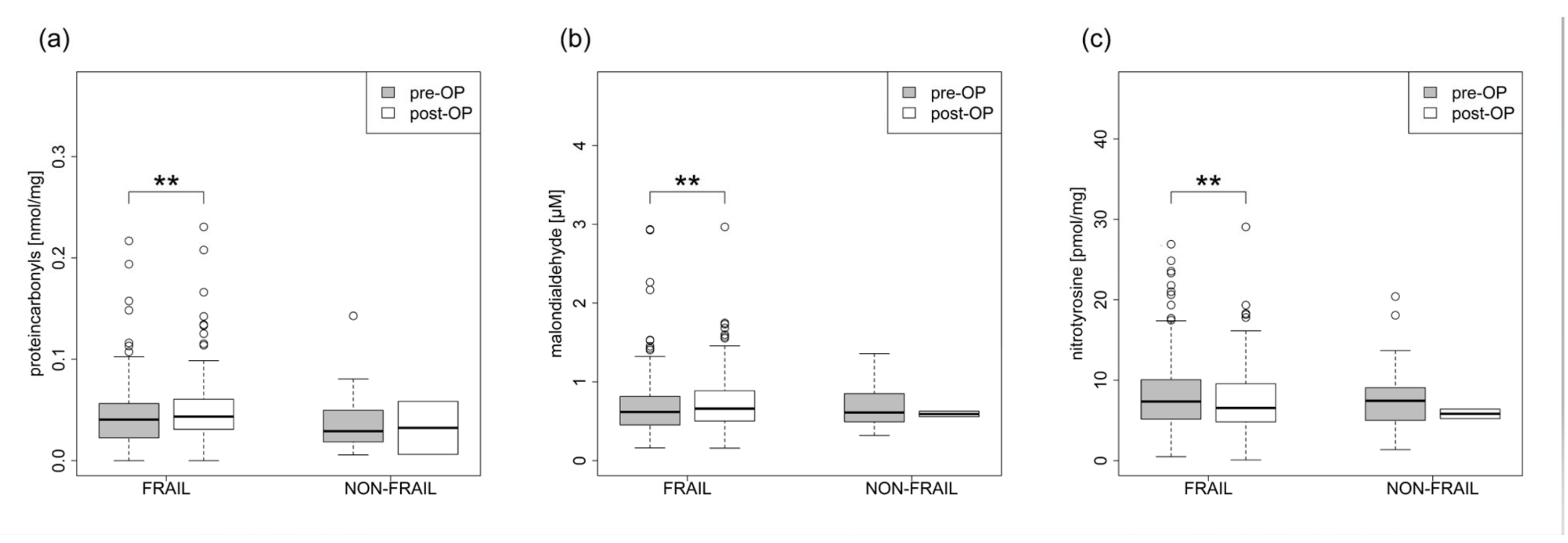
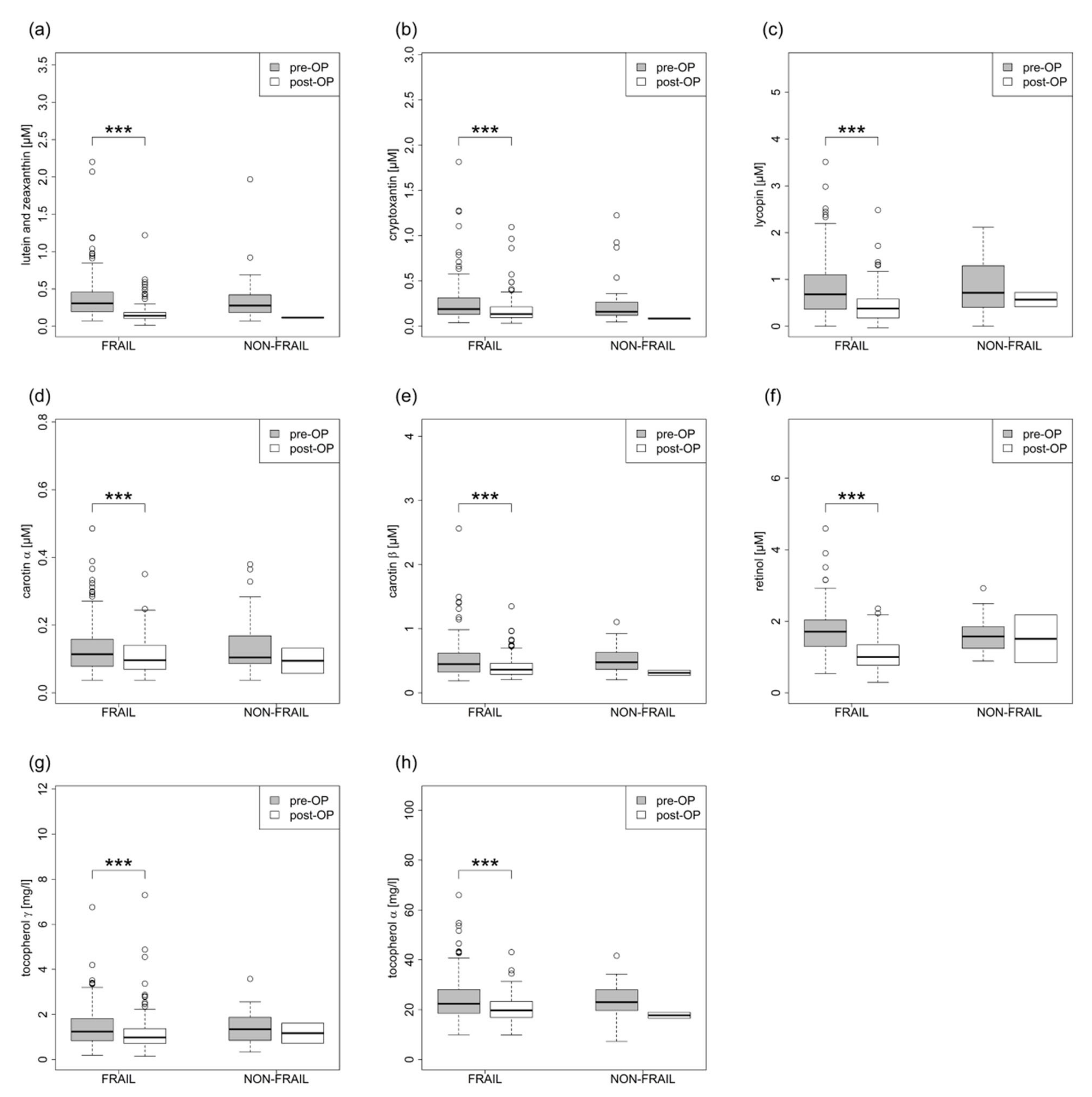
3.3. Laboratory Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Deutscher Herzbericht. German Heart Report. 2021. Available online: https://epaper.herzstiftung.de/#0 (accessed on 3 September 2022).
- Antonazzo, B.; Biondi-Zoccai, G.; Marullo, A.G.M.; Frati, G.; Ronzoni, S.; Chiariello, G.A.; Versaci, F.; Giordano, A. Transcatheter aortic valve implantation in the elderly: An umbrella review. Vessel. Plus. 2020, 4, 3. [Google Scholar] [CrossRef]
- Ferrante, L.E.; Pisani, M.A.; Murphy, T.E.; Gahbauer, E.A.; Leo-Summers, L.S.; Gill, T.M. The Association of Frailty with Post-ICU Disability, Nursing Home Admission, and Mortality. Chest 2018, 153, 1378–1386. [Google Scholar] [CrossRef] [PubMed]
- Sprung, C.L.; Artigas, A.; Kesecioglu, J.; Pezzi, A.; Wiis, J.; Pirracchio, R.; Baras, M.; Edbrooke, D.L.; Pesenti, A.; Bakker, J.; et al. The Eldicus prospective, observational study of triage decision making in European intensive care units. Part II. Crit. Care Med. 2012, 40, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Bagshaw, S.M.; Stelfox, H.T.; McDermid, R.C.; Rolfson, D.B.; Tsuyuki, R.T.; Baig, N.; Artiuch, B.; Ibrahim, Q.; Stollery, D.E.; Rokosh, E.; et al. Association between frailty and short- and long-term outcomes among critically ill patients: A multicentre prospective cohort study. Can. Med. Assoc. J. 2013, 186, E95–E102. [Google Scholar] [CrossRef] [PubMed]
- Ostovar, R.; Schröter, F.; Kühnerl, R.U.; Hartrumpf, M.; Albes, J.M. What Exactly Makes Age a Risk Factor for an Unfavorable Outcome after Mitral Valve Surgery? J. Clin. Med. 2022, 11, 6907. [Google Scholar] [CrossRef] [PubMed]
- Nashef, S.A.; Roques, F.; Sharples, L.D.; Nilsson, J.; Smith, C.; Goldstone, A.R.; Lockowandt, U. Euroscore ii. Eur. J. Cardio-Thoracic Surg. 2012, 41, 734–744. [Google Scholar] [CrossRef]
- Gogbashian, A.; Sedrakyan, A.; Treasure, T. EuroSCORE: A systematic review of international performance. Eur. J. Cardio-Thoracic Surg. 2004, 25, 695–700. [Google Scholar] [CrossRef]
- Dewey, T.M.; Brown, D.; Ryan, W.H.; Herbert, M.A.; Prince, S.L.; Mack, M.J. Reliability of risk algorithms in predicting early and late operative outcomes in high-risk patients undergoing aortic valve replacement. J. Thorac. Cardiovasc. Surg. 2008, 135, 180–187. [Google Scholar] [CrossRef]
- Ostovar, R.; Schroeter, F.; Erb, M.; Hartrumpf, M.; Chopsonidou, S.; Albes, J.M. Liver cirrhosis: Still an elusive risk factor in the current EuroSCORE system. Eur. J. Cardio-Thoracic Surg. 2022, 62, ezac128. [Google Scholar] [CrossRef]
- Wendt, D.; Osswald, B.R.; Kayser, K.; Thielmann, M.; Tossios, P.; Massoudy, P.; Kamler, M.; Jakob, H. Society of Thoracic Surgeons Score Is Superior to the EuroSCORE Determining Mortality in High Risk Patients Undergoing Isolated Aortic Valve Replacement. Ann. Thorac. Surg. 2009, 88, 468–475. [Google Scholar] [CrossRef]
- Rockwood, K.; Abeysundera, M.J.; Mitnitski, A. How should we grade frailty in nursing home patients? J. Am. Med. Dir. Assoc. 2007, 8, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Topinkova, E. Ageing, Disability and Frailty. Ann. Nutr. Metab. 2008, 52, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in Older adults: Evidence for a phenotype. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.; Bruno, R.R.; Wernly, B.; Wolff, G.; Beil, M.; Kelm, M. Frailty as a Prognostic Indicator in Intensive Care. Dtsch. Ärzteblatt Int. 2020, 117, 668–673. [Google Scholar] [CrossRef]
- Rockwood, K.; Song, X.; MacKnight, C.; Bergman, H.; Hogan, D.B.; McDowell, I.; Mitnitski, A. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005, 173, 489–495. [Google Scholar] [CrossRef]
- Cornette, P.; Swine, C.; Malhomme, B.; Gillet, J.-B.; Meert, P.; D’Hoore, W. Early evaluation of the risk of functional decline following hospitalization of older patients: Development of a predictive tool. Eur. J. Public Health 2005, 16, 203–208. [Google Scholar] [CrossRef]
- Sager, M.A.; Franke, T.; Inouye, S.K.; Landefeld, C.S.; Morgan, T.M.; Rudberg, M.A.; Siebens, H.; Winograd, C.H. Functional Outcomes of Acute Medical Illness and Hospitalization in Older Persons. Arch. Intern. Med. 1996, 156, 645–652. [Google Scholar] [CrossRef]
- Afilalo, J.; Mottillo, S.; Eisenberg, M.J.; Alexander, K.P.; Noiseux, N.; Perrault, L.P.; Morin, J.-F.; Langlois, Y.; Ohayon, S.M.; Monette, J.; et al. Addition of Frailty and Disability to Cardiac Surgery Risk Scores Identifies Elderly Patients at High Risk of Mortality or Major Morbidity. Circ. Cardiovasc. Qual. Outcomes 2012, 5, 222–228. [Google Scholar] [CrossRef]
- Sündermann, S.; Dademasch, A.; Rastan, A.; Praetorius, J.; Rodriguez, H.; Walther, T.; Mohr, F.-W.; Falk, V. One-year follow-up of patients undergoing elective cardiac surgery assessed with the Comprehensive Assessment of Frailty test and its simplified form. Interact. Cardiovasc. Thorac. Surg. 2011, 13, 119–123. [Google Scholar] [CrossRef]
- Sepehri, A.; Beggs, T.; Hassan, A.; Rigatto, C.; Shaw-Daigle, C.; Tangri, N.; Arora, R.C. The impact of frailty on outcomes after cardiac surgery: A systematic review. J. Thorac. Cardiovasc. Surg. 2014, 148, 3110–3117. [Google Scholar] [CrossRef]
- Hiraoka, A.; Saito, K.; Chikazawa, G.; Totsugawa, T.; Tamura, K.; Ishida, A.; Sakaguchi, T.; Yoshitaka, H. Modified predictive score based on frailty for mid-term outcomes in open total aortic arch surgery. Eur. J. Cardio-Thoracic Surg. 2018, 54, 42–47. [Google Scholar] [CrossRef] [PubMed]
- McCusker, J.; Bellavance, F.; Cardin, S.; Trepanier, S.; Verdon, J.; Ardman, O.; McCusker, D.J.; Msc, S.T.; Msc, O.O.A. Detection of Older People at Increased Risk of Adverse Health Outcomes After an Emergency Visit: The ISAR Screening Tool. J. Am. Geriatr. Soc. 1999, 47, 1229–1237. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.A.; Yanagawa, B.; An, K.R.; Arora, R.C.; Verma, S.; Friedrich, J.O.; On behalf of the Canadian Cardiovascular Surgery Meta-Analysis Working Group. Frailty and pre-frailty in cardiac surgery: A systematic review and meta-analysis of 66,448 patients. J. Cardiothorac. Surg. 2021, 16, 184. [Google Scholar] [CrossRef]
- Kane, A.E.; Sinclair, D.A. Frailty biomarkers in humans and rodents: Current approaches and future advances. Mech. Ageing Dev. 2019, 180, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Bilfinger, T.V. A Blood Test to Predict the Future. Thorac. Cardiovasc. Surg. 2019, 67, 426–427. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria; Available online: https://www.R-project.org/ (accessed on 10 August 2021).
- Afilalo, J.; Eisenberg, M.J.; Morin, J.-F.; Bergman, H.; Monette, J.; Noiseux, N.; Perrault, L.P.; Alexander, K.P.; Langlois, Y.; Dendukuri, N.; et al. Gait Speed as an Incremental Predictor of Mortality and Major Morbidity in Elderly Patients Undergoing Cardiac Surgery. J. Am. Coll. Cardiol. 2010, 56, 1668–1676. [Google Scholar] [CrossRef]
- Lytwyn, J.; Stammers, A.N.; Kehler, D.S.; Jung, P.; Alexander, B.; Hiebert, B.M.; Dubiel, C.; Kimber, D.; Hamm, N.; Clarke, M.; et al. The impact of frailty on functional survival in patients 1 year after cardiac surgery. J. Thorac. Cardiovasc. Surg. 2017, 154, 1990–1999. [Google Scholar] [CrossRef]
- Sündermann, S.H.; Dademasch, A.; Seifert, B.; Biefer, H.R.C.; Emmert, M.Y.; Walther, T.; Jacobs, S.; Mohr, F.-W.; Falk, V.; Starck, C.T. Frailty is a predictor of short- and mid-term mortality after elective cardiac surgery independently of age. Interact. Cardiovasc. Thorac. Surg. 2014, 18, 580–585. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, C.A.; Placide, S.; Lipsitz, L.A.; Marcantonio, E.R. Preoperative frailty assessment and outcomes at 6 months or later in older adults undergoing cardiac surgical procedures: A systematic review. Ann. Intern. Med. 2016, 165, 650–660. [Google Scholar] [CrossRef]
- Jung, P.; Pereira, M.A.; Hiebert, B.; Song, X.; Rockwood, K.; Tangri, N.; Arora, R.C. The impact of frailty on postoperative delirium in cardiac surgery patients. J. Thorac. Cardiovasc. Surg. 2015, 149, 869–875.e2. [Google Scholar] [CrossRef]
- Green, P.; Arnold, S.V.; Cohen, D.J.; Kirtane, A.J.; Kodali, S.K.; Brown, D.L.; Rihal, C.S.; Xu, K.; Lei, Y.; Hawkey, M.C.; et al. Relation of Frailty to Outcomes After Transcatheter Aortic Valve Replacement (from the PARTNER Trial). Am. J. Cardiol. 2015, 116, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Schoenenberger, C.-M.; Bischoff, S.; Kalesan, B.; Stuck, A.E.; Moser, A.; Wenaweser, P.; Jüni, P.; Windecker, S.; Carrel, T.; Schoenenberger, A.W.; et al. Evaluation of multidimensional geriatric assessment as a predictor of mortality and cardiovascular events after transcatheter aortic valve implantation. JACC Cardiovasc. Interv. 2012, 5, 489–496. [Google Scholar] [CrossRef]
- Codner, P.; Orvin, K.; Assali, A.; Sharony, R.; Vaknin-Assa, H.; Shapira, Y.; Schwartzenberg, S.; Bental, T.; Sagie, A.; Kornowski, R. Long-Term Outcomes for Patients with Severe Symptomatic Aortic Stenosis Treated With Transcatheter Aortic Valve Implantation. Am. J. Cardiol. 2015, 116, 1391–1398. [Google Scholar] [CrossRef]
- Reis, P.; Moro, A.; Ely, V.B.; Fernandes, C.; Vilagra, J.; Peres, L.; Junior, O.F.; Merino, E. Universal design and accessibility: An approach of the influence of muscle strength loss in the risk of falls in the elderly. Work 2012, 41, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Rijk, J.M.; Roos, P.R.; Deckx, L.; Akker, M.V.D.; Buntinx, F. Prognostic value of handgrip strength in people aged 60 years and older: A systematic review and meta-analysis. Geriatr. Gerontol. Int. 2015, 16, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Reichart, D.; Rosato, S.; Nammas, W.; Onorati, F.; Dalén, M.; Castro, L.; Gherli, R.; Gatti, G.; Franzese, I.; Faggian, G.; et al. Clinical frailty scale and outcome after coronary artery bypass grafting. Eur. J. Cardio-Thoracic Surg. 2018, 54, 1102–1109. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, J.A.; Lett, H.S.; Babyak, M.A.; White, W.; Smith, P.K.; Mark, D.B.; Jones, R.; Mathew, J.P.; Newman, M.F. Depression as a risk factor for mortality after coronary artery bypass surgery. Lancet 2003, 362, 604–609. [Google Scholar] [CrossRef]
- Afilalo, J.; Lauck, S.; Kim, D.H.; Lefèvre, T.; Piazza, N.; Lachapelle, K.; Martucci, G.; Lamy, A.; Labinaz, M.; Peterson, M.D.; et al. Frailty in Older Adults Undergoing Aortic Valve Replacement: The FRAILTY-AVR Study. J. Am. Coll. Cardiol. 2017, 70, 689–700. [Google Scholar] [CrossRef]
- Goldfarb, M.; Bendayan, M.; Rudski, L.G.; Morin, J.-F.; Langlois, Y.; Ma, F.; Lachapelle, K.; Cecere, R.; DeVarennes, B.; Tchervenkov, C.I.; et al. Cost of Cardiac Surgery in Frail Compared with Nonfrail Older Adults. Can. J. Cardiol. 2017, 33, 1020–1026. [Google Scholar] [CrossRef]


| Comorbidities | All (246) | Frail (146) | Non-Frail (100) | p-Value |
|---|---|---|---|---|
| Age (years) | 66.5 ± 9.05 | 66.68 ± 9.33 | 66.23 ± 8.67 | 0.566 |
| Sex (female) | 21.14% (52) | 25.34% (37) | 15% (15) | 0.073 |
| Hypertension | 72.36%(178) | 71.92% (105) | 73% (73) | 0.967 |
| Diabetes | 30.49% (75) | 34.93% (51) | 24.% (24) | 0.091 |
| LVEF | 56.86 ± 8.3 | 56.64 ± 8.94 | 57.18 ± 7.29 | 0.914 |
| EuroSCORE II | 2.59 ± 3.28 | 2.94 ± 3.93 | 2.08 ± 1.92 | 0.009 |
| BMI (kg/m2) | 29.71 ± 5.2 | 29.95 ± 5.57 | 29.37 ± 4.64 | 0.712 |
| Surgery | ||||
| CABG | 40.24% (98) | 36.3% (53) | 46% (46) | |
| sAVR | 32.11% (79) | 31.51% (46) | 33% (33) | |
| Combination CAGB/sAVR | 13.41% (33) | 13.01% (19) | 14% (14) | |
| Other operation | 14.23% (35) | 19.18% (28) | 7% (7) |
| All (246) | Frail (146) | Non-Frail (100) | p-Value | |
|---|---|---|---|---|
| In-hospital mortality | 4.88% (12) | 4.79% (7) | 5% (5) | 1 |
| 1-year mortality | 6.1% (13) | 6.45% (8) | 5.62 (5) | 1 |
| Wound healing disorder | 11.48% (28) | 11.81% (17) | 11% (11) | 1 |
| Stroke during stay | 3.28% (8) | 3.47% (5) | 3% (3) | 1 |
| Arrythmias | 25.61% (63) | 26.39% (38) | 25% (25) | 0.924 |
| Re-thoracotomy | 5.69% (14) | 5.59% (8) | 6% (6) | 1 |
| Myocardial infarction | 0.82% (2) | 0.69% (1) | 1% (1) | 1 |
| Pneumonia | 5.33% (13) | 4.86% (7) | 6% (6) | 0.921 |
| Delirium | 10.25% (25) | 11.81% (17) | 8% (8) | 0.454 |
| Intensive/intermediate care | 5.18 ± 4.5 | 5.4 ± 4.33 d | 4.86 ± 4.78 d | 0.014 |
| In-hospital stay | 14.79 ± 8.7 d | 15.53 ± 8.5 d | 13.71 ± 8.94 d | 0.004 |
| Plasma Redox Biomarkers | Frail Pre-Surgery | Frail Post-Surgery | p | Non-Frail Pre-Surgery | Non-Frail Post-Surgery | p |
|---|---|---|---|---|---|---|
| Protein carbonyls nmol/mg | 0.043 ± 0.03 | 0.049 ± 0.032 | 0.003 | 0.036 ± 0.027 | 0.032 ± 0.037 | 1.000 |
| Malondialdehyde µM | 0.695 ± 0.394 | 0.73 ± 0.339 | 0.009 | 0.699 ± 0.303 | 0.594 ± 0.05 | 0.500 |
| Nitrotyrosine pmol/mg | 8.002 ± 4.463 | 7.309 ± 3.996 | 0.010 | 7.881 ± 4.112 | 5.832 ± 0.834 | 1.000 |
| Fat-soluble micronutrients/antioxidants | ||||||
| Lutein / Zeaxanthin µM | 0.37 ± 0.272 | 0.165 ± 0.118 | <0.001 | 0.38 ± 0.341 | 0.117 ± 0.003 | 0.500 |
| Beta-Cryptoxanthine µM | 0.255 ± 0.215 | 0.172 ± 0.133 | <0.001 | 0.256 ± 0.26 | 0.085 ± 0.007 | 0.500 |
| Lycopin µM | 0.805 ± 0.603 | 0.424 ± 0.339 | <0.001 | 0.877 ± 0.569 | 0.57 ± 0.217 | 0.500 |
| Alpha-Carotene µM | 0.133 ± 0.077 | 0.109 ± 0.05 | <0.001 | 0.14 ± 0.088 | 0.095 ± 0.052 | 0.500 |
| Beta-Carotene µM | 0.508 ± 0.274 | 0.395 ± 0.155 | <0.001 | 0.517 ± 0.199 | 0.313 ± 0.056 | 0.500 |
| Retinol µM | 1.719 ± 0.587 | 1.053 ± 0.392 | <0.001 | 1.58 ± 0.468 | 1.511 ± 0.945 | 0.743 |
| Gamma-Tocopherol µM | 1.421 ± 0.854 | 1.155 ± 0.77 | <0.001 | 1.394 ± 0.712 | 1.168 ± 0.626 | 0.587 |
| Alpha-Tocopherol µM | 24.425 ± 8.548 | 20.295 ± 5.055 | <0.001 | 23.6 ± 6.751 | 17.748 ± 1.68 | 0.205 |
| In-house laboratory | ||||||
| CRP mg/L | 5.555 ± 10.153 | 84.38 ± 58.67 | <0.001 | 4.241 ± 5.01 | 63.281 ± 36.178 | <0.001 |
| Hemoglobin mmol/L | 8.801 ± 0.919 | 6.49 ± 0.822 | <0.001 | 8.674 ± 1.082 | 6.374 ± 0.952 | <0.001 |
| Creatintine µmol/L | 90.09 ± 76.075 | 81.628 ± 63.889 | <0.001 | 81.235 ± 22.969 | 86.955 ± 65.741 | 0.242 |
| Urea mmol/L | 6.93 ± 4.34 | 6.985 ± 4.285 | 0.400 | 9.997 ± 19.306 | 6.375 ± 3.14 | 0.194 |
| NT-proBNP pg/ml | 1123.361 ± 3146.059 | 2953.946 ± 4782.174 | <0.001 | 1106.085 ± 2729.072 | 4009.077 ± 9320.021 | 0.001 |
| Albumin g/L | 43.455 ± 3.001 | 33.812 ± 23.283 | <0.001 | 44.216 ± 6.102 | 30.667 ± 6.502 | <0.001 |
| Cholesterol mmol/L | 4.954 ± 1.385 | 4.043 ± 0.928 | <0.001 | 4.97 ± 0.961 | 3.873 ± 0.894 | 0.002 |
| HDL mmol/L | 1.22 ± 0.333 | 0.791 ± 0.17 | <0.001 | 1.341 ± 0.32 | 0.764 ± 0.174 | <0.001 |
| LDL mmol/L | 2.802 ± 1.196 | 2.394 ± 0.755 | <0.001 | 2.911 ± 0.951 | 2.293 ± 0.78 | 0.339 |
| Triglyceride mmol/L | 2.254 ± 1.637 | 1.948 ± 0.77 | 0.083 | 1.725 ± 0.863 | 1.774 ± 0.504 | 0.373 |
| Total protein g/L | 70.985 ± 5.075 | 58.513 ± 6.508 | <0.001 | 69.994 ± 4.737 | 55.492 ± 9.146 | 0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laux, M.L.; Braun, C.; Schröter, F.; Weber, D.; Moldasheva, A.; Grune, T.; Ostovar, R.; Hartrumpf, M.; Albes, J.M. How Can We Best Measure Frailty in Cardiosurgical Patients? J. Clin. Med. 2023, 12, 3010. https://doi.org/10.3390/jcm12083010
Laux ML, Braun C, Schröter F, Weber D, Moldasheva A, Grune T, Ostovar R, Hartrumpf M, Albes JM. How Can We Best Measure Frailty in Cardiosurgical Patients? Journal of Clinical Medicine. 2023; 12(8):3010. https://doi.org/10.3390/jcm12083010
Chicago/Turabian StyleLaux, Magdalena L., Christian Braun, Filip Schröter, Daniela Weber, Aiman Moldasheva, Tilman Grune, Roya Ostovar, Martin Hartrumpf, and Johannes Maximilian Albes. 2023. "How Can We Best Measure Frailty in Cardiosurgical Patients?" Journal of Clinical Medicine 12, no. 8: 3010. https://doi.org/10.3390/jcm12083010
APA StyleLaux, M. L., Braun, C., Schröter, F., Weber, D., Moldasheva, A., Grune, T., Ostovar, R., Hartrumpf, M., & Albes, J. M. (2023). How Can We Best Measure Frailty in Cardiosurgical Patients? Journal of Clinical Medicine, 12(8), 3010. https://doi.org/10.3390/jcm12083010









