Diagnostic Value of Serum Lactate Dehydrogenase Level Measured in the Emergency Department in Predicting Clinical Outcome in Out-of-Hospital Cardiac Arrest: A Multicenter, Observational Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
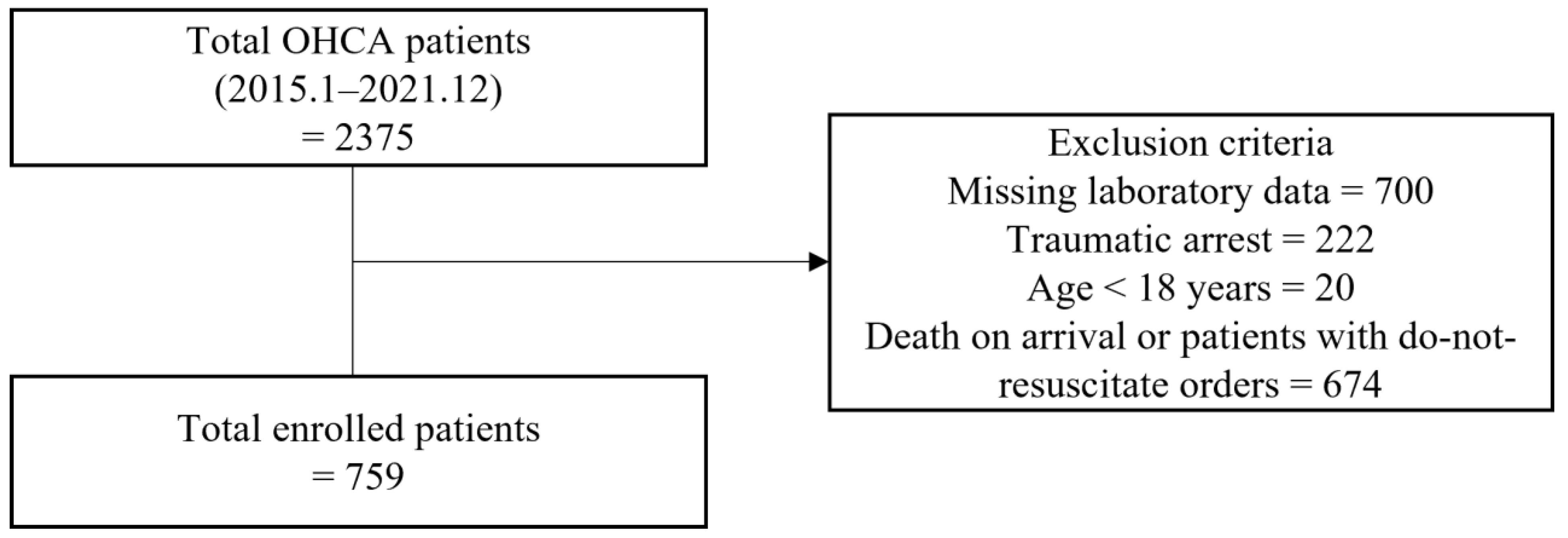
2.2. Participants
2.3. Study Variables
2.4. Study Endpoints
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
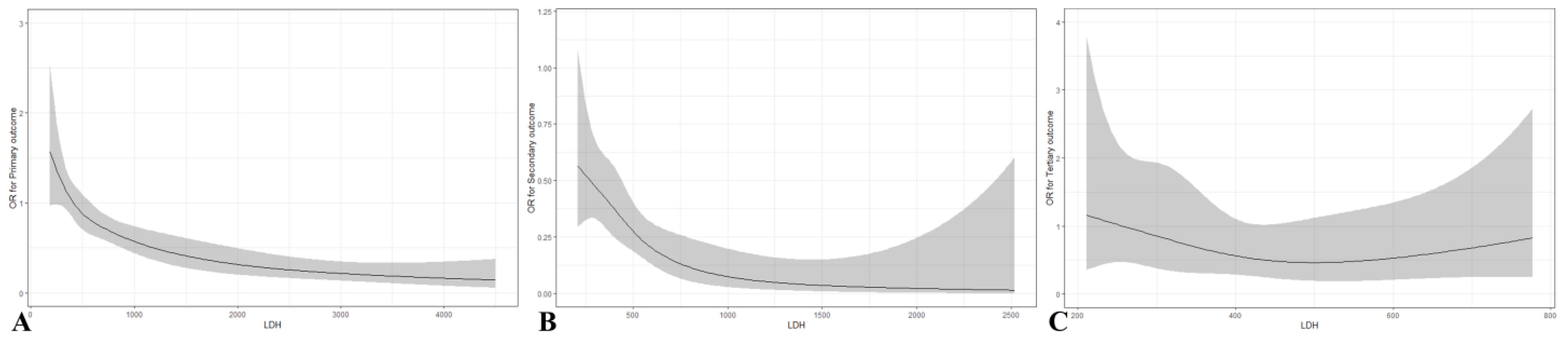
3.2. Diagnostic Value of LDH in the Clinical Outcomes of OHCA
3.3. Predictive Value of LDH in the Clinical Outcomes of OHCA
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kong, M.H.; Fonarow, G.C.; Peterson, E.D.; Curtis, A.B.; Hernandez, A.F.; Sanders, G.D.; Thomas, K.L.; Hayes, D.L.; Al-Khatib, S.M. Systematic Review of the Incidence of Sudden Cardiac Death in the United States. J. Am. Coll. Cardiol. 2011, 57, 794–801. [Google Scholar] [CrossRef]
- Wijdicks, E.F.M.; Hijdra, A.; Young, G.B.; Bassetti, C.L.; Wiebe, S. Practice Parameter: Prediction of outcome in comatose survivors after cardiopulmonary resuscitation (an evidence-based review): Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2006, 67, 203–210. [Google Scholar] [CrossRef]
- Chang, H.C.-H.; Tsai, M.-S.; Kuo, L.-K.; Hsu, H.-H.; Huang, W.-C.; Lai, C.-H.; Shih, M.-C.; Huang, C.-H. Factors affecting outcomes in patients with cardiac arrest who receive target temperature management: The multi-center TIMECARD registry. J. Formos. Med. Assoc. 2021, 121, 294–303. [Google Scholar] [CrossRef]
- Sandroni, C.; D’Arrigo, S.; Nolan, J.P. Prognostication after cardiac arrest. Crit. Care 2018, 22, 150. [Google Scholar] [CrossRef]
- Kim, T.Y.; Hwang, S.O.; Jung, W.J.; Roh, Y.I.; Kim, S.; Kim, H.; Cha, K.-C. Early neuro-prognostication with the Patient State Index and suppression ratio in post-cardiac arrest patients. J. Crit. Care 2021, 65, 149–155. [Google Scholar] [CrossRef]
- van Wijnen, H.G.; Rasquin, S.M.C.; van Heugten, C.M.; Verbunt, J.A.; Moulaert, V.R.M. The impact of cardiac arrest on the long-term wellbeing and caregiver burden of family caregivers: A prospective cohort study. Clin. Rehabil. 2017, 31, 1267–1275. [Google Scholar] [CrossRef]
- Sasson, C.; Rogers, M.A.M.; Dahl, J.; Kellermann, A.L. Predictors of survival from out-of-hospital cardiac arrest: A systematic review and meta-analysis. Circ. Cardiovasc. Qual. Outcomes 2010, 3, 63–81. [Google Scholar] [CrossRef]
- Han, S.I.; Cha, K.-C.; Roh, Y.I.; Hwang, S.O.; Jung, W.J.; Kim, T.Y. Association between Novel Marker (Platelet-Lymphocyte Ratio, Neutrophil-Lymphocyte Ratio, and Delta Neutrophil Index) and Outcomes in Sudden Cardiac Arrest Patients. Emerg. Med. Int. 2021, 2021, 6650958. [Google Scholar] [CrossRef]
- Cotoia, A.; Franchi, F.; De Fazio, C.; Vincent, J.-L.; Creteur, J.; Taccone, F.S. Platelet indices and outcome after cardiac arrest. BMC Emerg. Med. 2018, 18, 31. [Google Scholar] [CrossRef]
- Isenschmid, C.; Kalt, J.; Gamp, M.; Tondorf, T.; Becker, C.; Tisljar, K.; Locher, S.; Schuetz, P.; Marsch, S.; Hunziker, S. Routine blood markers from different biological pathways improve early risk stratification in cardiac arrest patients: Results from the prospective, observational COMMUNICATE study. Resuscitation 2018, 130, 138–145. [Google Scholar] [CrossRef]
- Røsjø, H.; Vaahersalo, J.; Hagve, T.-A.; Pettilä, V.; Kurola, J.; Omland, T. Prognostic value of high-sensitivity troponin T levels in patients with ventricular arrhythmias and out-of-hospital cardiac arrest: Data from the prospective FINNRESUSCI study. Crit. Care 2014, 18, 605. [Google Scholar] [CrossRef]
- Aarsetøy, R.; Omland, T.; Røsjø, H.; Strand, H.; Lindner, T.; Aarsetøy, H.; Staines, H.; Nilsen, D.W.T. N-terminal pro-B-type natriuretic peptide as a prognostic indicator for 30-day mortality following out-of-hospital cardiac arrest: A prospective observational study. BMC Cardiovasc. Disord. 2020, 20, 382. [Google Scholar] [CrossRef]
- Agusala, V.; Khera, R.; Cheeran, D.; Mody, P.; Reddy, P.P.; Link, M.S. Diagnostic and prognostic utility of cardiac troponin in post-cardiac arrest care. Resuscitation 2019, 141, 69–72. [Google Scholar] [CrossRef]
- Havmöller, R.; Chugh, S.S. Plasma biomarkers for prediction of sudden cardiac death: Another piece of the risk stratification puzzle? Circ. Arrhythm. Electrophysiol. 2012, 5, 237–243. [Google Scholar] [CrossRef]
- Lin, L.; Gao, R.; Chen, L.; Wu, Z.; Wei, X.; Xie, Y. Relationship between serum lactate dehydrogenase and mortality after cardiac arrest: A retrospective cohort study. Medicine 2022, 101, e31499. [Google Scholar] [CrossRef]
- Watanabe, T.; Sugawara, H.M.; Fukuchi, T.M.; Omoto, K.M. Correlation between the 72-hour fatality ratios and out-of-hospital cardiac arrest ratios in patients with extremely high outlier values of 57 laboratory test items: A single-center retrospective inception cohort study. Medicine 2022, 101, e31300. [Google Scholar] [CrossRef]
- He, H.; Chai, X.; Zhou, Y.; Pan, X.; Yang, G. Association of Lactate Dehydrogenase with In-Hospital Mortality in Patients with Acute Aortic Dissection: A Retrospective Observational Study. Int. J. Hypertens. 2020, 2020, 1347165. [Google Scholar] [CrossRef]
- Zan, X.; Deng, H.; Zhang, Y.; Wang, P.; Chong, W.; Hai, Y.; You, C.; Fang, F. Lactate dehydrogenase predicting mortality in patients with aneurysmal subarachnoid hemorrhage. Ann. Clin. Transl. Neurol. 2022, 9, 1565–1573. [Google Scholar] [CrossRef]
- Jin, H.; Bi, R.; Hu, J.; Xu, D.; Su, Y.; Huang, M.; Peng, Q.; Li, Z.; Chen, S.; Hu, B. Elevated Serum Lactate Dehydrogenase Predicts Unfavorable Outcomes After rt-PA Thrombolysis in Ischemic Stroke Patients. Front. Neurol. 2022, 13, 816216. [Google Scholar] [CrossRef]
- Forkasiewicz, A.; Dorociak, M.; Stach, K.; Szelachowski, P.; Tabola, R.; Augoff, K. The usefulness of lactate dehydrogenase measurements in current oncological practice. Cell. Mol. Biol. Lett. 2020, 25, 35. [Google Scholar] [CrossRef]
- Ceci, F.M.; Fiore, M.; Gavaruzzi, F.; Angeloni, A.; Lucarelli, M.; Scagnolari, C.; Bonci, E.; Gabanella, F.; Di Certo, M.G.; Barbato, C.; et al. Early routine biomarkers of SARS-CoV-2 morbidity and mortality: Outcomes from an emergency section. Diagnostics 2022, 12, 176. [Google Scholar] [CrossRef]
- You, Y.H.; In, Y.N.; Park, J.S.; Yoo, I.; Kim, S.W.; Lee, J.; Ryu, S.; Min, J.H.; Jeong, W.J.; Cho, Y.C.; et al. Relationships between serum levels of lactate dehydrogenase and neurological outcomes of patients who underwent targeted temperature management after out-of-hospital cardiac arrest. Medicine 2021, 100, e26260. [Google Scholar] [CrossRef]
- Farhana, A.; Lappin, S.L. Biochemistry, lactate dehydrogenase. In StatPearls [Internet]; StatPearls Publishing: Tampa, FL, USA, 2022. [Google Scholar]
- Marti, H.H.; Jung, H.H.; Pfeilschifter, J.; Bauer, C. Hypoxia and cobalt stimulate lactate dehydrogenase (LDH) activity in vascular smooth muscle cells. Pflug. Arch. 1994, 429, 216–222. [Google Scholar] [CrossRef]
- Park, J.S.; You, Y.; Ahn, H.J.; Min, J.H.; Jeong, W.; Yoo, I.; Cho, Y.; Ryu, S.; Lee, J.; Kim, S.; et al. Cerebrospinal fluid lactate dehydrogenase as a potential predictor of neurologic outcomes in cardiac arrest survivors who underwent target temperature management. J. Crit. Care 2020, 57, 49–54. [Google Scholar] [CrossRef]
- Hwang, S.O.; Cha, K.-C.; Jung, W.J.; Roh, Y.-I.; Kim, T.Y.; Chung, S.P.; Kim, Y.-M.; Park, J.D.; Kim, H.-S.; Lee, M.J.; et al. 2020 Korean Guidelines for Cardiopulmonary Resuscitation. Part 1. Update process and highlights. Clin. Exp. Emerg. Med. 2021, 8, S1–S7. [Google Scholar] [CrossRef]
- Annborn, M.; Dankiewicz, J.; Erlinge, D.; Hertel, S.; Rundgren, M.; Smith, J.G.; Struck, J.; Friberg, H. Procalcitonin after cardiac arrest–an indicator of severity of illness, ischemia-reperfusion injury and outcome. Resuscitation 2013, 84, 782–787. [Google Scholar] [CrossRef]
- Frydland, M.; Kjaergaard, J.; Erlinge, D.; Stammet, P.; Nielsen, N.; Wanscher, M.; Pellis, T.; Friberg, H.; Hovdenes, J.; Horn, J.; et al. Usefulness of Serum B-Type Natriuretic Peptide Levels in Comatose Patients Resuscitated from Out-of-Hospital Cardiac Arrest to Predict Outcome. Am. J. Cardiol. 2016, 118, 998–1005. [Google Scholar] [CrossRef]
- Naik, R.; Mandal, I.; Gorog, D.A. Scoring Systems to Predict Survival or Neurological Recovery after Out-of-hospital Cardiac Arrest. Eur. Cardiol. Rev. 2022, 17, e20. [Google Scholar] [CrossRef]
- Kupari, P.; Skrifvars, M.; Kuisma, M. External validation of the ROSC after cardiac arrest (RACA) score in a physician staffed emergency medical service system. Scand. J. Trauma, Resusc. Emerg. Med. 2017, 25, 34. [Google Scholar] [CrossRef]
- Maupain, C.; Bougouin, W.; Lamhaut, L.; Deye, N.; Diehl, J.-L.; Geri, G.; Perier, M.-C.; Beganton, F.; Marijon, E.; Jouven, X.; et al. The CAHP (Cardiac Arrest Hospital Prognosis) score: A tool for risk stratification after out-of-hospital cardiac arrest. Eur. Heart J. 2015, 37, 3222–3228. [Google Scholar] [CrossRef]
| Primary Outcome | Secondary Outcome | Tertiary Outcome | |||||||
|---|---|---|---|---|---|---|---|---|---|
| No-ROSC (n = 431) | ROSC (n = 328) | p-Value | Death (n = 230) | Survival to Discharge (n = 64) | p-Value | Poor (n = 37) | Favorable (n = 27) | p-Value | |
| Age (years) a | 70.49 ± 15.55 | 66.88 ± 15.73 | 0.001 | 67.96 ± 15.30 | 58.83 ± 15.62 | 0.000 | 63.46 ± 16.56 | 52.48 ± 11.80 | 0.003 |
| Male sex, n (%) b | 265 (61.5%) | 191 (58.2%) | 0.406 | 132 (57.4%) | 42 (65.6%) | 0.298 | 25 (67.6%) | 17 (63.0%) | 0.907 |
| Hypertension, n (%) b | 197 (45.7%) | 140 (42.7%) | 0.449 | 99 (43.0%) | 26 (40.6%) | 0.839 | 18 (48.6%) | 8 (29.6%) | 0.203 |
| Diabetes mellitus, n (%) b | 120 (27.8%) | 102 (31.1%) | 0.370 | 68 (29.6%) | 21 (32.8%) | 0.729 | 14 (37.8%) | 7 (25.9%) | 0.464 |
| Bystander CPR, n (%) b | 227 (52.7%) | 191 (58.2%) | 0.146 | 129 (56.1%) | 39 (60.9%) | 0.582 | 21 (56.8%) | 18 (66.7%) | 0.587 |
| Witnessed, n (%) b | 206 (47.8%) | 197 (60.1%) | 0.001 | 134 (58.3%) | 41 (64.1%) | 0.489 | 22 (59.5%) | 19 (70.4%) | 0.526 |
| Initial shockable rhythm, n (%) b | 27 (6.3%) | 39 (11.9%) | 0.009 | 22 (9.6%) | 16 (25.0%) | 0.002 | 3 (8.1%) | 13 (48.1%) | 0.001 |
| Out-of-hospital CPR time a | 27 (0–174) | 21 (1–75) | <0.001 | 22 (1–75) | 13.5 (1–68) | 0.010 | 20 (1–52) | 4 (1–68) | 0.001 |
| In-hospital CPR time a | 24 (0–179) | 9(0–102) | <0.001 | 10.5 (1–102) | 7 (0–35) | 0.002 | 7 (2–35) | 7 (0–33) | 0.728 |
| Total CPR time (min) a | 52 (3–194) | 31 (2–134) | <0.001 | 32 (2–134) | 25.5 (2–72) | 0.001 | 29 (3–67) | 13 (2–72) | 0.016 |
| Total epinephrine dose (mg) a | 8 (0–60) | 3 (0–34) | <0.001 | 4 (1–34) | 3 (0–12) | 0.005 | 3 (1–12) | 3 (0–11) | 0.660 |
| LDH (U/L), reference value (<290 U/L) a | 623 (112–4500) | 448 (117–4500) | <0.001 | 486 (112–4500) | 376 (171–1620) | <0.001 | 388 (211–829) | 354 (171–1620) | 0.362 |
| Primary Outcome (ROSC) | Secondary Outcome | Tertiary Outcome | ||||
|---|---|---|---|---|---|---|
| Univariable (OR, 95% CI) | Multivariable (OR, 95% CI) | Univariable (OR, 95% CI) | Multivariable (OR, 95% CI) | Univariable (OR, 95% CI) | Multivariable (OR, 95% CI) | |
| Age (years) | 0.985 (0.976–0.995) | 0.971 (0.959–0.982) | 0.965 (0.948–0.982) | 0.968 (0.948–0.988) | 0.950 (0.915–0.987) | 0.961 (0.918–1.005) |
| Male sex | 1.145 (0.854–1.535) | - | 1.417 (0.795–2.527) | - | 1.225 (0.433–3.471) | - |
| Hypertension | 0.885 (0.662–1.181) | - | 0.905 (0.516–1.590) | - | 0.444 (0.156–1.267) | - |
| Diabetes mellitus | 1.170 (0.854–1.602) | - | 1.163 (0.643–2.107) | - | 0.575 (0.194–1.706) | - |
| Bystander CPR | 1.253 (0.938–1.674) | - | 1.221 (0.694–2.150) | - | 1.524 (0.543–4.273) | - |
| Witnessed | 1.643 (1.228–2.197) | 1.496 (1.045–2.141) | 1.277 (0.719–2.267) | - | 1.619 (0.564–4.651) | - |
| Initial shockable rhythm | 2.019 (1.208–3.374) | 1.501 (0.771–2.925) | 3.152 (1.540–6.451) | 2.749 (1.173–6.443) | 10.524 (2.592–42.727) | 9.826 (1.807–53.438) |
| Out-of-hospital CPR time | 0.965 (0.955–0.975) | 1.076 (0.874–1.325) | 0.975 (0.956–0.996) | 1.301 (0.509–3.327) | 0.944 (0.905–0.986) | 0.934 (0.854–1.023) |
| In-hospital CPR time | 0.906 (0.890–0.921) | 0.789 (0.596–1.045) | 0.955 (0.922–0.989) | 1.156 (0.431–3.098) | 1.014 (0.955–1.076) | - |
| Total CPR time | 0.947 (0.938–0.956) | 0.901 (0.732–1.109) | 0.972 (0.955–0.988) | 0.753 (0.295–1.922) | 0.963 (0.930–0.997) | 0.997 (0.928–1.072) |
| Total epinephrine dose (mg) | 0.748 (0.711–0.788) | 2.049 (1.195–3.515) | 0.875 (0.789–0.971) | 1.350 (0.536–3.398) | 1.021 (0.853–1.223) | - |
| LDH (U/L), cut-off | 2.760 (2.021–3.770) a | 2.418 (1.665–3.513) a | 5.197 (2.369–11.400) b | 4.961(2.184–11.269) b | 2.678 (0.826–8.686) c | 3.192 (0.691–14.743) c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Kim, Y.W.; Kim, T.-Y. Diagnostic Value of Serum Lactate Dehydrogenase Level Measured in the Emergency Department in Predicting Clinical Outcome in Out-of-Hospital Cardiac Arrest: A Multicenter, Observational Study. J. Clin. Med. 2023, 12, 3006. https://doi.org/10.3390/jcm12083006
Kim J, Kim YW, Kim T-Y. Diagnostic Value of Serum Lactate Dehydrogenase Level Measured in the Emergency Department in Predicting Clinical Outcome in Out-of-Hospital Cardiac Arrest: A Multicenter, Observational Study. Journal of Clinical Medicine. 2023; 12(8):3006. https://doi.org/10.3390/jcm12083006
Chicago/Turabian StyleKim, Jihyun, Yong Won Kim, and Tae-Youn Kim. 2023. "Diagnostic Value of Serum Lactate Dehydrogenase Level Measured in the Emergency Department in Predicting Clinical Outcome in Out-of-Hospital Cardiac Arrest: A Multicenter, Observational Study" Journal of Clinical Medicine 12, no. 8: 3006. https://doi.org/10.3390/jcm12083006
APA StyleKim, J., Kim, Y. W., & Kim, T.-Y. (2023). Diagnostic Value of Serum Lactate Dehydrogenase Level Measured in the Emergency Department in Predicting Clinical Outcome in Out-of-Hospital Cardiac Arrest: A Multicenter, Observational Study. Journal of Clinical Medicine, 12(8), 3006. https://doi.org/10.3390/jcm12083006






