Abstract
ECMO has become a therapeutic modality for in- and out-of-hospital scenarios and is also suitable as a bridging therapy until further decisions and interventions can be made. Case report: A 27-year-old male patient with mechanical aortic valve prothesis had a sudden cardiac arrest (SCA). ROSC had been achieved after more than 60 min of CPR and eight DC shocks due to ventricular fibrillation (VF). The National Ambulance Service unit transported the patient to our clinic for further treatment. Due to the trauma and therapeutic INR, a CT scan was performed and ruled out bleeding. Echocardiography described severely decreased left ventricular function. Coronary angiography was negative. Due to the therapeutic refractory circulatory and respiratory failure against intensive care, VA-ECMO implantation was indicated. After four days of ECMO treatment, the patient’s circulation was stabilized without neurological deficit, and the functions of the end organs were normalized. Cardiac MRI showed no exact etiology behind SCA. ICD was implanted due to VF and SCA. The patient was discharged after 19 days of hospitalization. Conclusion: This case report points out that the early application of mechanical circulatory support could be an outcome-determinant therapeutic modality. Post-resuscitation care includes cardiorespiratory stabilization, treatment of reversible causes of malignant arrhythmia, and secondary prevention.
1. Introduction
The availability and application numbers of extracorporeal life support (ECLS) and extracorporeal membrane oxygenation (ECMO) are continuously increasing in cardiac surgery centers [,]. Even though ECLS and ECMO are complex therapies that require high-level specialized centers, the first multidisciplinary guideline for the organization and application of ECMO therapy for cardiocirculatory support was published in 2021 []. The most common indications for ECLS or ECMO are therapy-refractory cardiogenic shock, acute pulmonary embolism, respiratory failure, and hypothermia. ECLS and ECMO have become therapeutic modalities for in- and out-of-hospital scenarios and are also suitable as bridging therapies in emergency situations until further decisions and interventions can be made [,].
Based on the international literature, the utilization of ECLS has increased in both in-hospital and out-of-hospital resuscitation in recent years [,,,]. The Extracorporeal Life Support Organization (ELSO) database reports that the incidence of extracorporeal cardiopulmonary resuscitation (eCPR) increased from 35 cases per year to 400 cases per year between 2003 and 2014 []. The current recommendation suggests that eCPR initiation should be considered as a last resort in selected patient populations [,].
Based on the length of time that mechanical circulatory support is used, a difference can be made between devices suitable for short-term and long-term support. Short-term devices are used in high-risk percutaneous coronary intervention (CHIP), cardiogenic shock, and post-cardiac arrest, while durable left ventricular assist devices (LVADs) are used in bridge-to-transplant and bridge-to-decision clinical situations, or as a bridge-to-destination therapy. The use of INTERMACS profiles guides the decision of the optimal timing of MCS implantation and the associated risk based on clinical presentation [].
In addition to ECMO, other new devices for MCS with powerful continuous flow (e.g., Impella®) are becoming increasingly available and are in common use in cardiac surgeries and cardiovascular centers. We focus on ECMO in the following [].
There are two types of peripheral ECMO based on the type of cannulated vessels: venoarterial (VA) and venovenous (VV). Depending on the allocation of the vessels that have been cannulated, VV-ECMO can be further subdivided into femoro-jugular and femoro-femoral VV-ECMO. Both types of peripheral ECMO can provide respiratory support, but the VA-ECMO can assist with acute cardiorespiratory support in cardiogenic shock or cardiac arrest patients as well [].
Peripheral arterial cannulation is frequently used in non-cardiac surgical scenarios when immediate ECMO support is necessary. The common femoral artery is the most common site for peripheral arterial cannulation in VA-ECMO, but another option can be the axillary artery. Conversely, the aorta serves as the site for the inflow cannula in the case of central VA-ECMO. Implantation of central VA-ECMO is a more invasive procedure that involves opening the chest, although there have been reports of central cannulation using minimally invasive techniques. Surgeons often express concerns regarding the potential risks of bleeding and infection. However, a notable advantage of central VA-ECMO is the ability to achieve sufficient perfusion flow, enabling the use of larger diameter arterial cannulas and the offloading of the left ventricle [,].
Peripheral cannulation for VA ECMO is commonly performed by accessing the common femoral artery and vein below the inguinal ligament but above their bifurcations. The size of the arterial cannula typically ranges from 17 to 19 French, which is usually sufficient for providing the required flow based on the patient’s needs. In certain clinical scenarios, such as sepsis, larger cannulas of 19 or 21 French may be necessary to achieve higher flow rates. However, it is important to note that the larger the arterial cannula is, the higher the risk of vascular complication, including limb ischemia. To avoid lower limb ischemia in the case of femoral arterial cannulation, one should add a distally directed shunt, and the perfusion of the distal lower limb should be monitored continuously []. While the recommendation of placing the arterial and venous cannulas in separate limbs is based on expert opinion, it is considered preferable in order to minimize vascular complications and facilitate decannulation. Whenever possible, the venous cannula should be placed in the right femoral vein, as it offers the most direct venous path to the inferior vena cava and right atrium [].
In the context of postcardiotomy scenarios, central cannulation is frequently applied, especially when severe peripheral vascular disease is present at the femoral/iliac levels. Both central and peripheral cannulation approaches have advantages and disadvantages (detailed in Table 1); however, accumulating evidence demonstrates a stronger association between peripheral cannulation and superior overall outcomes [,,].
An important aspect of ECMO treatment is the use of anticoagulant medication. Currently, there are limited high level evidence-based recommendations for the choice of anticoagulant agent (heparin, bivalirudin, argatroban, or nafamostat mesilate) during ECMO treatment. In a recent meta-analysis, bivalirudin was shown to be superior to heparin in reducing mortality and the risk of thrombosis. In the future, anti-adsorbent and anti-coagulant coatings of the MCS and ECLS circuits and bio-hybrid materials could be promising to prevent the initiation of the thrombogenic and inflammatory response [,,,].


Table 1.
Advantages and disadvantages of peripheral and central canulation based on ELSO guideline [].
Table 1.
Advantages and disadvantages of peripheral and central canulation based on ELSO guideline [].
| Advantages | Disadvantages | |
|---|---|---|
| Peripheral cannulation | No surgical incision | Lower limb ischemia |
| Lower bleeding risk | Retrograde flow | |
| No sternotomy | Short-term support | |
| No cardiac compression | Lower flow | |
| Easier switch to LVAD | Higher risk of vascular complication | |
| No re-sternotomy for removal | Reduced options for LV venting | |
| Central cannulation | Use of originally implanted cannulas | Sternotomy to implant |
| Antegrade flow | Higher bleeding risk | |
| Better drainage | Cardiac compression | |
| Long-term support | Re-sternotomy to remove | |
| Higher flow | Higher infection risk | |
| Easier patient mobilization | Higher rate of cerebral embolization | |
| More options for LV venting | Higher risk of closed aortic valve |
The weaning off of VA-ECMO should be contemplated once patients have substantial cardiac improvement, particularly when there is minimal reliance on vasoactive and inotropic drugs to maintain appropriate pulse pressure, mean arterial pressure, and mixed venous/central saturation at a flow rate of 2–2.5 L/min on VA-ECMO. For those patients who fail the weaning process within 5–7 days, temporary LVAD support may be considered to give more time for left ventricular recovery [].
2. Case Presentation
We present a case of a successfully resuscitated 27-year-old male patient due to out-of-hospital cardiac arrest. The patient’s progressive cardiogenic shock and oxygenation failure did not respond to conservative ICU therapy, and he was ultimately stabilized with peripheral VA-ECMO.
The patient suffered sudden cardiac arrest in a public area during his regular 3-hour-long bicycle tour. Approximately after 2 min of no flow anoxic time, a healthcare worker started basic life support (BLS). After 19 min of BLS, the Hungarian National Ambulance Service staff overtook the resuscitation using an automated chest compression device (LUCAS) for a further 39 min of advanced life support (ALS). Overall, after 58 min of cardiopulmonary resuscitation and application of eight direct current shocks, intubation, and mechanical ventilation, spontaneous circulation returned. The Hungarian National Ambulance Service unit transported the patient with continuous intravenous circulatory support to the Heart and Vascular Centre, Semmelweis University, for further treatment (see details in Table 2.).

Table 2.
Timeline from sudden cardiac arrest to VA-ECMO implantation.
3. Patient Information
In the patient’s medical history, there was a familiar polycystic kidney disease with left-sided dominance and surgical mechanical aortic valve replacement (Sorin®, 25 mm, date of implantation: 2017) surgery due to a congenital bicuspid aortic valve. He has been regularly taking acenocoumarin medication only.
4. Acute Diagnostic Assessment
As the first step, a cine and coronary angiography were performed: symmetrically opening mechanical aortic valve leaflets without any disabled movement and intact coronary artery anatomy (Figure 1). We ruled out pneumothorax, pericardial, pleural, and abdominal fluid with emergency bedside ultrasound after ICU admission. Echocardiography showed severely reduced left and right ventricular function with diffuse hypokinesis, and mild paravalvular regurgitation of the mechanical aortic valve, and ruled out left ventricular outflow tract obstruction and pericardial fluid. Due to the mechanism of his bicycle fall, prolonged CPR using LUCAS, and therapeutic INR level, a cranial, thoracic and abdominal computed tomography (CT) scan and as well as pulmonary CT angiography (Figure 2) were performed to exclude bleeding and pulmonary contusion and rule out acute pulmonary embolism and aortic dissection. The cranial CT ruled out severe cerebral ischemic damage, postischemic edema, and cerebral herniation.
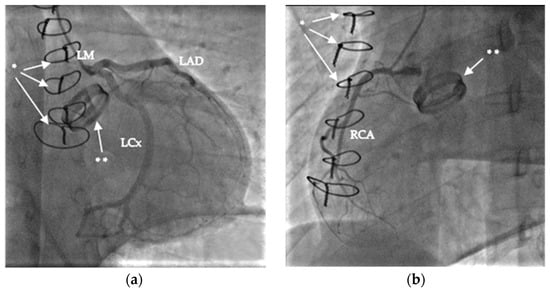
Figure 1.
Images of coronary angiography: (a) left coronary arteries (left main (LM), left anterior descendent (LAD), and left circumflex (LCx); (b) right coronary artery [RCA]. The sternal sutures are marked with *, and the mechanical aortic valve with **.
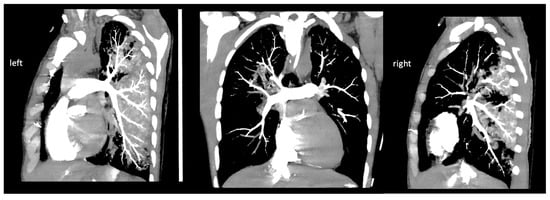
Figure 2.
Images (left and right pulmonary artery) of pulmonary CT angiography.
5. Indication of VA-ECMO during Post Resuscitation ICU Care
Due to the rapidly increasing combined catecholamine demand, gas exchange failure with invasive mechanical ventilatory support, and therapy-refractory cardiogenic shock in the first few hours of the post-resuscitation care, the multidisciplinary team decided to immediately implant a peripheral VA-ECMO. Sheaths were placed in the femoral artery and vein in the cardiac intensive care unit to save time, and the peripheral VA-ECMO cannulas were inserted by a cardiac surgeon in the operating room, and mechanical circulatory support was initiated.
Indication was set for short-mechanical circulation support (MCS) as a bridge to a diagnosis and decision due to progressively worsening refractory cardiogenic shock against inotropic and vasopressor support to buy time for ruling out or treating all the potentially reversible or surgically correctable causes [,]. Of the commercially available percutaneous temporary MCS, only an intra-aortic balloon pump (IABP) and a VA-ECMO was available in our institute. Even with up-titrated inotropic and vasopressor support, IABP alone is not sufficient for physiologic circulatory maintenance for patients with severe post cardiac arrest biventricular failure due to malignant arrhythmia. VA-ECMO should be considered preemptively in postcardiac arrest shock, particularly to prevent severe multiorgan failure as the consequence of hypoperfusion.
The patient’s young age and practically lack of comorbidities forecast a good prognosis if short term MCS was decided for starting bridging for short decision making or for upgrading to an LVAD or heart transplantation [].
Aggravating prognostic factors were excluded, such as poor life expectancy, severe liver impairment, acute brain injury, vascular disease, and immuno-deficiency before ECMO application. We used the SAVE score as a decision-support system; the patient scored 7 points (Class I), which predicted 75% in-hospital survival with ECMO (detailed in Supplementary Material) [].
6. ICU Treatment
During four days of VA-ECMO treatment and five days of invasive mechanical ventilation, combined catecholamine support was successfully withdrawn (Figure 3). Initially, significant elevations in creatine kinase and cardiac enzyme levels were observed, which showed rapidly decreasing kinetics. The high serum lactate levels normalized after ECMO treatment started within hours. Progressive anemia was observed after the start of ECMO, but no transfusion was required, and no source of hemorrhage was confirmed. It was considered to be a side effect of appropriate volume loading for ECMO. The circulation and the target organ functions of the patients were normalized with ECMO support (see details in Figure 4). Intact neurological function was observed during the daily awakenings and after extubation.
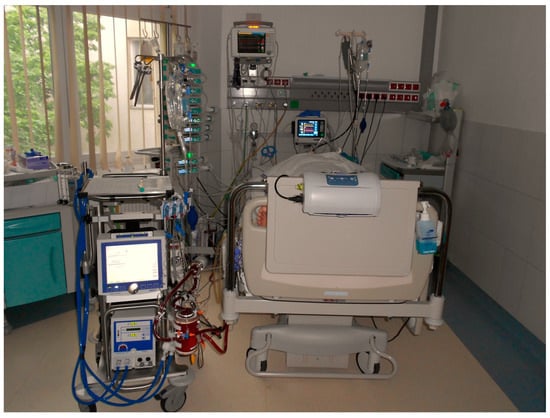
Figure 3.
Set up of VA-ECMO treatment in the cardiac intensive care unit.
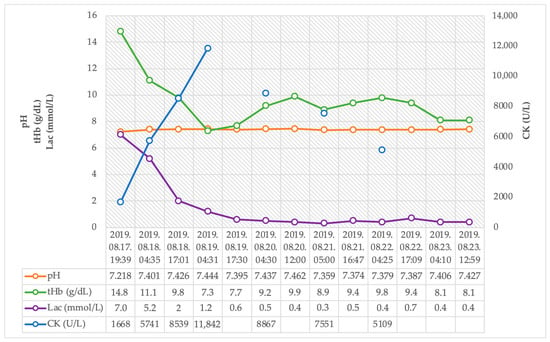
Figure 4.
Trends of pH, total hemoglobin, serum lactate, and creatine-kinase after hospital admission.
Compared to the echocardiogram performed at admission (LVEF: 15%, TAPSE: 12 mm), controls showed improved left and right ventricular function (LVEF: 53%, TAPSE: 22 mm) and proper valvular functions (see details in Figure 5).
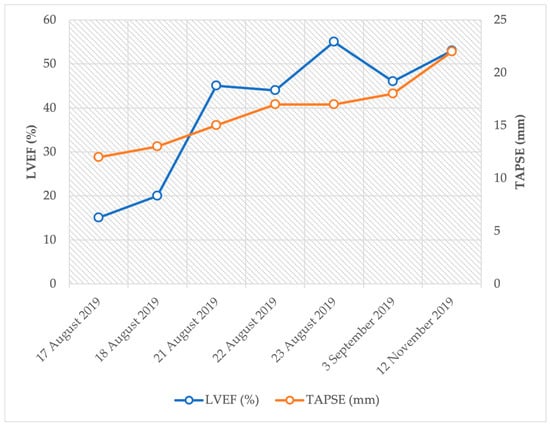
Figure 5.
Trends of left and right ventricular function after sudden cardiac arrest.
After the patient was stabilized, cardiac magnetic resonance imaging (MRI) was performed to clarify the etiology of the cardiac arrest. The cardiac MRI showed mildly reduced left ventricular function, concentric left ventricular hypertrophy, and non-specific patterns of late enhancement in contrast-enhanced cardiac MRI.
An implantable cardioverter defibrillator (ICD) device was implanted due to idiopathic ventricular fibrillation after all reversible causes were excluded.
7. Discharge and Follow up
After successful rehabilitation, the patient was discharged to his home after 19 days of hospital treatment. During the ambulatory follow-up visits, no ventricular tachycardia or fibrillation episodes were observed in the device memory (Table 3).

Table 3.
Timeline of post-resuscitation care, rehabilitation, and follow-up.
8. Conclusions
This case report points out that in the case of a progressively increasing demand for circulatory and respiratory support and therapy-refractory cardiogenic shock, the early application of mechanical circulatory support could be an outcome-determinant therapeutic modality.
The urgently implanted peripheral VA-ECMO was an appropriate clinical, multidisciplinary decision for hemodynamic stabilization, providing extra time to rule out the reversible causes of sudden cardiac arrest and ultimately serving as a bridge to recovery for the patient. Peripheral VA-ECMO does not require re-thoracotomy and has a lower risk of bleeding compared to the centrally inserted ECMO. In clinical situations where the physician is faced with rapidly deteriorating cardiogenic shock despite inotropes and vasopressors, inserting femoral arterial and venous cannulas onsite should be considered in the cardiac ICU or catheterization laboratory to save time.
Post-resuscitation care includes cardiorespiratory care, including the use of ECLS or ECMO if necessary, treatment of reversible causes of malignant arrhythmia, and secondary prevention.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm12134249/s1.
Author Contributions
Conceptualization, B.K., B.N., Á.P.-J., B.L., Á.S., I.O., K.H., E.N., I.F.É. and E.Z.; methodology, B.K. and E.Z.; investigation, B.K. and E.Z.; resources, B.M. and E.Z.; data curation, B.K.; writing—original draft preparation, B.K. and E.Z.; writing—review and editing, B.N., Á.P.-J., B.L., Á.S., I.O., K.H., I.F.É. and E.N.; visualization, B.K.; supervision, B.M. and E.Z.; project administration, B.K.; funding acquisition, B.M. All authors certify that they have participated sufficiently in the work to take public responsibility for the content, including participation in the concept, design, analysis, writing, or revision of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
No ethical approval was required for this case report.
Informed Consent Statement
The authors confirm that consent for submission and publication of this case report has been obtained from the patient in line with COPE guidance. Written informed consent was obtained from the patient for publication of this case report accompanying images. A copy of written consent is available for review by the Editor-in-Chief of this journal upon request.
Data Availability Statement
The data underlying this article will be shared upon reasonable request to the corresponding author.
Conflicts of Interest
The authors declare that they have no competing interests.
References
- Assmann, A.; Boeken, U.; Klotz, S.; Harringer, W.; Beckmann, A. Organization and Application of ECLS Therapy-A Nationwide Survey in German Cardiosurgical Departments. Thorac. Cardiovasc. Surg. 2019, 67, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Édes, I.F.; Németh, B.T.; Hartyánszky, I.; Szilveszter, B.; Kulyassa, P.; Fazekas, L.; Pólos, M.; Németh, E.; Becker, D.; Merkely, B. Predictors of mortality following extracorporeal membrane oxygenation support in an unselected, critically ill patient population. Adv. Interv. Cardiol. W Kardiol. Interwencyjnej 2021, 17, 290–297. [Google Scholar] [CrossRef]
- Boeken, U.; Assmann, A.; Beckmann, A.; Schmid, C.; Werdan, K.; Michels, G.; Miera, O.; Schmidt, F.; Klotz, S.; Starck, C.; et al. S3 Guideline of Extracorporeal Circulation (ECLS/ECMO) for Cardiocirculatory Failure. Thorac. Cardiovasc. Surg. 2021, 69, S121–S212. [Google Scholar] [CrossRef]
- Kovács, E.; Németh, E.; Prigya, J.; Szvath, P.; Édes, I.; Hartyánszky, I.; Soltész, Á.; Csikós, G.R.; Fazekas, L.; Gál, J.; et al. The role of extracorporeal life support in cardiopulmonary resuscitation. Orvosi. Hetilap. 2023, 164, 510–514. [Google Scholar] [CrossRef] [PubMed]
- Dennis, M.; Lal, S.; Forrest, P.; Nichol, A.; Lamhaut, L.; Totaro, R.J.; Burns, B.; Sandroni, C. In-depth extracorporeal cardiopulmonary resuscitation in adult out-of-hospital cardiac arrest. J. Am. Heart Assoc. 2020, 9, e016521. [Google Scholar] [CrossRef]
- Swol, J.; Belohlávek, J.; Brodie, D.; Bellezzo, J.; Weingart, S.D.; Shinar, Z.; Schober, A.; Bachetta, M.; Haft, J.W.; Ichiba, S.; et al. Extracorporeal life support in the emergency department: A narrative review for the emergency physician. Resuscitation 2018, 133, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Richardson, A.S.C.; Schmidt, M.; Bailey, M.; Pellegrino, V.A.; Rycus, P.T.; Pilcher, D.V. ECMO cardiopulmonary resuscitation (ECPR), trends in survival from an international multicentre cohort study over 12-years. Resuscitation 2017, 112, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Hutin, A.; Abu-Habsa, M.; Burns, B.; Bernard, S.; Bellezzo, J.; Shinar, Z.; Torres, E.C.; Gueugniaud, P.-Y.; Carli, P.; Lamhaut, L. Early ECPR for out-of-hospital cardiac arrest: Best practice in 2018. Resuscitation 2018, 130, 44–48. [Google Scholar] [CrossRef]
- Lott, C.; Truhlář, A.; Alfonzo, A.; Barelli, A.; González-Salvado, V.; Hinkelbein, J.; Nolan, J.P.; Paal, P.; Perkins, G.D.; Thies, K.C.; et al. European Resuscitation Council guidelines 2021: Cardiac arrest in special circumstances. Resuscitation 2021, 161, 152–219. [Google Scholar] [CrossRef]
- Vieira, J.L.; Ventura, H.O.; Mehra, M.R. Mechanical circulatory support devices in advanced heart failure: 2020 and beyond. Prog. Cardiovasc. Dis. 2020, 63, 630–639. [Google Scholar] [CrossRef]
- Dudzinsk, J.E.; Gnall, E.; Kowey, P.R. A Review of percutaneous mechanical support devices and strategies. Rev. Cardiovasc. Med. 2018, 19, 21–26. [Google Scholar] [CrossRef]
- Rao, P.; Khalpey, Z.; Smith, R.; Burkhoff, D.; Kociol, R.D.; Mehmood, M.; Alhussein, M.; Moayedi, Y.; Posada, J.D.; Ross, H.; et al. Venoarterial Extracorporeal Membrane Oxygenation for Cardiogenic Shock and Cardiac Arrest. Circ. Heart Fail. 2018, 11, e004905. [Google Scholar] [CrossRef]
- Brogan, T. (Ed.) ELSO Specialist Manual, 4th ed.; Extracorporeal Life Support Organization (ELSO): Ann Arbor, MI, USA, 2018. [Google Scholar]
- Keenan, J.E.; Schechter, M.A.; Bonadonna, D.K.; Bartz, R.R.; Milano, C.A.; Schroder, J.N.; Daneshmand, M.A. Early experience with a novel cannulation strategy for left ventricular decompression during nonpostcardiotomy venoarterial ECMO. ASAIO J 2016, 62, e30–e34. [Google Scholar] [CrossRef] [PubMed]
- Rupprecht, L.; Lunz, D.; Philipp, A.; Lubnow, M.; Schmid, C. Pitfalls in percutaneous ECMO cannulation. Heart Lung Vessel. 2015, 7, 320–326. [Google Scholar] [PubMed]
- Brogan, T.; Lequier, L.; Lorusso, R.; MacLaren, G.; Peek, G. The ELSO Red Book, 5th ed.; ELSO, Ed.; ELSO: Ann Arbor, MI, USA, 2017. [Google Scholar]
- Raffa, G.M.; Kowalewski, M.; Brodie, D.; Ogino, M.; Whitman, G.; Meani, P.; Pilato, M.; Arcadipane, A.; Delnoij, T.; Natour, E.; et al. Meta-analysis of peripheral or central extracorporeal membrane oxygenation in postcardiotomy and non-postcardiotomy shock. Ann. Thorac. Surg. 2019, 107, 311–321. [Google Scholar] [CrossRef]
- Mariscalco, G.; Salsano, A.; Fiore, A.; Dalen, M.; Ruggieri, V.G.; Saeed, D.; Jonsson, K.; Gatti, G.; Zipfel, S.; Dell’Aquila, A.M.; et al. PC-ECMO group: Peripheral versus central extracorporeal membrane oxygenation for postcardiotomy shock: Multicenter registry, systematic review, and meta-analysis. J. Thorac. Cardiovasc. Surg. 2020, 160, 1207–1216. [Google Scholar] [CrossRef] [PubMed]
- Willers, A.; Arens, J.; Mariani, S.; Pels, H.; Maessen, J.G.; Hackeng, T.M.; Lorusso, R.; Swol, J. New Trends, Advantages and Disadvantages in Anticoagulation and Coating Methods Used in Extracorporeal Life Support Devices. Membranes 2021, 11, 617. [Google Scholar] [CrossRef] [PubMed]
- Sanfilippo, F.; Currò, J.M.; La Via, L.; Dezio, V.; Martucci, G.; Brancati, S.; Murabito, P.; Pappalardo, F.; Astuto, M. Use of nafamostat mesilate for anticoagulation during extracorporeal membrane oxygenation: A systematic review. Artif. Organs 2022, 46, 2371–2381. [Google Scholar] [CrossRef] [PubMed]
- Sanfilippo, F.; La Via, L.; Murabito, P.; Pappalardo, F.; Astuto, M. More evidence available for the use of Bivalirudin in patients supported by extracorporeal membrane oxygenation. Thromb. Res. 2022, 211, 148–149. [Google Scholar] [CrossRef]
- Li, D.H.; Sun, M.W.; Zhang, J.C.; Zhang, C.; Deng, L.; Jiang, H. Is bivalirudin an alternative anticoagulant for extracorporeal membrane oxygenation (ECMO) patients? A systematic review and meta-analysis. Thromb. Res. 2022, 210, 53–62. [Google Scholar] [CrossRef]
- Richardson, A.S.C.; Tonna, J.E.; Nanjayya, V.; Nixon, P.; Abrams, D.C.; Raman, L.; Bernard, S.; Finney, S.J.; Grunau, B.; Youngquist, S.T.; et al. Extracorporeal Cardiopulmonary Resuscitation in Adults. Interim Guideline Consensus Statement from the Extracorporeal Life Support Organization. ASAIO J. 2021, 67, 221–228. [Google Scholar] [CrossRef] [PubMed]
- McMurray, J.J.; Adamopoulos, S.; Anker, S.D.; Auricchio, A.; Böhm, M.; Dickstein, K.; Falk, V.; Filippatos, G.; Fonseca, C.; Gomez-Sanchez, M.A.; et al. ESC Committee for Practice Guidelines: ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2012, 33, 1787–1847. [Google Scholar]
- Lorusso, R.; Whitman, G.; Milojevic, M.; Raffa, G.; McMullan, D.M.; Boeken, U.; Haft, J.; Bermudez, C.A.; Shah, A.S.; D’Alessandro, D.A. 2020 EACTS/ELSO/STS/ AATS expert consensus on postcardiotomy extracorporeal life support. ASAIO J. 2021, 67, e1–e43. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Burrell, A.; Roberts, L.; Bailey, M.; Sheldrake, J.; Rycus, P.T.; Hodgson, C.; Scheinkestel, C.; Cooper, D.J.; Thiagarajan, R.R.; et al. Predicting survival after ECMO for refractory cardiogenic shock: The survival after venoarterial- ECMO (SAVE) score. Eur. Heart J. 2015, 36, 2246–2256. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).