Abstract
This study investigated age-related differences in trunk kinematics during walking in healthy men. Secondary aims were to investigate the covarying effects of physical activity (PA) and lumbar paravertebral muscle (LPM) morphology on trunk kinematics, and the effect of age on interplanar coupling between the trunk and pelvis. Three-dimensional (3D) trunk and pelvis motion data were obtained for 12 older (67.3 ± 6.0 years) and 12 younger (24.7 ± 3.1 years) healthy men during walking at a self-selected speed along a 10 m walkway. Phase-specific differences were observed in the coronal and transverse planes, with midstance and swing phases highlighted as instances when trunk and pelvic kinematics differed significantly (p < 0.05) between the younger group and older group. Controlling for age, fewer significant positive correlations were revealed between trunk and pelvic ranges and planes of motion. LPM morphology and PA were not significant covariates of age-related differences in trunk kinematics. Age-related differences in trunk kinematics were most apparent in the coronal and transverse planes. The results further indicate ageing causes an uncoupling of interplanar upper body movements during gait. These findings provide important information for rehabilitation programmes in older adults designed to improve trunk motion, as well as enable identification of higher-risk movement patterns related to falling.
1. Introduction
It is well-known that gait characteristics are modified with ageing, either as a compensatory mechanism to reduce injury risk [1] or as a result of deteriorated muscle activity [2]. Whilst age-induced changes in the biomechanical function of the lower limbs during walking are well understood, the quality and quantity of evidence on age-related adaptations in trunk function are relatively low. This is a likely consequence of clinical gait analyses primarily focusing on lower limb biomechanics [3]. As the proportion of older adults continues to increase globally [4], understanding trunk kinematic changes during gait has become increasingly important and is a critical step in devising effective strategies that preserve independence in older adults.
The trunk muscles, particularly the lumbar paravertebral muscles (LPMs), play a crucial role in walking gait, and actively contribute to dynamic balance during functional activities [5,6]. Dynamic margins of stability are reduced considerably during single-limb support [7]. Therefore, this phase of the gait cycle presents a greater challenge to dynamic stability, particularly in older adults for whom reduced single-limb balance increases the risk of injurious falls [8]. Due to the large mass and moment of inertia of the upper body, particularly the trunk, controlling its position relative to the base of support is critical in maintaining dynamic stability [9]. Older adults may not have the ability to recover from dynamic instability given that their trunk movement control is already being challenged by higher mechanical energy demands, and a forward-leaning position to preserve walking speed and upper body posture [10]. Control of the trunk throughout single limb support is therefore a critical phase in maintaining dynamic stability and reducing falls risk in older adults. Despite this, few studies have considered phase-specific differences in the trunk during the gait cycle as a function of age [10,11,12]. Indeed, previous research [13,14,15] has typically focused on discrete data (e.g., peak trunk flexion angle). To explore the age response in trunk kinematics, analysing phase-specific effects may be more informative than comparing peaks and troughs. Therefore, statistical inferencing methods, such as Statistical Parametric Mapping (SPM), may be more valuable in detecting age-related differences in gait kinematics as the whole time-series waveform is considered.
To separate comorbidities from the effects of ageing, establishing normal age-related changes in trunk kinematics during gait is needed. Therefore, the primary aim of this study was to investigate age-related differences in trunk kinematics during walking between healthy younger and older men. Furthermore, it is important to understand the relationship between degenerative muscle morphology and kinematic changes in the trunk with age, as skeletal muscle atrophy and fat infiltration accompany the ageing process [16,17]. Secondary aims were therefore, to investigate the covarying effects of LPM morphology and physical activity (PA) on trunk kinematics, and the effect of age on interplanar coupling between the trunk and pelvis.
2. Materials and Methods
Reporting of this prospective observational case-matched study is based on the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement [18]. Coventry University Ethics Committee approved the study (P70399) on 13 September 2018.
2.1. Participants
Following informed written consent, twenty-four community-dwelling volunteers and university staff and students were stratified into the young group (YG; n = 12) or older group (OG; n = 12). Inclusion criteria were generally healthy males (e.g., free from disease, musculoskeletal injury or functional impairment) aged between 18 and 30 years, or above 60 years. Exclusion criteria were BMI outside of 18.5–29.9 kg·m−2, smokers, consumption of alcohol on a daily basis, use of assistive walking equipment, and an existing or past medical history of metabolic diseases, neuromuscular disorders, or musculoskeletal impairments that may affect muscle function.
To ensure factors such as pain-related disability and PA levels did not confound the results, participants completed the Modified Oswestry Low Back Pain Disability Questionnaire (ODQ-m) [19] and moderate-to-vigorous physical activity (MVPA) was recorded prior to 3D motion analysis using accelerometery. ActiGraph GT9X accelerometers, sampling at 90 Hz, were worn on participants’ dominant wrist for 10 consecutive days based on the recommendations of Migueles et al., [20]. Accelerometer data were processed using ActiLife software (version 6.13). To be eligible for processing, the accelerometer must have been worn for a minimum of 4 days, including one weekend day and at least 10 h of awake time daily [20]. Valid data were divided into 1 s epochs, and a cut-off value of 1031 counts/minute was used to calculate average time spent per day in MVPA [21]. Although no studies have investigated the influence of epoch length on accelerometer outcomes, unpublished data suggest that shorter epochs (1 s vs. 60 s) are more sensitive in detecting time spent in MVPA [20].
2.2. 3D Motion Analysis Protocol
Anthropometric measurements and passive retro-reflective marker placement were performed by an experienced researcher (AD). Thirty-nine markers were attached to anatomical landmarks according to the PiG Full Body Marker Model [22] to acquire data on gait cycles and spatiotemporal parameters.
3D motion analysis was performed using a Vicon motion capture system consisting of 14 infrared cameras (Vero2.2, VMS, Oxford, UK) sampling at 100 Hz. All participants walked barefoot along a 10 m walkway at a self-selected speed. Three trials were required to complete data collection for each participant. All gait trials were processed in Vicon Nexus (Version 2.10.1, VMS, Oxford, UK) using standard operations to generate the PiG biomechanical model. Marker trajectory data were low-pass filtered using a 4th order, zero-lag Butterworth filter with a 10 Hz cut-off frequency. The cut-off frequency was based on the recommendations of Sinclair et al. [23], where 99% of the signal power was contained below. Each trial was manually truncated to include one full gait cycle (GC) (heel strike to heel strike) for each limb. Trials were exported into Vicon Polygon (Version 4.4.5, VMS, Oxford, UK) for further analysis. All outcomes were normalised to one GC (100%) using linear interpolation to 101 data samples. Each trial consisted of two GCs, and three trials were processed for each participant (i.e., six steps per participant). Ensemble averages were generated for trunk kinematics in the global reference frame (trunk-G), pelvis kinematics in the global reference frame, and also for trunk kinematics in the pelvic reference frame (trunk-P), as well as spatiotemporal gait parameters.
Mean peak minima and maxima angle data were obtained during the GC for each participant. Mean ROM was also obtained for the trunk-G, trunk-P, and pelvis throughout the GC for each participant. Kinematic data were generated for the trunk and pelvis segments relative to the global reference frame (trunk-G) in the sagittal (anterior/posterior tilt), coronal (obliquity), and transverse (axial rotation) planes. Trunk motion data in the pelvic reference frame (trunk-P), relative rotations between the pelvis and trunk segments were also generated in the sagittal (flexion/extension), coronal (lateral flexion) and transverse (axial rotation) planes. For the trunk-G and pelvis, positive angular values were defined for forward (anterior) bending in tilt, bending to the contralateral side in obliquity, and protraction in axial rotation; negative values represent the opposite movements. Protraction was defined as rotation away from the reference limb, whilst retraction was rotation toward the reference limb. For the trunk-P, positive angular values were defined for flexion in the sagittal plane, bending to the ipsilateral side in the coronal plane, and retraction in the transverse plane; negative values represent the opposite movements.
2.3. Muscle Morphology
All participants underwent T2-weighted axial MRI of the lumbar spine. Methods have been described in detail previously [24]. Briefly, normalised muscle volume (NMV) and muscle fat infiltrate (MFI) were determined bilaterally for the LPMs, from the superior endplate L3 to the superior endplate L4, consisting of the psoas, quadratus lumborum, erector spinae, and multifidus. Total LPM volume was normalised to the straight-line distance measured between the anterior superior border of the L1 vertebra and the anterior superior border of the S1 vertebra on T2-weighted mid-sagittal MRI images. MFI was calculated as the mean signal intensity (MSI) of the LPMs as a percentage of the MSI of a homogenous region of subcutaneous back fat.
2.4. Statistical Analysis
For spatiotemporal parameters and discrete data (kinematic peaks and ROM), independent sample t-tests were performed in SPSS 24.0 (IBM Corp, Armonk, NY, USA) to compare statistical differences between the OG and YG. Muscle morphology (LPM mean MFI and total NMV) and MVPA covariates were assessed using univariate ANCOVA. For each discrete variable, differences between groups were analysed and reported using the ANCOVA test if a significant covariate was found. Zero-order and partial correlations, controlling for age group, were also performed between ROMs in the trunk-G, trunk-P, and pelvis. Alpha level was set at 5% for all statistical tests, and effect sizes (Cohen’s d) calculated where appropriate. All data were normally distributed, as assessed by Shapiro–Wilks test (p > 0.05). Where the assumption of homogeneity of variances was violated, as assessed by Levene’s Test of Equality of Variances (p < 0.05), the Welch–Satterthwaite correction was used.
To identify phase-specific differences in kinematic waveforms during the GC between age groups, SPM two-tailed independent t-tests [25] were performed in MATLAB (R2019a, version 9.6.0.1072779, The MathWorks, Inc., Natick, MA, USA) and implemented using the open-source one-dimensional SPM code (spm1D-package, version 0.4.3, http://spm1d.org/index.html, accessed on 15 July 2020). The primary advantage of SPM is that abstraction of the originally sampled time series does not need to be performed to statistically analyse the data. Interpretation is therefore, more intuitive for time-series data where the statistical result is also a time-series; in this case, a time-series of t-values. If instantaneous values of the kinematic waveforms crossed the critical threshold (at which α = 5% of smooth random curves would be expected to traverse), a supra-threshold cluster depicted by grey shading indicated a significant difference (p < 0.05) between groups at a specific phase in the GC. Graphical presentation of the kinematic waveforms was performed using GraphPad Prism (Version 8.3.1, San Diego, CA, USA). Data are presented as means with standard deviations (mean ± SD) unless otherwise stated.
3. Results
Apart from age, there were no significant differences (p > 0.05) in participant characteristics and any of the spatiotemporal gait parameters between the YG and OG (Table 1).

Table 1.
Participant characteristics and spatiotemporal parameters during normal gait (mean ± SD) for the younger and older groups.
3.1. Discrete Measures
There were significant age effects in trunk-G kinematics during the GC, predominantly in the transverse plane. The YG had significantly greater trunk-G ROM than the OG in the sagittal (2.96 ± 0.88° vs. 1.99 ± 0.39°, p = 0.002) and transverse planes (6.87 ± 2.21° vs. 5.04 ± 1.29°, p = 0.024). Peak trunk-G protraction and retraction were also significantly greater (p < 0.05) in the YG compared to the OG (Table 2). All significant differences were large according to effect size estimates (Cohen’s d = 0.9–1.4).

Table 2.
Trunk and pelvic kinematic peaks and ROMs (mean ± SD) for the younger and older groups during normal walking gait.
Significant trunk-P kinematic differences between the YG and OG were predominantly in the coronal plane, although transverse plane kinematics also showed large age-related differences despite not reaching significance. Peak trunk-P retraction was significantly greater (p = 0.047) in the YG (6.30 ± 1.88°) compared to the OG (4.77 ± 1.65°). The YG also demonstrated greater peak protraction and axial rotation ROM than the OG, although not statistically significant. However, the magnitudes of these differences were large and comparable to that of internal rotation. During stance, the YG (7.19 ± 1.50°) exhibited significantly greater (p = 0.004) peak contralateral flexion compared to the OG (5.19 ± 1.64°). The YG (−7.13 ± 1.59°) also demonstrated significantly greater (p = 0.005) peak ipsilateral flexion than the OG (−5.03 ± 1.66°) during swing phase, resulting in the YG possessing a lateral flexion ROM 40% greater than the OG (14.31 ± 3.08° vs. 10.22 ± 3.29°, p = 0.005). Similar differences were found in pelvic obliquity, where peak upward and downward tilt were significantly greater in the YG compared to the OG (p < 0.001). Pelvic obliquity ROM as a result, was also significantly greater (p < 0.001) in the YG compared to the OG by 74%. Significant age-related differences in lower back lateral flexion and pelvic obliquity were large according to effect size estimates (Cohen’s d = 1.3–2.0). ANCOVA revealed that MVPA, as well as MFI and NMV of the LPMs, were not significant covariates of any of the discrete spatiotemporal and kinematic variables (p > 0.05).
3.2. Continuous Measures
SPM revealed no significant phase-specific differences in trunk-G kinematic waveform patterns between the OG and YG throughout the GC (Figure 1). Two age-related phase-specific differences were identified for trunk-P kinematics in the coronal plane (Figure 2). Two clusters at 13.3–17.7% and 62.5–67.2% exceeded the critical threshold indicating that lateral flexion in the YG was significantly greater during midstance and initial swing than in the OG (t(22) = 3.247, p = 0.039; t(22) = 3.247, p = 0.038, respectively). In the transverse plane, one supra-threshold cluster (66.1–76.9%) exceeded the critical threshold of t(22) = 3.346 as the YG exhibited significantly greater (p = 0.004) trunk-P axial rotation than the OG during swing phase (Figure 3).
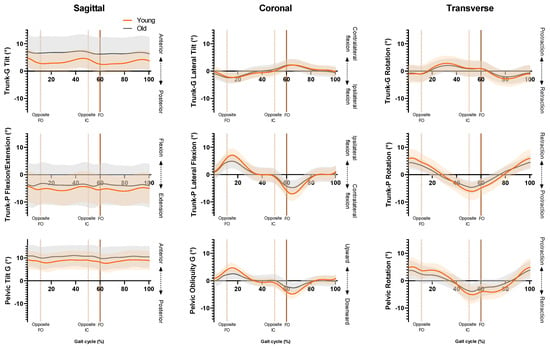
Figure 1.
Ensemble averages for trunk-G (trunk relative to global reference frame), trunk-P (trunk relative to pelvic reference frame) and pelvis kinematics. Orange line = YG mean, grey line = OG mean, orange shaded area = YG SD, grey shaded area = OG SD. FO = Foot-off, IC = Initial contact.
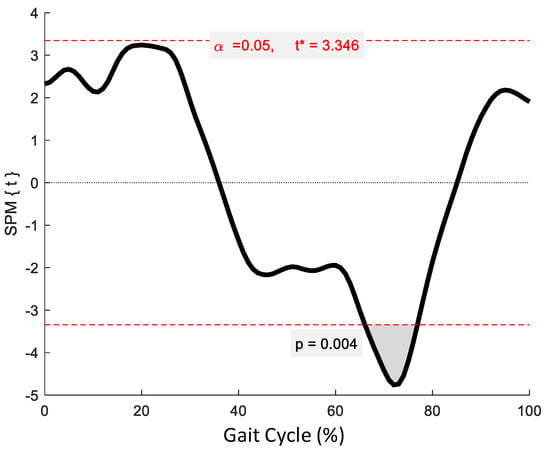
Figure 2.
Statistical Parametric Mapping (SPM) output for trunk-P kinematics in the coronal plane. The horizontal red dashed lines represent the critical t* based on α = 0.05 and random field theory calculations of residual smoothness. t* is the critical threshold.
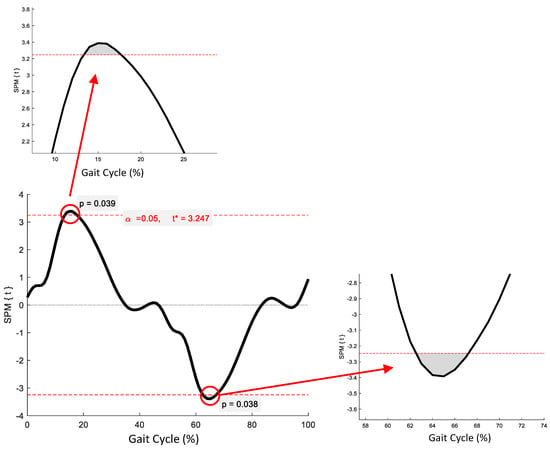
Figure 3.
Statistical Parametric Mapping (SPM) output for trunk-P kinematics in the transverse plane. The horizontal red dashed lines represent the critical t* based on α = 0.05 and random field theory calculations of residual smoothness. t* is the critical threshold.
No significant phase-specific differences were revealed for pelvic tilt between age groups. Two clusters for pelvic obliquity (Figure 4) at 10.3–24.6% and 59.4–73.8% exceeded the critical threshold indicating that pelvic obliquity in the YG was significantly greater during midstance and initial swing than in the OG (t(22) = 3.274, p = 0.003; t(22) = 3.247, p = 0.002, respectively). One supra-threshold cluster (67.8–76.3%) exceeded the critical threshold of t(22) = 3.222 for pelvic axial rotation, as the YG exhibited significantly greater pelvic rotation than the OG during swing phase (p = 0.023) (Figure 5).
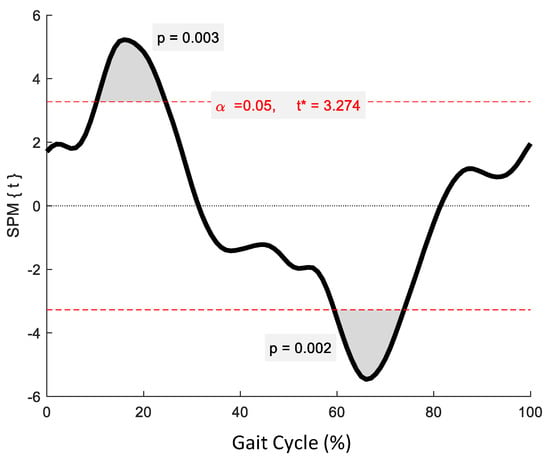
Figure 4.
Statistical Parametric Mapping (SPM) output for pelvic kinematics in the coronal plane. The horizontal red dashed lines represent the critical t* based on α = 0.05 and random field theory calculations of residual smoothness. t* is the critical threshold.
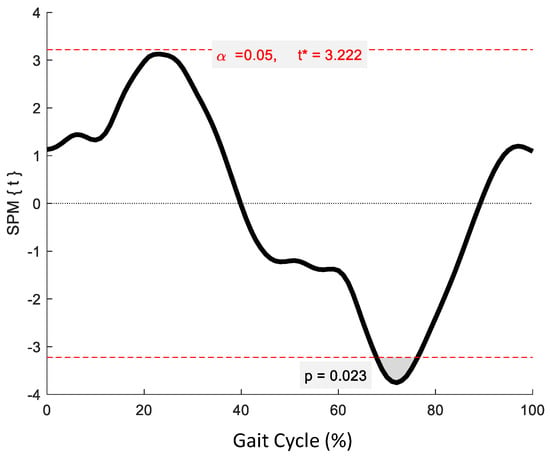
Figure 5.
Statistical Parametric Mapping (SPM) output for pelvic kinematics in the transverse plane. The horizontal red dashed lines represent the critical t* based on α = 0.05 and random field theory calculations of residual smoothness. t* is the critical threshold.
3.3. Correlations between Planes of Motion and Intersegment Motion
There were multiple significant interplanar and intersegment correlations in ROMs (Table 3). However, after controlling for age group, only significant partial correlations remained between trunk-P lateral flexion ROM and trunk-G lateral tilt ROM (r(21) = 0.43, p = 0.043), trunk-P lateral flexion ROM and pelvic obliquity ROM (r(21) = 0.56, p = 0.005), and trunk-P axial rotation ROM and pelvic axial rotation ROM (r(21) = 0.63, p = 0.001).

Table 3.
Zero-order correlation coefficients for ROM between the trunk-P, trunk-G, and pelvis in all cardinal planes.
4. Discussion
This study identifies age-related differences in trunk kinematics between healthy older men and younger counterparts. To date, differences in trunk kinematics between younger and older participants have not been fully examined. As a consequence, the present study adds new knowledge in relation to ageing gait biomechanics to the literature base. The key findings from the present study were that age-related differences in trunk-P kinematics were greatest in the coronal and transverse planes, and that the initial periods of single limb support were identified as important phases during the GC, where significant differences in trunk kinematics between younger and older adults were detected. This suggests that age-related differences in the trunk are most apparent when dynamic balance is compromised during initial single limb support phases. Given that trunk kinematics are important to locomotor control and maintaining balance in older age [26], these observations highlight the importance of analysing the upper body during gait, and have potential benefits with regards to identifying falls risk in older adults.
4.1. Age-Related Changes in the Sagittal Plane
Sagittal plane trunk-G and pelvis kinematics were similar between the OG and YG, resulting in similar trunk-P movement patterns. However, there was a delayed phase shift in the YG, resulting in altered phase-specific flexion/extension movements of the trunk-P. Trunk-G tilt exhibited a biphasic oscillation, corresponding to one flexion/extension cycle for each step. These findings are supported by other gait studies [27,28], and substantiated by EMG studies that have shown peaks of paraspinal muscle activity at early midstance and around foot-off [29,30]. Indeed, ES muscle activity precedes corresponding kinematics indicating that the paravertebral muscles drive trunk movement by anticipating propulsive phases in walking [5].
The OG adopted a more forward-tilted trunk-G throughout the GC, possibly to account for reduced propulsive force [31]. A similar strategy has been observed in lower-extremity amputees [32]. According to Leroux et al., [33] tilting the trunk forward is the best strategy to generate greater forward propulsion during walking gait. Therefore, the OG may have adopted a greater anterior trunk-G angle, causing an anterior shift in centre of mass (COM) to facilitate their forward progression. Tilting the trunk anteriorly may also play an injury prevention role, reducing lower limb stress by damping COM oscillations [26]. The OG also exhibited significantly less trunk-G ROM than the YG. Reduced trunk-G ROM may be indicative of a conservative gait strategy to prevent larger destabilising forces in the sagittal plane [11], despite this being less efficient as more muscular work would be required for horizontal displacement of the COM.
4.2. Coronal Plane Changes in Older Age
Trunk motion in the coronal plane was less variable than in the sagittal plane and similar between age groups. Results from the SPM indicate that there is a phase-specific age effect in trunk-P coronal plane kinematics during the early midstance and early swing phase. This is supported by movement amplitudes being significantly greater in the YG than the OG. Given that trunk-G movements were similar in phase and amplitude between groups, and pelvic obliquity exhibited similar significant differences to trunk-P lateral flexion, it is likely that trunk-P motion in the coronal plane was simply a reflection of pelvic movement. This is supported by Crosbie, et al., [28] who state that spinal movements associated with walking are linked to the primary motions of the pelvis.
Pelvic motion in the coronal plane is particularly important for reducing trunk-G oscillations, which could excessively displace COM and cause lateral instability during walking. Instability during the GC may be minimised by permitting a relatively larger ROM in the trunk relative to the pelvis than in the global reference frame through the independent motions of the pelvis. The reduced pelvic, and thus trunk-P ROM in the OG may decrease stability during gait, and indeed explain why falls are more prevalent in the elderly [34]. Lower lateral flexion peaks in the OG may also lead to reduced foot clearance [35] and consequently increase the risk of falling [36]. However, older adults may prevent impact injuries by decreasing trunk movement in the coronal plane. Reducing peak lateral flexion during the early stages of single-limb support allowed the OG to decrease trunk-P angular velocity when it started to move towards the opposite side, which may be an effort to reduce impact from heel strike [14].
4.3. Kinematic Changes in the Transverse Plane
Whilst it appears that trunk-G axial rotation was reduced by ageing based on peak values and ROM, no phase-specific differences were observed. Furthermore, coinciding phase-specific differences between groups were identified in the pelvis (68–76% of GC) and trunk-P (66–77% of GC) during swing phase. Coupling between the trunk-P and pelvis was further supported by the significant correlation in their ROMs in the transverse plane. These results indicate that age-related changes in the transverse plane involve a complex interrelationship between the trunk and pelvis. It appears that instances of peak rotational excursion during gait immediately following heel strikes are affected by age-related kinematic changes in the trunk-G. Whereas, during single limb support, age-related differences in the pelvis appear to be more influential than in the trunk-G to trunk-P motion.
4.4. Age Effect on Interplanar Motions
The results of the present study suggest there is a change in planar motion with ageing. Zero-order correlations showed that there were numerous significant relationships between the ROM in the trunk-P, trunk-G, and pelvis in different planes. After controlling for age, only three significant correlations remained (trunk-P lateral flexion with trunk-G lateral tilt, trunk-P lateral flexion with pelvic obliquity, and trunk-P axial rotation with pelvic axial rotation), all exclusive to their respective planes. This indicates that ageing causes rotations of the upper body in any given plane to become independent of rotations in orthogonal planes. This finding is novel, although others have shown that coronal and transverse plane trunk motions are interconnected in younger adults [14,37], and that older adults exhibit reduced compensatory coordination between trunk and pelvis movements during walking [11].
In older age, disassociation of trunk and pelvic movements may increase the energetic demands of walking if angular momentum cannot be conserved from motions in orthogonal planes to assist in the forward progression of the body’s COM [38,39]. Whilst the exact mechanisms for the age-related disassociation of interplanar motions in the trunk are unknown, it has been suggested that the vector of the spinal muscles may be responsible for the association between coronal and transverse plane trunk movement [14]. Therefore, including data on muscle fibre orientation derived by MRI diffusion-tensor techniques [40] may elucidate mechanisms. Macroscopic features such as MFI and NMV are not likely responsible for interplanar decoupling in older age based on the current results.
4.5. Clinical and Practical Applications
In clinical gait analysis, the upper body is typically overlooked whilst the lower limbs receive much of the focus. Data from the present study indicate that the upper body’s contribution should be considered during gait to inform the design of more effective strategies to preserve mobility in older age. Understanding age-related changes in trunk biomechanics could assist clinical decision making and public health strategies with regards to incorporating motor skills training into exercise interventions and PA programmes. Exercise interventions generally focus on promoting strength, endurance, balance, and flexibility in older adults. Whilst useful, incorporating trunk motor skills training may improve physical function and mobility to a greater extent. The current findings could support a targeted approach by identifying movement patterns in the trunk which should be prioritised to improve physical function in older age. Routinely analysing the trunk will also increase the evidence base and establish normative data, which could be used to identify abnormal movement patterns that may not be apparent in the lower limbs. Furthermore, the full-body PiG model would be appropriate in clinical settings where a balance of accuracy and practicality is needed.
4.6. Limitations
There are limitations in this study that should be acknowledged. Whilst PiG has been used to estimate lumbar spine kinematics as the intersection between the modelled trunk and pelvic segments [14,41], it provides a gross understanding of trunk kinematics. More complex spinal models may offer greater accuracy at individual vertebral levels. However, they may also increase measurement error as the size of functional spinal units is small and limits our ability to position three non-colinear markers on the skin overlying multiple functional spinal units [42]. The increased data collection and computational requirements may also make more detailed approaches less clinically applicable [43]. Confidence in the current results is high since the movement patterns and peak values in all planes were highly comparable to a study using indwelling bone pins to assess the 3D motion of the lumbar spine during gait [44], which is considered the gold-standard approach. It should be noted that the current modelling approach for the pelvis, whilst commonly used, may introduce systematic error and a false anterior tilt. Normalising anterior pelvic tilt to zero by defining a pelvis angle that has a coronal plane parallel to the floor may be more convenient for describing pelvic motion.
Secondly, the cohort recruited in the current study comprised healthy, active, young, and older adult men. It has been shown that trunk kinematics are affected by disease and physical impairment such as stroke [41]. Therefore, caution should be taken when generalising the findings of the current study to populations other than healthy men. This study was also specific to walking gait biomechanics on a level surface. Age-related differences may be more pronounced for more challenging movements such as stair negotiation and sit-to-stand.
Group sample sizes were also somewhat smaller than the number generally targeted in most gait studies. This study was part of a larger project in which the MRI parameters were the primary outcome measures. Therefore, the a priori power calculation was based on MRI outcomes, which indicated a minimum of n = 8 participants per group was sufficient based on moderate to large effect sizes (α error = 0.05, β error = 0.8). Regarding the implications of sample size for SPM in the current study, it is likely that the analysis was underpowered to detect subtle differences in kinematics between groups. Research has shown that required sample sizes range widely from five to over thirty participants per group to detect differences of 10° to 5° using SPM methods [45]. Detecting more subtle differences (e.g., 2°) between groups would require an even greater sample size to ensure sufficient statistical power, which is atypical in biomechanical research.
5. Conclusions
This study offers new insight by providing trunk motion data in all cardinal planes and using one-dimensional SPM to uncover phase-specific differences with respect to ageing. In this study, ageing modified trunk kinematics primarily in the coronal and transverse planes, although this may be a reflection of changes in pelvic motion. Age-related kinematic changes were characterized by reductions in peak amplitudes and ROM in all planes, which were likely to be manifestations of a conservative gait strategy. Muscle degeneration in the lumbar spine and MVPA did not covary with age-related changes in trunk kinematics. Future research should focus on a range of age groups and diseased populations to further understand normal trunk motion during gait with ageing.
Author Contributions
A.D.: conceptualization, methodology, formal analysis, investigation, data curation, writing—original draft preparation, writing—review and editing, visualization, project administration; M.D.: supervision, conceptualization, writing—review and editing, funding acquisition; C.G.: writing—review and editing, project administration; J.T.: writing—review and editing, supervision; D.R.: writing—review and editing, supervision, funding acquisition; J.H.: supervision, conceptualization, writing—review and editing, funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was jointly funded by University Hospitals Coventry and Warwickshire NHS Trust and Coventry University and carried out with the support of the National Institute of Health Research (NIHR) Coventry and Warwickshire Clinical Research Facility. The views expressed are those of the author(s) and not necessarily those of University Hospitals Coventry and Warwickshire NHS Trust and Coventry University, the NIHR or the Department of Health. The APC was funded by the University of Wolverhampton.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of Coventry University (protocol code P70399, 13 September 2018).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are not publicly available due to privacy or ethical restrictions.
Acknowledgments
We would like to thank the following people for their support and assistance in collecting data. Ciara Dinneny and Bart Koning of the Coventry Gait Laboratory, University Hospitals Coventry and Warwickshire NHS Trust; Alison Campbell, Poonam Patel and David Dixon of the Human Metabolism Research Unit, University Hospitals Coventry and Warwickshire NHS Trust; Kathrine Whelan, Charli Jarvis and Niamh Ruffles of Coventry University.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no input into the study design, collection, analysis, interpretation of data, and writing of the report.
References
- Chamberlin, M.E.; Fulwider, B.D.; Sanders, S.L.; Medeiros, J.M. Does Fear of Falling Influence Spatial and Temporal Gait Parameters in Elderly Persons beyond Changes Associated with Normal Aging? J. Gerontol.-Ser. A Biol. Sci. Med. Sci. 2005, 60, 1163–1167. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.U.; Hausdorff, J.M.; Ferrucci, L. Age-Associated Differences in the Gait Pattern Changes of Older Adults during Fast-Speed and Fatigue Conditions: Results from the Baltimore Longitudinal Study of Ageing. Age Ageing 2010, 39, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Cimolin, V.; Galli, M. Summary Measures for Clinical Gait Analysis: A Literature Review. Gait Posture 2014, 39, 1005–1010. [Google Scholar] [CrossRef]
- Unitied Nations; Department of Economic and Social Affairs: Population Division; Department of Economic and Social Affairs Population Division, U.N. World Population Ageing 2019: Highlights; United Nations: New York, NY, USA, 2020.
- Ceccato, J.C.; de Sèze, M.; Azevedo, C.; Cazalets, J.R. Comparison of Trunk Activity during Gait Initiation and Walking in Humans. PLoS ONE 2009, 4, e8193. [Google Scholar] [CrossRef]
- Hicks, G.E.; Simonsick, E.M.; Harris, T.B.; Newman, A.B.; Weiner, D.K.; Nevitt, M.A.; Tylavsky, F.A. Trunk Muscle Composition as a Predictor of Reduced Functional Capacity in the Health, Aging and Body Composition Study: The Moderating Role of Back Pain. J. Gerontol.-Ser. A Biol. Sci. Med. Sci. 2005, 60, 1420–1424. [Google Scholar] [CrossRef]
- Young PM, M.; Wilken, J.M.; Dingwell, J.B. Dynamic Margins of Stability during Human Walking in Destabilizing Environments. J. Biomech. 2012, 45, 1053–1059. [Google Scholar] [CrossRef]
- Vellas, B.J.; Wayne, S.J.; Romero, L.; Baumgartner, R.N.; Rubenstein, L.Z.; Garry, P.J. One-Leg Balance Is an Important Predictor of Injurious Falls in Older Persons. J. Am. Geriatr. Soc. 1997, 45, 735–738. [Google Scholar] [CrossRef]
- Winter, D.A.; Patla, A.E.; Frank, J.S.; Walt, S.E. Biomechanical Walking Pattern Changes in the Fit and Healthy Elderly. Phys. Ther. 1990, 70, 340–347. [Google Scholar] [CrossRef] [PubMed]
- McGibbon, C.A.; Krebs, D.E. Age-Related Changes in Lower Trunk Coordination and Energy Transfer during Gait. J. Neurophysiol. 2001, 85, 1923–1931. [Google Scholar] [CrossRef]
- Van Emmerik, R.E.A.; McDermott, W.J.; Haddad, J.M.; Van Wegen, E.E.H. Age-Related Changes in Upper Body Adaptation to Walking Speed in Human Locomotion. Gait Posture 2005, 22, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Van Criekinge, T.; Hallemans, A.; Van de Walle, P.; Sloot, L.H. Age-Related Changes in Trunk Kinematics and Mechanical Work during Gait. Gait Posture 2021, 90, 281–282. [Google Scholar] [CrossRef]
- Schmid, S.; Bruhin, B.; Ignasiak, D.; Romkes, J.; Taylor, W.R.; Ferguson, S.J.; Brunner, R.; Lorenzetti, S. Spinal Kinematics during Gait in Healthy Individuals across Different Age Groups. Hum. Mov. Sci. 2017, 54, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.Y.; Park, M.S.; Lee, S.H.; Kong, S.J.; Lee, K.M. Kinematic Aspects of Trunk Motion and Gender Effect in Normal Adults. J. Neuroeng. Rehabil. 2010, 7, 9. [Google Scholar] [CrossRef]
- Leardini, A.; Berti, L.; Begon, M.; Allard, P. Effect of Trunk Sagittal Attitude on Shoulder, Thorax and Pelvis Three-Dimensional Kinematics in Able-Bodied Subjects during Gait. PLoS ONE 2013, 8, e77168. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European Consensus on Definition and Diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Dallaway, A.; Kite, C.; Griffen, C.; Duncan, M.; Tallis, J.; Renshaw, D.; Hattersley, J. Age-Related Degeneration of the Lumbar Paravertebral Muscles: Systematic Review and Three-Level Meta-Regression. Exp. Gerontol. 2020, 133, 110856. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef] [PubMed]
- Fritz, J.M.; Irrgang, J.J. A Comparison of a Modified Oswestry Low Back Pain Disability Questionnaire and the Quebec Back Pain Disability Scale. Phys. Ther. 2001, 81, 776–788. [Google Scholar] [CrossRef]
- Migueles, J.H.; Cadenas-Sanchez, C.; Ekelund, U.; Delisle Nyström, C.; Mora-Gonzalez, J.; Löf, M.; Labayen, I.; Ruiz, J.R.; Ortega, F.B. Accelerometer Data Collection and Processing Criteria to Assess Physical Activity and Other Outcomes: A Systematic Review and Practical Considerations. Sport. Med. 2017, 47, 1821–1845. [Google Scholar] [CrossRef]
- Diaz, K.M.; Krupka, D.J.; Chang, M.J.; Kronish, I.M.; Moise, N.; Goldsmith, J.; Schwartz, J.E. Wrist-Based Cut-Points for Moderate- and Vigorous-Intensity Physical Activity for the Actical Accelerometer in Adults. J. Sports Sci. 2018, 36, 206–212. [Google Scholar] [CrossRef]
- Vicon Plug-in Gait. Product Guide—Foundation Notes. Available online: https://www.google.co.uk/search?q=Plug-in+Gait.+Product+Guide+-+Foundation+Notes+%5BOnline%5D.&ie=&oe= (accessed on 10 June 2020).
- Sinclair, J.; Edmundson, C.J.; Brooks, D.; Hobbs, S.J.; Taylor, P.J. The Influence of Footwear Kinetic, Kinematic and Electromyographical Parameters on the Energy Requirements of Steady State Running. Mov. Sport. Sci. 2013, 80, 39–49. [Google Scholar] [CrossRef]
- Dallaway, A.; Hattersley, J.; Diokno, M.; Tallis, J.; Renshaw, D.; Wilson, A.; Wayte, S.; Weedall, A.; Duncan, M. Age-Related Degeneration of Lumbar Muscle Morphology in Healthy Younger versus Older Men. Aging Male 2021, 23, 1583–1597. [Google Scholar] [CrossRef]
- Friston, K.J.; Ashburner, J.T.; Kiebel, S.J.; Nichols, T.E.; Penny, W.D. Statistical Parametric Mapping: The Analysis of Functional Brain Images; Elsevier: London, UK, 2007; ISBN 9780123725608. [Google Scholar]
- Krebs, D.E.; Wong, D.; Jevsevar, D.; Riley, P.O.; Hodge, W.A. Trunk Kinematics during Locomotor Activities. Phys. Ther. 1992, 72, 505–514. [Google Scholar] [CrossRef]
- Sartor, C.; Alderink, G.; Greenwald, H.; Elders, L. Critical Kinematic Events Occurring in the Trunk during Walking. Hum. Mov. Sci. 1999, 18, 669–679. [Google Scholar] [CrossRef]
- Crosbie, J.; Vachalathiti, R.; Smith, R. Patterns of Spinal Motion during Walking. Gait Posture 1997, 5, 6–12. [Google Scholar] [CrossRef]
- Lamoth, C.J.C.; Daffertshofer, A.; Meijer, O.G.; Lorimer Moseley, G.; Wuisman, P.I.J.M.; Beek, P.J. Effects of Experimentally Induced Pain and Fear of Pain on Trunk Coordination and Back Muscle Activity during Walking. Clin. Biomech. 2004, 19, 551–563. [Google Scholar] [CrossRef] [PubMed]
- Callaghan, J.P.; Patla, A.E.; McGill, S.M. Low Back Three-Dimensional Joint Forces, Kinematics, and Kinetics during Walking. Clin. Biomech. 1999, 14, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Hendershot, B.D.; Wolf, E.J. Three-Dimensional Joint Reaction Forces and Moments at the Low Back during over-Ground Walking in Persons with Unilateral Lower-Extremity Amputation. Clin. Biomech. 2014, 29, 235–242. [Google Scholar] [CrossRef]
- Goujon-Pillet, H.; Sapin, E.; Fodé, P.; Lavaste, F. Three-Dimensional Motions of Trunk and Pelvis during Transfemoral Amputee Gait. Arch. Phys. Med. Rehabil. 2008, 89, 87–94. [Google Scholar] [CrossRef]
- Leroux, A.; Fung, J.; Barbeau, H. Postural Adaptation to Walking on Inclined Surfaces: I. Normal Strategies. Gait Posture 2002, 15, 64–74. [Google Scholar] [CrossRef]
- Sharif, S.I.; Al-Harbi, A.B.; Al-Shihabi, A.M.; Al-Daour, D.S.; Sharif, R.S. Falls in the Elderly: Assessment of Prevalence and Risk Factors. Pharm. Pract. 2018, 16. [Google Scholar] [CrossRef] [PubMed]
- Prince, F.; Corriveau, H.; Hébert, R.; Winter, D.A. Gait in the Elderly. Gait Posture 1997, 5, 128–135. [Google Scholar] [CrossRef]
- Robinovitch, S.N.; Feldman, F.; Yang, Y.; Schonnop, R.; Leung, P.M.; Sarraf, T.; Sims-Gould, J.; Loughi, M. Video Capture of the Circumstances of Falls in Elderly People Residing in Long-Term Care: An Observational Study. Lancet 2013, 381, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Whittle, M.W.; Levine, D. Three-Dimensional Relationships between the Movements of the Pelvis and Lumbar Spine during Normal Gait. Hum. Mov. Sci. 1999, 18, 681–692. [Google Scholar] [CrossRef]
- Van Emmerik, R.E.A.; Wagenaar, R.C. Effects of Walking Velocity on Relative Phase Dynamics in the Trunk in Human Walking. J. Biomech. 1996, 29, 1175–1184. [Google Scholar] [CrossRef]
- Gracovetsky, S.A.; Iacono, S. Energy Transfers in the Spinal Engine. J. Biomed. Eng. 1987, 9, 99–114. [Google Scholar] [CrossRef]
- Farrow, M.; Biglands, J.; Tanner, S.F.; Clegg, A.; Brown, L.; Hensor, E.M.A.; O’Connor, P.; Emery, P.; Tan, A.L. The Effect of Ageing on Skeletal Muscle as Assessed by Quantitative MR Imaging: An Association with Frailty and Muscle Strength. Aging Clin. Exp. Res. 2020, 33, 291–301. [Google Scholar] [CrossRef]
- Titus, A.W.; Hillier, S.; Louw, Q.A.; Inglis-Jassiem, G. An Analysis of Trunk Kinematics and Gait Parameters in People with Stroke. Afr. J. Disabil. 2018, 7, a310. [Google Scholar] [CrossRef]
- Konz, R.J.; Fatone, S.; Stine, R.L.; Ganju, A.; Gard, S.A.; Ondra, S.L. A Kinematic Model to Assess Spinal Motion during Walking. Spine 2006, 31, E898–E906. [Google Scholar] [CrossRef]
- Gutierrez, E.M.; Bartonek, Å.; Haglund-Åkerlind, Y.; Saraste, H. Centre of Mass Motion during Gait in Persons with Myelomeningocele. Gait Posture 2003, 18, 37–46. [Google Scholar] [CrossRef]
- MacWilliams, B.A.; Rozumalski, A.; Swanson, A.N.; Wervey, R.A.; Dykes, D.C.; Novacheck, T.F.; Schwartz, M.H. Assessment of Three-Dimensional Lumbar Spine Vertebral Motion during Gait with Use of Indwelling Bone Pins. J. Bone Jt. Surg.-Ser. A 2013, 95, e184. [Google Scholar] [CrossRef] [PubMed]
- Luciano, F.; Ruggiero, L.; Pavei, G. Sample Size Estimation in Locomotion Kinematics and Electromyography for Statistical Parametric Mapping. J. Biomech. 2021, 122, 110481. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).