Mobile App and Digital System for Patients after Myocardial Infarction (afterAMI): Results from a Randomized Trial
Abstract
1. Introduction
2. Materials and Methods
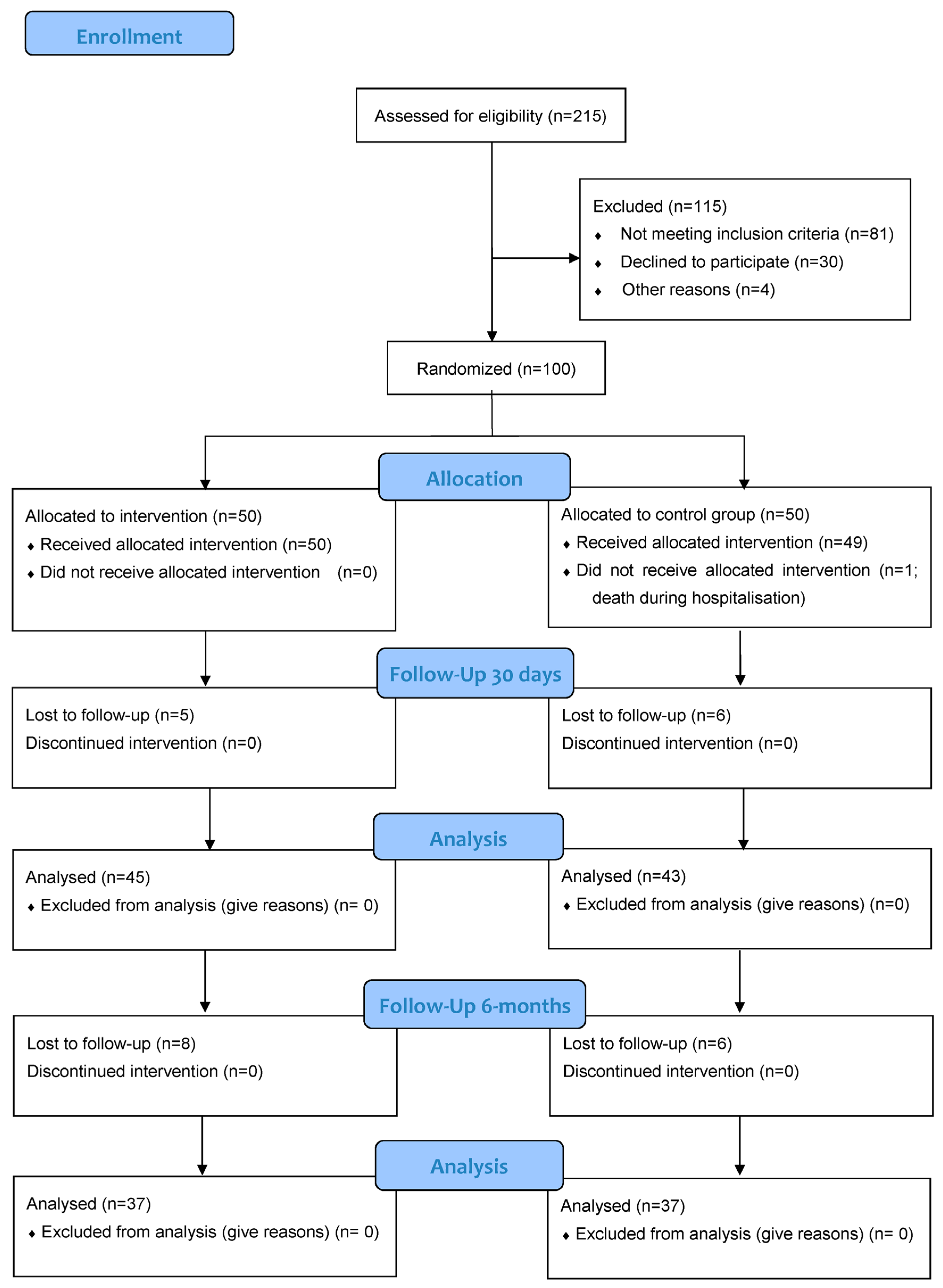
2.1. Study Design
2.2. Study Endpoints
2.3. Statistical Analysis
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lozano, R.; Naghavi, M.; Foreman, K.; Lim, S.; Shibuya, K.; Aboyans, V.; Abraham, J.; Adair, T.; Aggarwal, R.; Ahn, S.Y.; et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2095–2128. [Google Scholar] [CrossRef] [PubMed]
- Degano, I.R.; Salomaa, V.; Veronesi, G.; Ferrieres, J.; Kirchberger, I.; Laks, T.; Havulinna, A.S.; Ruidavets, J.B.; Ferrario, M.M.; Meisinger, C.; et al. Twenty-five-year trends in myocardial infarction attack and mortality rates, and case-fatality, in six European populations. Heart 2015, 101, 1413–1421. [Google Scholar] [CrossRef] [PubMed]
- Santos, I.S.; Goulart, A.C.; Brandao, R.M.; Santos, R.C.; Bittencourt, M.S.; Sitnik, D.; Pereira, A.C.; Pastore, C.A.; Samesima, N.; Lotufo, P.A.; et al. One-year Mortality after an Acute Coronary Event and its Clinical Predictors: The ERICO Study. Arq. Bras. Cardiol. 2015, 105, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Back, M.; Benetos, A.; Biffi, A.; Boavida, J.M.; Capodanno, D.; et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef] [PubMed]
- Jankowski, P.; Kosior, D.A.; Sowa, P.; Szostak-Janiak, K.; Koziel, P.; Krzykwa, A.; Sawicka, E.; Haberka, M.; Setny, M.; Kaminski, K.; et al. Secondary prevention of coronary artery disease in Poland. Results from the POLASPIRE survey. Cardiol. J. 2020, 27, 533–540. [Google Scholar] [CrossRef]
- Wojtyniak, B.; Gierlotka, M.; Opolski, G.; Rabczenko, D.; Ozierański, K.; Gąsior, M.; Chlebus, K.; Wierucki, Ł.; Rutkowski, D.; Dziełak, D.; et al. Observed and relative survival and 5-year outcomes of patients discharged after acute myocardial infarction: The nationwide AMI-PL database. Kardiol. Pol. 2020, 78, 990–998. [Google Scholar] [CrossRef]
- Kułach, A.; Wilkosz, K.; Wybraniec, M.; Wieczorek, P.; Gąsior, Z.; Mizia-Stec, K.; Wojakowski, W.; Zdrojewski, T.; Wojtyniak, B.; Gąsior, M.; et al. Managed Care after Acute Myocardial Infarction (MC-AMI)—Poland’s nationwide program of comprehensive post-MI care—improves prognosis in 2-year follow-up. A single high-volume center intention to treat analysis. Kardiol. Pol. 2022, 81, 121–123. [Google Scholar] [CrossRef]
- Jankowski, P.; Topór-Mądry, R.; Gąsior, M.; Cegłowska, U.; Gierlotka, M.; Kubica, J.; Kalarus, Z.; Lesiak, M.; Wojakowski, W.; Legutko, J.; et al. Management and predictors of clinical events in 75,686 patients with acute myocardial infarction. Kardiol. Pol. 2022, 80, 468–475. [Google Scholar] [CrossRef]
- How Many People Have Smartphones in 2022? Available online: https://www.oberlo.com/statistics/how-many-people-have-smartphones (accessed on 27 October 2022).
- Piotrowicz, R.; Krzesinski, P.; Balsam, P.; Piotrowicz, E.; Kempa, M.; Lewicka, E.; Glowczynska, R.; Grabowski, M.; Koltowski, L.; Peller, M.; et al. Telemedicine solutions in cardiology: A joint expert opinion by the Information Technology and Telemedicine Committee of the Polish Cardiac Society, the Section of Noninvasive Electrocardiology and Telemedicine of the Polish Cardiac Society, and the Clinical Research Committee of the Polish Academy of Sciences (short version, 2021). Kardiol. Pol. 2021, 79, 227–241. [Google Scholar] [CrossRef]
- Gonzalez, M.; Sjölin, I.; Bäck, M.; Ögmundsdottir Michelsen, H.; Tanha, T.; Sandberg, C.; Schiopu, A.; Leosdottir, M. Effect of a lifestyle-focused electronic patient support application for improving risk factor management, self-rated health, and prognosis in post-myocardial infarction patients: Study protocol for a multi-center randomized controlled trial. Trials 2019, 20, 76. [Google Scholar] [CrossRef]
- Alkamel, N.; Jamal, A.; Alnobani, O.; Househ, M.; Zakaria, N.; Qawasmeh, M.; Tharkar, S. Understanding the stakeholders’ preferences on a mobile application to reduce door to balloon time in the management of ST-elevated myocardial infarction patients—A qualitative study. BMC Med. Inform. Decis. Mak. 2020, 20, 205. [Google Scholar] [CrossRef] [PubMed]
- Garcia, H.; Springer, B.; Vengrenyuk, A.; Krishnamoorthy, P.; Pineda, D.; Wasielewski, B.; Tan, W.A.; D’Amiento, A.; Bastone, J.; Barman, N.; et al. Deploying a novel custom mobile application for STEMI activation and transfer in a large healthcare system to improve cross-team workflow. STEMIcathAID implementation project. Am. Heart J. 2022, 253, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Ögmundsdóttir Michelsen, H.; Sjölin, I.; Bäck, M.; Gonzalez Garcia, M.; Olsson, A.; Sandberg, C.; Schiopu, A.; Leósdóttir, M. Effect of a Lifestyle-Focused Web-Based Application on Risk Factor Management in Patients Who Have Had a Myocardial Infarction: Randomized Controlled Trial. J. Med. Internet Res. 2022, 24, e25224. [Google Scholar] [CrossRef]
- Widmer, R.J.; Allison, T.G.; Lerman, L.O.; Lerman, A. Digital Health Intervention as an Adjunct to Cardiac Rehabilitation Reduces Cardiovascular Risk Factors and Rehospitalizations. J. Cardiovasc. Transl. Res. 2015, 8, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Coorey, G.M.; Neubeck, L.; Mulley, J.; Redfern, J. Effectiveness, acceptability and usefulness of mobile applications for cardiovascular disease self-management: Systematic review with meta-synthesis of quantitative and qualitative data. Eur. J. Prev. Cardiol. 2018, 25, 505–521. [Google Scholar] [CrossRef]
- Gallagher, R.; Roach, K.; Sadler, L.; Glinatsis, H.; Belshaw, J.; Kirkness, A.; Zhang, L.; Gallagher, P.; Paull, G.; Gao, Y.; et al. Mobile Technology Use Across Age Groups in Patients Eligible for Cardiac Rehabilitation: Survey Study. JMIR Mhealth Uhealth 2017, 5, e161. [Google Scholar] [CrossRef]
- Steinberg, J.S.; Varma, N.; Cygankiewicz, I.; Aziz, P.; Balsam, P.; Baranchuk, A.; Cantillon, D.J.; Dilaveris, P.; Dubner, S.J.; El-Sherif, N.; et al. 2017 ISHNE-HRS expert consensus statement on ambulatory ECG and external cardiac monitoring/telemetry. Heart Rhythm 2017, 14, e55–e96. [Google Scholar] [CrossRef]
- Krzowski, B.; Peller, M.; Boszko, M.; Hoffman, P.; Żurawska, N.; Jaruga, K.; Skoczylas, K.; Osak, G.; Kołtowski, Ł.; Grabowski, M.; et al. Mobile app and digital system for patients after myocardial infarction (afterAMI): Study protocol for a randomized controlled trial. Trials 2022, 23, 522. [Google Scholar] [CrossRef] [PubMed]
- Collet, J.P.; Thiele, H.; Barbato, E.; Barthelemy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2020, 93, 575. [Google Scholar] [CrossRef]
- Kubica, A.; Kasprzak, M.; Siller-Matula, J.; Koziński, M.; Pio Navarese, E.; Obońska, K.; Andruszkiewicz, A.; Sztuba, B.; Fabiszak, T.; Swiątkiewicz, I.; et al. Time-related changes in determinants of antiplatelet effect of clopidogrel in patients after myocardial infarction. Eur. J. Pharmacol. 2014, 742, 47–54. [Google Scholar] [CrossRef]
- Antoniou, V.; Xanthopoulos, A.; Giamouzis, G.; Davos, C.; Batalik, L.; Stavrou, V.; Gourgoulianis, K.I.; Kapreli, E.; Skoularigis, J.; Pepera, G. Efficacy, efficiency and safety of a cardiac telerehabilitation programme using wearable sensors in patients with coronary heart disease: The TELEWEAR-CR study protocol. BMJ Open 2022, 12, e059945. [Google Scholar] [CrossRef] [PubMed]
- Batalik, L.; Pepera, G.; Papathanasiou, J.; Rutkowski, S.; Líška, D.; Batalikova, K.; Hartman, M.; Felšőci, M.; Dosbaba, F. Is the Training Intensity in Phase Two Cardiovascular Rehabilitation Different in Telehealth versus Outpatient Rehabilitation? J. Clin. Med. 2021, 10, 4069. [Google Scholar] [CrossRef] [PubMed]
- Chow, C.K.; Klimis, H.; Thiagalingam, A.; Redfern, J.; Hillis, G.S.; Brieger, D.; Atherton, J.; Bhindi, R.; Chew, D.P.; Collins, N.; et al. Text Messages to Improve Medication Adherence and Secondary Prevention After Acute Coronary Syndrome: The TEXTMEDS Randomized Clinical Trial. Circulation 2022, 145, 1443–1455. [Google Scholar] [CrossRef] [PubMed]
- Korzeniowska-Kubacka, I.; Dobraszkiewicz-Wasilewska, B.; Bilińska, M.; Rydzewska, E.; Piotrowicz, R. Two models of early cardiac rehabilitation in male patients after myocardial infarction with preserved left ventricular function: Comparison of standard out-patient versus hybrid training programmes. Kardiol. Pol. 2011, 69, 220–226. [Google Scholar]
- Park, L.G.; Elnaggar, A.; Lee, S.J.; Merek, S.; Hoffmann, T.J.; Von Oppenfeld, J.; Ignacio, N.; Whooley, M.A. Mobile Health Intervention Promoting Physical Activity in Adults Post Cardiac Rehabilitation: Pilot Randomized Controlled Trial. JMIR Form. Res. 2021, 5, e20468. [Google Scholar] [CrossRef]
- Lunde, P.; Bye, A.; Bergland, A.; Grimsmo, J.; Jarstad, E.; Nilsson, B.B. Long-term follow-up with a smartphone application improves exercise capacity post cardiac rehabilitation: A randomized controlled trial. Eur. J. Prev. Cardiol. 2020, 27, 1782–1792. [Google Scholar] [CrossRef]
- Márquez Contreras, E.; Márquez Rivero, S.; Rodríguez García, E.; López-García-Ramos, L.; Carlos Pastoriza Vilas, J.; Baldonedo Suárez, A.; Gracia Diez, C.; Gil Guillén, V.; Martell Claros, N. Specific hypertension smartphone application to improve medication adherence in hypertension: A cluster-randomized trial. Curr. Med. Res. Opin. 2019, 35, 167–173. [Google Scholar] [CrossRef]
- Guerriero, C.; Cairns, J.; Roberts, I.; Rodgers, A.; Whittaker, R.; Free, C. The cost-effectiveness of smoking cessation support delivered by mobile phone text messaging: Txt2stop. Eur. J. Health Econ. 2013, 14, 789–797. [Google Scholar] [CrossRef]
- Aceti, V.M.; Santoro, R.V.; Velarde, L.G.C.; Brandão, D.N.; da Cruz, R.A.F.; Taboada, G.F. Educating diabetic patients through an SMS intervention: A randomized controlled trial at a Brazilian public hospital. Arch. Endocrinol. Metab. 2021, 65, 695–703. [Google Scholar] [CrossRef]
- Cho, M.J.; Sim, J.L.; Hwang, S.Y. Development of smartphone educational application for patients with coronary artery disease. Healthc Inform Res 2014, 20, 117–124. [Google Scholar] [CrossRef]
- Johnston, N.; Bodegard, J.; Jerström, S.; Åkesson, J.; Brorsson, H.; Alfredsson, J.; Albertsson, P.A.; Karlsson, J.E.; Varenhorst, C. Effects of interactive patient smartphone support app on drug adherence and lifestyle changes in myocardial infarction patients: A randomized study. Am. Heart J. 2016, 178, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Indraratna, P.; Biswas, U.; McVeigh, J.; Mamo, A.; Magdy, J.; Vickers, D.; Watkins, E.; Ziegl, A.; Liu, H.; Cholerton, N.; et al. A Smartphone-Based Model of Care to Support Patients With Cardiac Disease Transitioning From Hospital to the Community (TeleClinical Care): Pilot Randomized Controlled Trial. JMIR Mhealth Uhealth 2022, 10, e32554. [Google Scholar] [CrossRef]
- Pandey, A.C.; Golbus, J.R.; Topol, E.J. Cardiac rehabilitation in the digital era. Lancet 2021, 398, 16. [Google Scholar] [CrossRef] [PubMed]
- Setny, M.; Jankowski, P.; Kamiński, K.; Gąsior, Z.; Haberka, M.; Czarnecka, D.; Pająk, A.; Kozieł, P.; Szóstak-Janiak, K.; Sawicka, E.; et al. Secondary prevention of coronary heart disease in Poland: Does sex matter? Results from the POLASPIRE survey. Pol. Arch. Intern. Med. 2022, 132, 16179. [Google Scholar] [CrossRef] [PubMed]
- Schorr, E.N.; Gepner, A.D.; Dolansky, M.A.; Forman, D.E.; Park, L.G.; Petersen, K.S.; Still, C.H.; Wang, T.Y.; Wenger, N.K. Harnessing Mobile Health Technology for Secondary Cardiovascular Disease Prevention in Older Adults: A Scientific Statement From the American Heart Association. Circ. Cardiovasc. Qual. Outcomes 2021, 14, e000103. [Google Scholar] [CrossRef]

| Variable | afterAMI | Control | p-Value | |
|---|---|---|---|---|
| Clinical data | ||||
| Age (years) | 56.8 ± 9.23 | 63.42 ± 11.4 | 0.0019 | |
| BMI (kg/m2) | 28.5 ± 4.06 | 28.11 ±5.38 | 0.7247 | |
| Body weight (kg) | 88.95 ± 13.86 | 85.47 ± 24.33 | 0.4625 | |
| Sex [1] | 34 (68%) | 31 (61%) | 0.6753 | |
| KOS—rehabilitation | 17 (34%) | 9 (18%) | 0.1095 | |
| Hospitalization (days) | 6 (4–8) | 7 (5–11) | 0.2143 | |
| STEMI | 25 (50%) | 20 (40%) | 0.4176 | |
| NSTEMI | 25 (50%) | 30 (60%) | 0.4176 | |
| Infarction artery | LAD | 26 (52%) | 24 (48%) | 1 |
| LCA | 15 (30%) | 17 (34%) | 0.5218 | |
| RCA | 16 (32%) | 24 (48%) | 0.1438 | |
| PTCA | 39 (78%) | 39 (78%) | 0.6222 | |
| Bypass surgery | 5 (10%) | 6 (12%) | 0.7589 | |
| Body weight (kg) | 88.9459 ± 13.8663 | 85.4657 ± 24.3327 | 0.4625 | |
| Height (cm) | 176.3 ± 7.2328 | 171.6 ± 8.9729 | 0.0186 | |
| Nicotinism | 33 (66%) | 32 (64%) | 1 | |
| Packet years | 20 (0–30) | 14 (0–32.5) | 0.7934 | |
| Diabetes, type I | 2 (4%) | 0 (0%) | 0.4949 | |
| Diabetes, type II | 11 (22%) | 11 (22%) | 1 | |
| Hypertension | 30 (60%) | 34 (68%) | 0.3828 | |
| Dyslipidemia | 36 (72%) | 39 (78%) | 0.3069 | |
| Atrial fibrillation/atrial flutter | 1 (2%) | 7 (14%) | 0.0288 | |
| Heart failure | 6 (12%) | 15 (30%) | 0.0274 | |
| Implanted pacemaker or ICD | 1 (2%) | 5 (10%) | 0.1112 | |
| Chronic kidney disease | 1 (2%) | 1 (2%) | 1 | |
| Peripheral artery disease | 1 (2%) | 1 (2%) | 1 | |
| EF in hospital (%) | 51.78 ± 8.42 | 48.0 ± 9.22 | 0.0394 | |
| CVD risk factors knowledge | 8 (6–9) | 8 (4–9) | 0.4131 | |
| Employed | 27 (54%) | 17 (34%) | 0.1261 | |
| Lab tests at hospital | ||||
| Troponin I (μg/L) | 0.7930 (0.2250–5.5710) | 0.694 (0.111–4.350) | 0.7248 | |
| Troponin II (μg/L) | 2.2550 (0.7145–8.7340) | 5.640 (0.437–34.635) | 0.1702 | |
| Creatinine (mg/dL) | 0.98 ± 0.21 | 1.05 ± 0.34 | 0.1991 | |
| eGFR (mL/(min × 1.72 m2))) | 79.16 ± 17.22 | 73.28 ± 20.93 | 0.1351 | |
| Na (mmol/L) | 139.1 ± 3.05 | 139.6 ± 4.36 | 0.5399 | |
| K (mmol/L) | 4.17 ± 0.45 | 4.38 ± 0.51 | 0.0363 | |
| WBC (×109/L) | 10.27 ± 3.04 | 10.19 ± 2.94 | 0.9052 | |
| HbA1C (%) | 5.8 (5.4–7.1) | 5.6 (5.4–6.0) | 0.4593 | |
| NTproBNP (pg/mL) | 422 (133–1256) | 886.5 (230–2250) | 0.0735 | |
| HgB (g/dL) | 14.58 ± 1.49 | 14.14 ± 1.83 | 0.1989 | |
| Total cholesterol (mg/dL) | 191.3 ± 71. 57 | 192.1 ± 52.29 | 0.9523 | |
| HDL (mg/dL) | 39.55 ± 10.02 | 46.78 ± 10.65 | 0.0010 | |
| LDL (mg/dL) | 117.5 ± 68.59 | 111.7 ± 61.56 | 0.6621 | |
| Tg (mg/dL) | 146 (92–233) | 136.5 (87–201) | 0.2423 | |
| Drugs at discharge | ||||
| ACEi | 42 (84%) | 40 (80%) | 0.5229 | |
| ARB | 4 (8%) | 2 (4%) | 0.2314 | |
| ARNI | 0 (0%) | 0 (0%) | ||
| MRA | 9 (18%) | 15 (30%) | 0.2366 | |
| B-blocker | 42 (84%) | 41 (82%) | 0.7398 | |
| CCB | 20 (40%) | 10 (20%) | 0.0257 | |
| Statin | 46 (92%) | 45 (90%) | 1 | |
| Ezetimibe | 5 (10%) | 2 (4%) | 0.2673 | |
| VKA | 0 (0%) | 0 (0%) | ||
| NOAC | 1 (2%) | 2 (4%) | 1 | |
| ASA | 45 (90%) | 43 (86%) | 1 | |
| Clopidogrel | 12 (24%) | 13 (26%) | 1 | |
| Prasugrel | 2 (4%) | 0 (0%) | 0.2419 | |
| Ticagrelor | 28 (56%) | 28 (56%) | 1 | |
| Digoxin | 0 (0%) | 0 (0%) | ||
| Endpoint | afterAMI | Control Group | p-Value |
|---|---|---|---|
| Creatinine (mg/dL) | 0.945 (0.84–1.26) | 0.95 (0.80–1.01) | 0.4510 |
| eGFR (mL/(min × 1.72 m2)) | 78.18 ± 17.11 | 69.77 ± 20.10 | 0.0940 |
| HbA1C (%) | 5.8 (5.5–7.7) | 5.7 (5.6–6.0) | 0.7491 |
| NTproBNP (pg/mL) | 119 (44–257) | 244 (130–696) | 0.0286 |
| HgB (g/dL) | 14.4 (13.3–14.9) | 13.85 (13.3–14.6) | 0.3587 |
| Total cholesterol (mg/dL) | 130 (114–145) | 134 (116–153) | 0.5112 |
| HDL (mg/dL) | 44 (39–54) | 46 (41–61) | 0.1990 |
| LDL (mg/dL) | 58 (45–75) | 64.5 (48.5–83.5) | 0.3226 |
| Tg (mg/dL) | 98 (71–181) | 100 (82.5–135) | 0.8800 |
| LDL difference vs. baseline | 38.4 ± 50.75 | 49.44 ± 64.51 | 0.4721 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krzowski, B.; Boszko, M.; Peller, M.; Hoffman, P.; Żurawska, N.; Skoczylas, K.; Osak, G.; Kołtowski, Ł.; Grabowski, M.; Opolski, G.; et al. Mobile App and Digital System for Patients after Myocardial Infarction (afterAMI): Results from a Randomized Trial. J. Clin. Med. 2023, 12, 2886. https://doi.org/10.3390/jcm12082886
Krzowski B, Boszko M, Peller M, Hoffman P, Żurawska N, Skoczylas K, Osak G, Kołtowski Ł, Grabowski M, Opolski G, et al. Mobile App and Digital System for Patients after Myocardial Infarction (afterAMI): Results from a Randomized Trial. Journal of Clinical Medicine. 2023; 12(8):2886. https://doi.org/10.3390/jcm12082886
Chicago/Turabian StyleKrzowski, Bartosz, Maria Boszko, Michał Peller, Paulina Hoffman, Natalia Żurawska, Kamila Skoczylas, Gabriela Osak, Łukasz Kołtowski, Marcin Grabowski, Grzegorz Opolski, and et al. 2023. "Mobile App and Digital System for Patients after Myocardial Infarction (afterAMI): Results from a Randomized Trial" Journal of Clinical Medicine 12, no. 8: 2886. https://doi.org/10.3390/jcm12082886
APA StyleKrzowski, B., Boszko, M., Peller, M., Hoffman, P., Żurawska, N., Skoczylas, K., Osak, G., Kołtowski, Ł., Grabowski, M., Opolski, G., & Balsam, P. (2023). Mobile App and Digital System for Patients after Myocardial Infarction (afterAMI): Results from a Randomized Trial. Journal of Clinical Medicine, 12(8), 2886. https://doi.org/10.3390/jcm12082886







