Role of Chemerin and Perivascular Adipose Tissue Characteristics on Cardiovascular Risk Assessment by Arterial Stiffness Markers in Patients with Morbid Obesity
Abstract
1. Introduction
Potential Biomarkers for Assessing Cardiovascular Risk in Morbid Obesity
2. Materials and Methods
2.1. Study Design and Population
2.2. Laboratory Measurements
2.3. Arterial Stiffness Evaluation
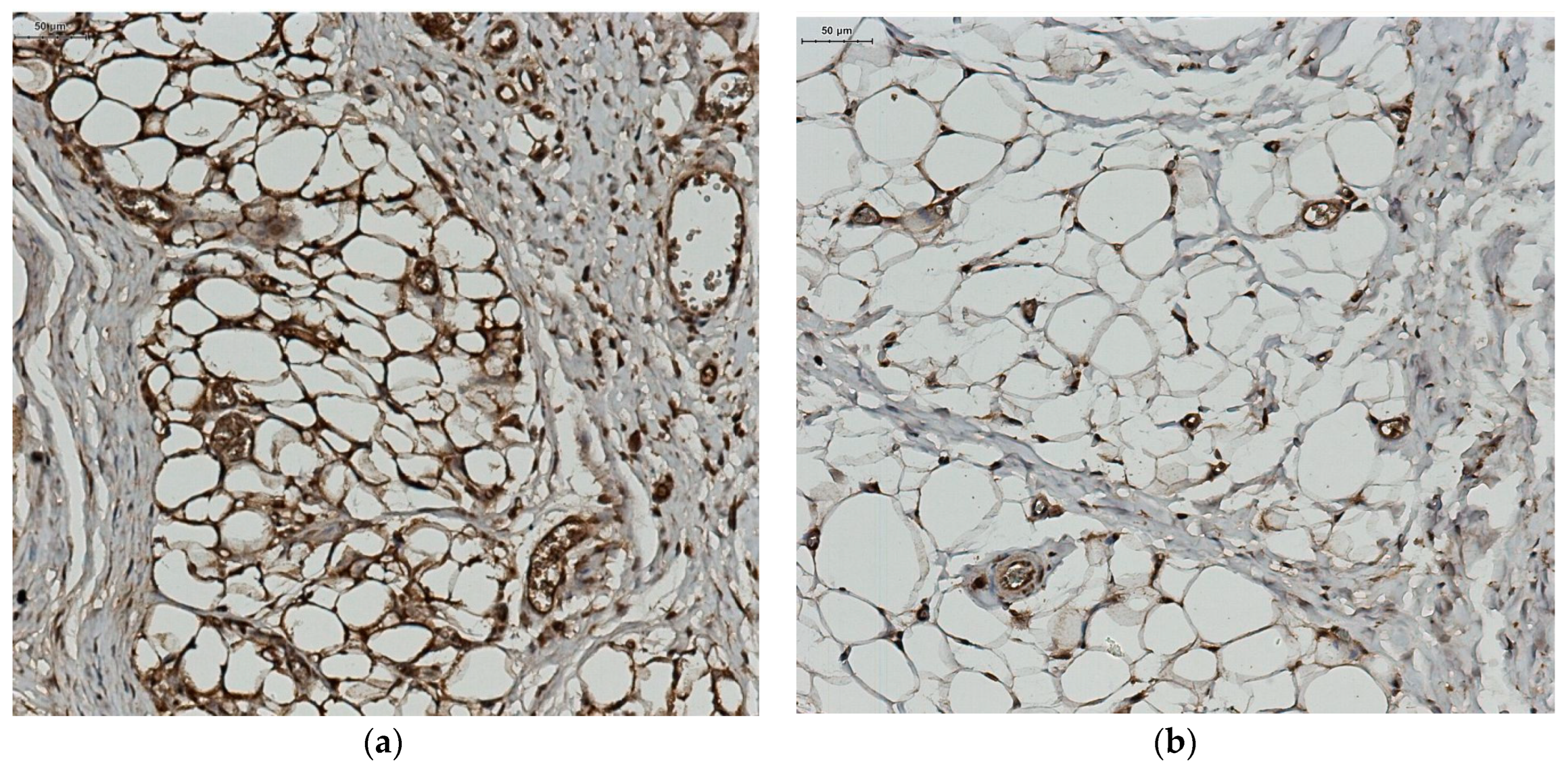
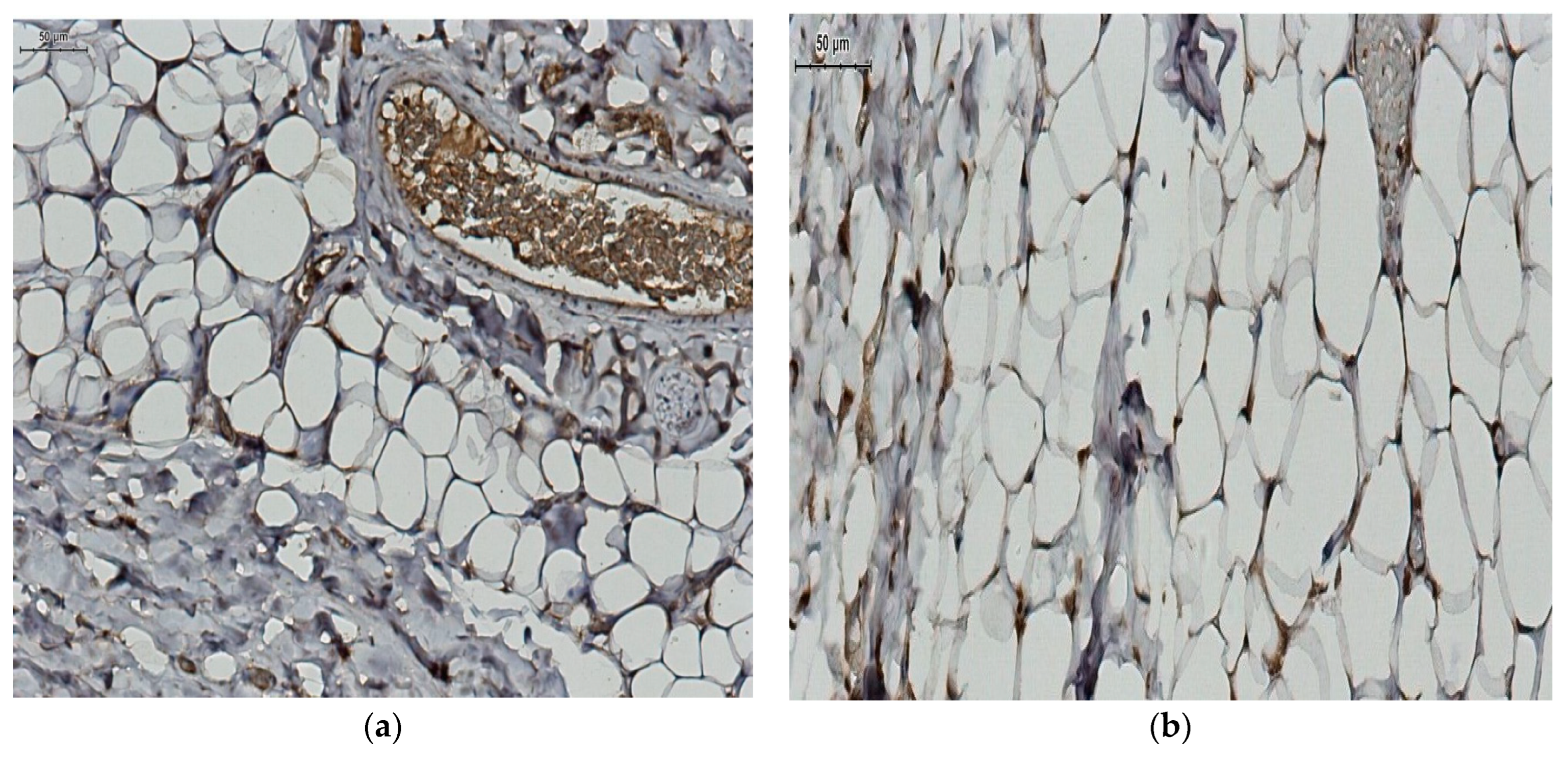
2.4. Local Adipocytes and Adiponectin Expression Evaluation
2.5. Statistical Analysis
2.6. Ethics
3. Results
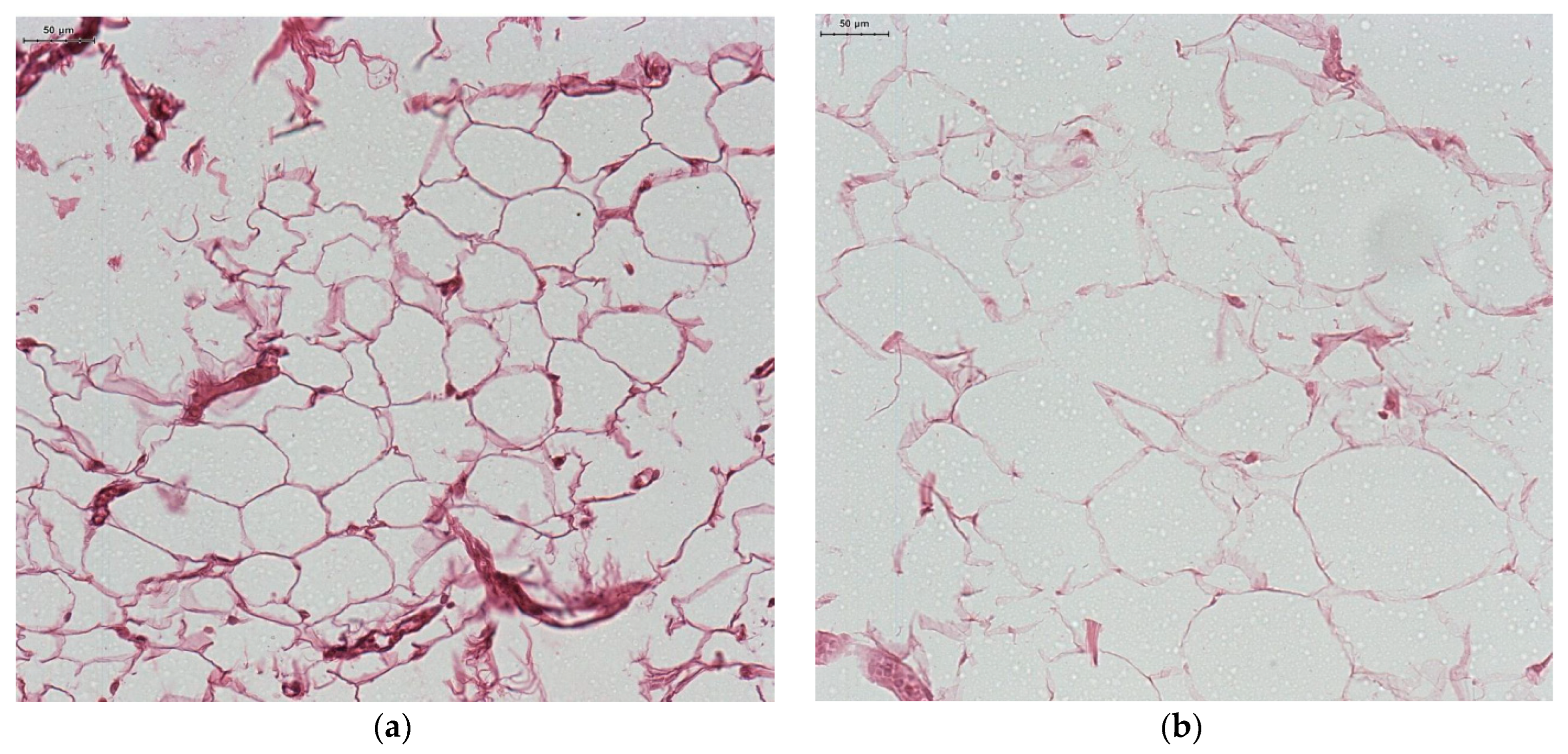
Histopathological Study
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GBD 2019 Diseases and Injuries Collaborators Global Burden of 369 Diseases and Injuries in 204 Countries and Territories, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet Lond. Engl. 2020, 396, 1204–1222. [CrossRef] [PubMed]
- Adam, C.A.; Șalaru, D.L.; Prisacariu, C.; Marcu, D.T.M.; Sascău, R.A.; Stătescu, C. Novel Biomarkers of Atherosclerotic Vascular Disease—Latest Insights in the Research Field. Int. J. Mol. Sci. 2022, 23, 4998. [Google Scholar] [CrossRef] [PubMed]
- Anghel, R.; Adam, C.; Marcu, D.; Mitu, O.; Mitu, F. Impact of Comorbidities on the Long-Term Prognosis of Patients with Intermittent Claudication. Intern. Med. Interna 2021, 18, 7–19. [Google Scholar] [CrossRef]
- Adam, C.A.; Anghel, R.; Marcu, D.T.M.; Mitu, O.; Roca, M.; Mitu, F. Impact of Sodium–Glucose Cotransporter 2 (SGLT2) Inhibitors on Arterial Stiffness and Vascular Aging—What Do We Know So Far? (A Narrative Review). Life 2022, 12, 803. [Google Scholar] [CrossRef] [PubMed]
- Macvanin, M.T.; Rizzo, M.; Radovanovic, J.; Sonmez, A.; Paneni, F.; Isenovic, E.R. Role of Chemerin in Cardiovascular Diseases. Biomedicines 2022, 10, 2970. [Google Scholar] [CrossRef]
- Anghel, R.; Adam, C.A.; Mitu, O.; Marcu, D.T.M.; Onofrei, V.; Roca, M.; Costache, A.D.; Miftode, R.S.; Tinica, G.; Mitu, F. Cardiac Rehabilitation and Mortality Risk Reduction in Peripheral Artery Disease at 6-Month Outcome. Diagnostics 2022, 12, 1500. [Google Scholar] [CrossRef]
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Després, J.-P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P.; et al. Obesity and Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation 2021, 143, e984–e1010. [Google Scholar] [CrossRef]
- Anghel, R.; Adam, C.A.; Marcu, D.T.M.; Mitu, O.; Roca, M.; Tinica, G.; Mitu, F. Cardiac Rehabilitation in Peripheral Artery Disease in a Tertiary Center—Impact on Arterial Stiffness and Functional Status after 6 Months. Life 2022, 12, 601. [Google Scholar] [CrossRef]
- Anghel, R.; Adam, C.A.; Marcu, D.T.M.; Mitu, O.; Mitu, F. Cardiac Rehabilitation in Patients with Peripheral Artery Disease—A Literature Review in COVID-19 Era. J. Clin. Med. 2022, 11, 416. [Google Scholar] [CrossRef]
- Rizvi, A.A.; Stoian, A.P.; Rizzo, M. Metabolic Syndrome: From Molecular Mechanisms to Novel Therapies. Int. J. Mol. Sci. 2021, 22, 10038. [Google Scholar] [CrossRef]
- Helfer, G.; Wu, Q.-F. Chemerin: A Multifaceted Adipokine Involved in Metabolic Disorders. J. Endocrinol. 2018, 238, R79–R94. [Google Scholar] [CrossRef] [PubMed]
- Karastergiou, K.; Mohamed-Ali, V. The Autocrine and Paracrine Roles of Adipokines. Mol. Cell. Endocrinol. 2010, 318, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Pardo, M.; Roca-Rivada, A.; Seoane, L.M.; Casanueva, F.F. Obesidomics: Contribution of Adipose Tissue Secretome Analysis to Obesity Research. Endocrine 2012, 41, 374–383. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Fuster, J.J.; Walsh, K. Adipokines: A Link between Obesity and Cardiovascular Disease. J. Cardiol. 2014, 63, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, A.A.; Nikolic, D.; Sallam, H.S.; Montalto, G.; Rizzo, M.; Abate, N. Adipokines and Lipoproteins: Modulation by Antihyperglycemic and Hypolipidemic Agents. Metab. Syndr. Relat. Disord. 2014, 12, 1–10. [Google Scholar] [CrossRef]
- Kirichenko, T.V.; Markina, Y.V.; Bogatyreva, A.I.; Tolstik, T.V.; Varaeva, Y.R.; Starodubova, A.V. The Role of Adipokines in Inflammatory Mechanisms of Obesity. Int. J. Mol. Sci. 2022, 23, 14982. [Google Scholar] [CrossRef]
- Hamjane, N.; Benyahya, F.; Nourouti, N.G.; Mechita, M.B.; Barakat, A. Cardiovascular Diseases and Metabolic Abnormalities Associated with Obesity: What Is the Role of Inflammatory Responses? A Systematic Review. Microvasc. Res. 2020, 131, 104023. [Google Scholar] [CrossRef]
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in Inflammation and Metabolic Disease. Nat. Rev. Immunol. 2011, 11, 85–97. [Google Scholar] [CrossRef]
- Rourke, J.L.; Dranse, H.J.; Sinal, C.J. Towards an Integrative Approach to Understanding the Role of Chemerin in Human Health and Disease. Obes. Rev. 2013, 14, 245–262. [Google Scholar] [CrossRef]
- Yan, Q.; Zhang, Y.; Hong, J.; Gu, W.; Dai, M.; Shi, J.; Zhai, Y.; Wang, W.; Li, X.; Ning, G. The Association of Serum Chemerin Level with Risk of Coronary Artery Disease in Chinese Adults. Endocrine 2012, 41, 281–288. [Google Scholar] [CrossRef]
- Dong, B.; Ji, W.; Zhang, Y. Elevated Serum Chemerin Levels Are Associated with the Presence of Coronary Artery Disease in Patients with Metabolic Syndrome. Intern. Med. Tokyo Jpn. 2011, 50, 1093–1097. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Kou, W.; Ji, S.; Shen, R.; Ji, H.; Zhuang, J.; Zhao, Y.; Li, B.; Peng, W.; Yu, X.; et al. Prognostic Value of Plasma Adipokine Chemerin in Patients with Coronary Artery Disease. Front. Cardiovasc. Med. 2022, 9, 968349. [Google Scholar] [CrossRef] [PubMed]
- Spiroglou, S.G.; Kostopoulos, C.G.; Varakis, J.N.; Papadaki, H.H. Adipokines in Periaortic and Epicardial Adipose Tissue: Differential Expression and Relation to Atherosclerosis. J. Atheroscler. Thromb. 2010, 17, 115–130. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.J.; Choi, H.Y.; Yang, S.J.; Kim, H.Y.; Seo, J.A.; Kim, S.G.; Kim, N.H.; Choi, K.M.; Choi, D.S.; Baik, S.H. Circulating Chemerin Level Is Independently Correlated with Arterial Stiffness. J. Atheroscler. Thromb. 2012, 19, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Molica, F.; Morel, S.; Kwak, B.R.; Rohner-Jeanrenaud, F.; Steffens, S. Adipokines at the Crossroad between Obesity and Cardiovascular Disease. Thromb. Haemost. 2015, 113, 553–566. [Google Scholar] [CrossRef]
- Fontes, V.S.; Neves, F.S.; Cândido, A.P.C. Quemerina e fatores relacionados ao risco cardiovascular em crianças e adolescentes: Uma revisão sistemática. Rev. Paul. Pediatr. 2018, 36, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Aroor, A.R.; Jia, G.; Sowers, J.R. Cellular Mechanisms Underlying Obesity-Induced Arterial Stiffness. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 314, R387–R398. [Google Scholar] [CrossRef]
- Fuster, J.J.; Ouchi, N.; Gokce, N.; Walsh, K. Obesity-Induced Changes in Adipose Tissue Microenvironment and Their Impact on Cardiovascular Disease. Circ. Res. 2016, 118, 1786–1807. [Google Scholar] [CrossRef]
- Fleenor, B.S.; Carlini, N.A.; Ouyang, A.; Harber, M.P. Perivascular Adipose Tissue-Mediated Arterial Stiffening in Aging and Disease: An Emerging Translational Therapeutic Target? Pharmacol. Res. 2022, 178, 106150. [Google Scholar] [CrossRef]
- DeVallance, E.; Branyan, K.W.; Lemaster, K.C.; Anderson, R.; Marshall, K.L.; Olfert, I.M.; Smith, D.M.; Kelley, E.E.; Bryner, R.W.; Frisbee, J.C.; et al. Exercise Training Prevents the Perivascular Adipose Tissue-Induced Aortic Dysfunction with Metabolic Syndrome. Redox Biol. 2019, 26, 101285. [Google Scholar] [CrossRef]
- Ouyang, A.; Garner, T.B.; Fleenor, B.S. Hesperidin Reverses Perivascular Adipose-Mediated Aortic Stiffness with Aging. Exp. Gerontol. 2017, 97, 68–72. [Google Scholar] [CrossRef]
- Ben-Shlomo, Y.; Spears, M.; Boustred, C.; May, M.; Anderson, S.G.; Benjamin, E.J.; Boutouyrie, P.; Cameron, J.; Chen, C.-H.; Cruickshank, J.K.; et al. Aortic Pulse Wave Velocity Improves Cardiovascular Event Prediction. J. Am. Coll. Cardiol. 2014, 63, 636–646. [Google Scholar] [CrossRef] [PubMed]
- Rochlani, Y.; Pothineni, N.V.; Kovelamudi, S.; Mehta, J.L. Metabolic Syndrome: Pathophysiology, Management, and Modulation by Natural Compounds. Ther. Adv. Cardiovasc. Dis. 2017, 11, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Aursulesei, V.; Timofte, D.; Tarau, L.M.; Mocanu, V.; Namat, R.A.; Aursulesei, V.C.; Costache, I.I. Circulating Chemerin Levels, Anthropometric Indices and Metabolic Profile in Morbid Obesity. Rev. Chim. 2018, 69, 1419–1423. [Google Scholar] [CrossRef]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the Concentration of Low-Density Lipoprotein Cholesterol in Plasma, without Use of the Preparative Ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Gutch, M.; Kumar, S.; Razi, S.; Gupta, K.; Gupta, A. Assessment of Insulin Sensitivity/Resistance. Indian J. Endocrinol. Metab. 2015, 19, 160. [Google Scholar] [CrossRef] [PubMed]
- Mattern, A.; Zellmann, T.; Beck-Sickinger, A.G. Processing, Signaling, and Physiological Function of Chemerin. IUBMB Life 2014, 66, 19–26. [Google Scholar] [CrossRef]
- Van Bortel, L.M.; Laurent, S.; Boutouyrie, P.; Chowienczyk, P.; Cruickshank, J.K.; De Backer, T.; Filipovsky, J.; Huybrechts, S.; Mattace-Raso, F.U.S.; Protogerou, A.D.; et al. Expert Consensus Document on the Measurement of Aortic Stiffness in Daily Practice Using Carotid-Femoral Pulse Wave Velocity. J. Hypertens. 2012, 30, 445–448. [Google Scholar] [CrossRef]
- Li, J.; Zhu, J.; Tan, Z.; Yu, Y.; Luo, L.; Zhou, W.; Zhu, L.; Wang, T.; Cao, T.; Liu, L.; et al. Visceral Adiposity Index Is Associated with Arterial Stiffness in Hypertensive Adults with Normal-Weight: The China H-Type Hypertension Registry Study. Nutr. Metab. 2021, 18, 90. [Google Scholar] [CrossRef]
- Kim, H.-L.; Ahn, D.-W.; Kim, S.H.; Lee, D.S.; Yoon, S.H.; Zo, J.-H.; Kim, M.-A.; Jeong, J.B. Association between Body Fat Parameters and Arterial Stiffness. Sci. Rep. 2021, 11, 20536. [Google Scholar] [CrossRef]
- Salvi, P.; Baldi, C.; Scalise, F.; Grillo, A.; Salvi, L.; Tan, I.; De Censi, L.; Sorropago, A.; Moretti, F.; Sorropago, G.; et al. Comparison Between Invasive and Noninvasive Methods to Estimate Subendocardial Oxygen Supply and Demand Imbalance. J. Am. Heart Assoc. 2021, 10, e021207. [Google Scholar] [CrossRef]
- Hoffman, J.I.E.; Buckberg, G.D. The Myocardial Oxygen Supply:Demand Index Revisited. J. Am. Heart Assoc. 2014, 3, e000285. [Google Scholar] [CrossRef]
- Tocci, N.D.; Collier, S.R.; Meucci, M. Measures of ejection duration and subendocardial viability ratio in normal weight and overweight adolescent children. Physiol. Rep. 2021, 9, e14852. [Google Scholar] [CrossRef] [PubMed]
- Fantin, F.; Giani, A.; Gasparini, L.; Rossi, A.P.; Zoico, E.; Mazzali, G.; Zamboni, M. Impaired subendocardial perfusion in patients with metabolic syndrome. Diabetes Vasc. Dis. Res. 2021, 18, 14791641211047135. [Google Scholar] [CrossRef]
- Khoshdel, A.R.; Eshtiaghi, R. Assessment of Arterial Stiffness in Metabolic Syndrome Related to Insulin Resistance in Apparently Healthy Men. Metab. Syndr. Relat. Disord. 2019, 17, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, R.P.; Copenhaver, M.M.; Zhou, D.; Yu, C.Y. Increased body fat and reduced insulin sensitivity are associated with impaired endothelial function and subendocardial viability in healthy, non-Hispanic white adolescents. Pediatr. Diab. 2019, 20, 842–848. [Google Scholar] [CrossRef] [PubMed]
- Gu, P.; Cheng, M.; Hui, X.; Lu, B.; Jiang, W.; Shi, Z. Elevating Circulation Chemerin Level Is Associated with Endothelial Dysfunction and Early Atherosclerotic Changes in Essential Hypertensive Patients. J. Hypertens. 2015, 33, 1624–1632. [Google Scholar] [CrossRef]
- Lu, B.; Zhao, M.; Jiang, W.; Ma, J.; Yang, C.; Shao, J.; Gu, P. Independent Association of Circulating Level of Chemerin With Functional and Early Morphological Vascular Changes in Newly Diagnosed Type 2 Diabetic Patients. Medicine 2015, 94, e1990. [Google Scholar] [CrossRef]
- Hu, W.; Zhang, H.; Liu, Z.; Duan, Q.; Liu, J.; Dong, Q.; You, L.; Wen, X.; Zhang, D. Relationship between Adipose Tissue Distribution and Arterial Stiffness in HFpEF. Nutrition 2022, 102, 111726. [Google Scholar] [CrossRef]
- Para, I.; Albu, A.; Porojan, M.D. Adipokines and Arterial Stiffness in Obesity. Medicina 2021, 57, 653. [Google Scholar] [CrossRef]
- Rodríguez, A.; Ezquerro, S.; Méndez-Giménez, L.; Becerril, S.; Frühbeck, G. Revisiting the Adipocyte: A Model for Integration of Cytokine Signaling in the Regulation of Energy Metabolism. Am. J. Physiol. Endocrinol. Metab. 2015, 309, E691–E714. [Google Scholar] [CrossRef] [PubMed]
- Arner, P.; Bäckdahl, J.; Hemmingsson, P.; Stenvinkel, P.; Eriksson-Hogling, D.; Näslund, E.; Thorell, A.; Andersson, D.P.; Caidahl, K.; Rydén, M. Regional Variations in the Relationship between Arterial Stiffness and Adipocyte Volume or Number in Obese Subjects. Int. J. Obes. 2005 2015, 39, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Bäckdahl, J.; Andersson, D.P.; Eriksson-Hogling, D.; Caidahl, K.; Thorell, A.; Mileti, E.; Daub, C.O.; Arner, P.; Rydén, M. Long-Term Improvement in Aortic Pulse Wave Velocity After Weight Loss Can Be Predicted by White Adipose Tissue Factors. Am. J. Hypertens. 2018, 31, 450–457. [Google Scholar] [CrossRef]
- Cecelja, M.; Jiang, B.; Keehn, L.; Hussain, T.; Silva Vieira, M.; Phinikaridou, A.; Greil, G.; Spector, T.D.; Chowienczyk, P. Arterial Stiffening Is a Heritable Trait Associated with Arterial Dilation but Not Wall Thickening: A Longitudinal Study in the Twins UK Cohort. Eur. Heart J. 2018, 39, 2282–2288. [Google Scholar] [CrossRef] [PubMed]
- Sabbatini, A.R.; Fontana, V.; Laurent, S.; Moreno, H. An Update on the Role of Adipokines in Arterial Stiffness and Hypertension. J. Hypertens. 2015, 33, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Siegel-Axel, D.I.; Häring, H.U. Perivascular Adipose Tissue: An Unique Fat Compartment Relevant for the Cardiometabolic Syndrome. Rev. Endocr. Metab. Disord. 2016, 17, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Greenstein, A.S.; Khavandi, K.; Withers, S.B.; Sonoyama, K.; Clancy, O.; Jeziorska, M.; Laing, I.; Yates, A.P.; Pemberton, P.W.; Malik, R.A.; et al. Local Inflammation and Hypoxia Abolish the Protective Anticontractile Properties of Perivascular Fat in Obese Patients. Circulation 2009, 119, 1661–1670. [Google Scholar] [CrossRef]
- Lim, S.; Meigs, J.B. Links between Ectopic Fat and Vascular Disease in Humans. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1820–1826. [Google Scholar] [CrossRef]
- Brown, N.K.; Zhou, Z.; Zhang, J.; Zeng, R.; Wu, J.; Eitzman, D.T.; Chen, Y.E.; Chang, L. Perivascular Adipose Tissue in Vascular Function and Disease: A Review of Current Research and Animal Models. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1621–1630. [Google Scholar] [CrossRef]
- Nosalski, R.; Guzik, T.J. Perivascular Adipose Tissue Inflammation in Vascular Disease. Br. J. Pharmacol. 2017, 174, 3496–3513. [Google Scholar] [CrossRef]
- Jia, G.; Aroor, A.R.; DeMarco, V.G.; Martinez-Lemus, L.A.; Meininger, G.A.; Sowers, J.R. Vascular Stiffness in Insulin Resistance and Obesity. Front. Physiol. 2015, 6, 231. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Hernández, A.; Beneit, N.; Díaz-Castroverde, S.; Escribano, Ó. Differential Role of Adipose Tissues in Obesity and Related Metabolic and Vascular Complications. Int. J. Endocrinol. 2016, 2016, 1216783. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.J.; Choi, K.M. Adipokines as a Novel Link between Obesity and Atherosclerosis. World J. Diabetes 2014, 5, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Ntaios, G.; Gatselis, N.K.; Makaritsis, K.; Dalekos, G.N. Adipokines as Mediators of Endothelial Function and Atherosclerosis. Atherosclerosis 2013, 227, 216–221. [Google Scholar] [CrossRef]
- Hajjar, D.P.; Gotto, A.M. Biological Relevance of Inflammation and Oxidative Stress in the Pathogenesis of Arterial Diseases. Am. J. Pathol. 2013, 182, 1474–1481. [Google Scholar] [CrossRef]
- Virdis, A.; Duranti, E.; Rossi, C.; Dell’Agnello, U.; Santini, E.; Anselmino, M.; Chiarugi, M.; Taddei, S.; Solini, A. Tumour Necrosis Factor-Alpha Participates on the Endothelin-1/Nitric Oxide Imbalance in Small Arteries from Obese Patients: Role of Perivascular Adipose Tissue. Eur. Heart J. 2015, 36, 784–794. [Google Scholar] [CrossRef]
- Lindberg, S.; Jensen, J.S.; Bjerre, M.; Pedersen, S.H.; Frystyk, J.; Flyvbjerg, A.; Galatius, S.; Jeppesen, J.; Mogelvang, R. Adiponectin, type 2 diabetes and cardiovascular risk. Eur. J. Prev. Cardiol. 2015, 22, 276–283. [Google Scholar] [CrossRef]
- Ouchi, N.; Shibata, R.; Walsh, K. Cardioprotection by Adiponectin. Trends Cardiovasc. Med. 2006, 16, 141–146. [Google Scholar] [CrossRef]
- Barandier, C.; Montani, J.-P.; Yang, Z. Mature Adipocytes and Perivascular Adipose Tissue Stimulate Vascular Smooth Muscle Cell Proliferation: Effects of Aging and Obesity. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H1807–H1813. [Google Scholar] [CrossRef]
- Sommer, G.; Kralisch, S.; Stangl, V.; Vietzke, A.; Köhler, U.; Stepan, H.; Faber, R.; Schubert, A.; Lössner, U.; Bluher, M.; et al. Secretory Products from Human Adipocytes Stimulate Proinflammatory Cytokine Secretion from Human Endothelial Cells. J. Cell. Biochem. 2009, 106, 729–737. [Google Scholar] [CrossRef]
- Turer, A.T.; Khera, A.; Ayers, C.R.; Turer, C.B.; Grundy, S.M.; Vega, G.L.; Scherer, P.E. Adipose Tissue Mass and Location Affect Circulating Adiponectin Levels. Diabetologia 2011, 54, 2515–2524. [Google Scholar] [CrossRef] [PubMed]
- Galic, S.; Oakhill, J.S.; Steinberg, G.R. Adipose Tissue as an Endocrine Organ. Mol. Cell. Endocrinol. 2010, 316, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Youn, J.-C.; Kim, C.; Park, S.; Lee, S.-H.; Kang, S.-M.; Choi, D.; Son, N.H.; Shin, D.-J.; Jang, Y. Adiponectin and Progression of Arterial Stiffness in Hypertensive Patients. Int. J. Cardiol. 2013, 163, 316–319. [Google Scholar] [CrossRef]
- Chen, M.-C.; Lee, C.-J.; Yang, C.-F.; Chen, Y.-C.; Wang, J.-H.; Hsu, B.-G. Low Serum Adiponectin Level Is Associated with Metabolic Syndrome and Is an Independent Marker of Peripheral Arterial Stiffness in Hypertensive Patients. Diabetol. Metab. Syndr. 2017, 9, 49. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, T.; Nio, Y.; Maki, T.; Kobayashi, M.; Takazawa, T.; Iwabu, M.; Okada-Iwabu, M.; Kawamoto, S.; Kubota, N.; Kubota, T.; et al. Targeted Disruption of AdipoR1 and AdipoR2 Causes Abrogation of Adiponectin Binding and Metabolic Actions. Nat. Med. 2007, 13, 332–339. [Google Scholar] [CrossRef]

| Non-Obese Patients (n = 25) | Obese Patients (n = 25) | p | |
|---|---|---|---|
| Demographics and anthropometric parameters | |||
| Age (y) | 43.36 ± 13.9 | 39.24 ± 8.74 | 0.021 |
| Female sex (%) | 17 (68%) | 21 (84%) | - |
| BMI (kg/m2) | 24.24 ± 3.15 | 43.9 ± 6.07 | 0.0001 |
| Waist circumference (cm) | 83.04 ± 8.75 | 125.5 ± 18.68 | 0.0001 |
| WHR | 0.83 ± 0.08 | 0.96 ± 0.10 | 0.0001 |
| Index of central obesity | 0.50 ± 0.06 | 0.75 ± 0.08 | 0.0001 |
| Systolic blood pressure (mmHg) | 118.04 ± 11.72 | 129.36 ± 13.03 | 0.002 |
| Diastolic blood pressure (mmHg) | 67.08 ± 7.89 | 75.28 ± 11.12 | 0.004 |
| Pulse pressure (mmHg) | 51.32 ± 9.98 | 59.08 ± 11.28 | 0.013 |
| Biological parameters | |||
| Total cholesterol (mg/dL) | 197.80 ± 41.39 | 201.4 ± 27.17 | 0.718 |
| HDL-cholesterol (mg/dL) | 50.36 ± 14.94 | 50 ± 9.98 | 0.92 |
| LDL-cholesteol (mg/dL) | 125.04 ± 39.97 | 127.68 ± 23.48 | 0.76 |
| VLDL-cholesterol (mg/dL) | 24.50 ± 10.13 | 24.86 ± 14.39 | 0.919 |
| Triglycerides (mg/dL) | 121.24 ± 25.74 | 124.32 ± 17.96 | 0.86 |
| Fasting glucose (mg/dL) | 88.32 ± 8.80 | 99.28 ± 14.62 | 0.002 |
| Insulinemia (µU/mL) | 8.23 ± 7.98 | 24.47 ± 6.16 | 0.0004 |
| Insulin sensitivity index * | 1.82 ± 1.87 | 6.45 ± 3.73 | 0.0001 |
| Insulin resistance (HOMA) (M/mU/L) | 0.16 ± 0.12 | 0.13 ± 0.02 | 0.001 |
| Uric acid (mg/dL) | 5.29 ± 1.48 | 6.79 ± 2.19 | 0.006 |
| TNF-ɑ (pg/mL) | 11.34 ± 11.42 | 7.49 ± 3.38 | 0.116 |
| Adiponectine (ng/mL) | 16.36 ± 1.49 | 18.05 ±1.155 | 0.0003 |
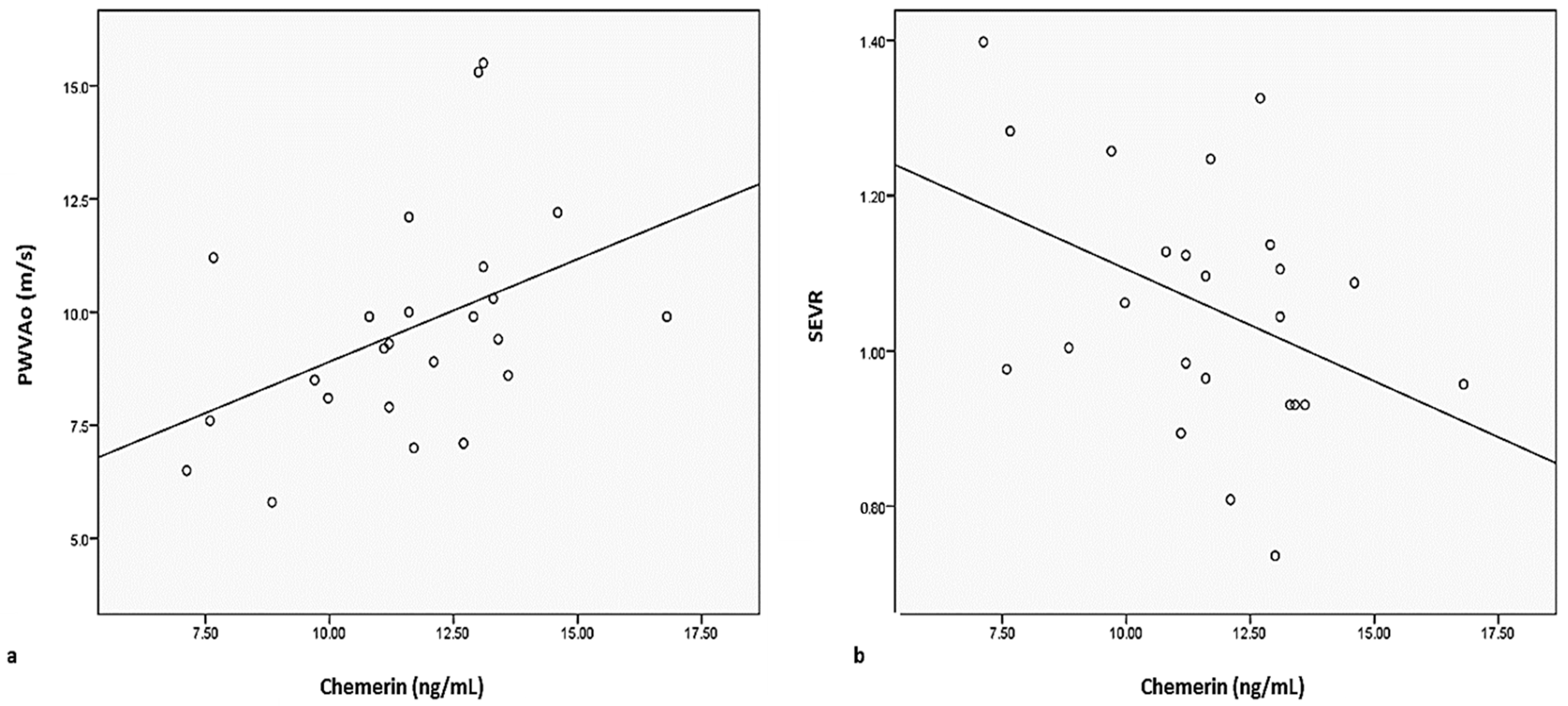
| Chemerin (ng/mL) | 9.10 ± 1.89 | 12.22 ± 3.80 | 0.0001 |
| Adiponectin/chemerin ratio | 0.55 ± 0.12 | 0.67 ± 0.18 | 0.005 |
| Arterial stiffness parameters | |||
| Aortic Aix (%) | 35.1 ± 16.2 | 24.1 ± 12.1 | 0.090 |
| Brachial Aix (%) | −5.5 ± 0.32 | −26.7 ± 0.24 | 0.110 |
| Aortic SBP (mmHg) | 119.42 ± 20.18 | 128.74 ± 20.81 | 0.114 |
| Aortic PP (mmHg) | 50.66 ± 12.69 | 52.26 ± 10.76 | 0.633 |
| DRA | 45.32 ± 18.82 | 49.68 ± 11.38 | 0.321 |
| SAI (%) | 46.41 ± 6.36 | 48.82 ± 3.81 | 0.112 |
| DAI (%) | 53.61 ± 6.06 | 51.28 ± 3.80 | 0.111 |
| SEVR | 1.19 ± 0.28 | 1.06 ± 0.16 | 0.054 |
| PWVAo (m/s) | 8.92 ± 2.14 | 9.59 ± 2.38 | 0.305 |
| Cardiovascular risk factors | |||
| Smoking | 10 (40.0%) | 9 (36.0%) | - |
| Fasting glucose above 100 mg/dL | - | 2 (8.0%) | - |
| Dyslipidemia | 9 (36.0%) | 11 (44.0%) | - |
| Perivascular adipose tissue parameters | |||
| Adipocyte size (µm) | 6.62 ± 1.78 | 9.34 ± 2.11 | 0.027 |
| Blood vessel wall thickness (µm) | 6.92 ± 1.48 | 8.79 ± 2.12 | 0.0001 |
| Non-Obese Patients | Patients with Morbid Obesity | |||||||
|---|---|---|---|---|---|---|---|---|
| Parameters | Adipocyte Size | Blood Vessel Wall Thickness | Adipocyte Size | Blood Vessel Wall Thickness | ||||
| r | p | r | p | r | p | r | p | |
| Biochemistry | ||||||||
| Adiponectin | −0.220 | 0.123 | −0.037 | 0.797 | 0.017 | 0.907 | 0.067 | 0.640 |
| Chemerin | 0.110 | 0.441 | −0.189 | 0.190 | −0.066 | 0.655 | 0.179 | 0.224 |
| Chemerin/Adiponectin ratio | 0.160 | 0.262 | −0.124 | 0.387 | −0.047 | 0.747 | 0.174 | 0.234 |
| TNF-α | 0.057 | 0.691 | −0.357 | 0.013 | 0.074 | 0.607 | 0.104 | 0.469 |
| Serum fibrinogen | −0.073 | 0.607 | 0.030 | 0.833 | 0.117 | 0.413 | −0.094 | 0.513 |
| HOMA | −0.093 | 0.513 | −0.165 | 0.252 | 0.060 | 0.674 | 0.237 | 0.097 |
| Insulinemia | −0.127 | 0.375 | −0.171 | 0.233 | 0.043 | 0.761 | 0.193 | 0.176 |
| Insulin sensitivity index | 0.093 | 0.513 | 0.165 | 0.252 | −0.060 | 0.674 | −0.237 | 0.097 |
| Fasting glucose | −0.034 | 0.815 | 0.287 | 0.049 | −0.017 | 0.907 | 0.304 | 0.035 |
| Total cholesterol | −0.144 | 0.427 | 0.185 | 0.198 | 0.181 | 0.207 | −0.050 | 0.726 |
| LDL-cholesterol | 0.010 | 0.944 | 0.182 | 0.206 | −0.044 | 0.761 | 0.007 | 0.963 |
| HDL-cholesterol | −0.081 | 0.574 | 0.027 | 0.851 | −0.176 | 0.223 | 0.010 | 0.944 |
| VLDL-cholesterol | −0.037 | 0.797 | 0.114 | 0.426 | 0.482 | 0.001 | −0.017 | 0.907 |
| Triglycerides | 0.003 | 0.981 | 0.101 | 0.483 | 0.482 | 0.001 | −0.017 | 0.907 |
| Uric acid | 0.071 | 0.623 | −0.082 | 0.574 | −0.047 | 0.744 | 0.003 | 0.981 |
| Serum creatinine | 0.081 | 0.574 | 0.038 | 0.796 | 0.125 | 0.387 | 0.222 | 0.123 |
| Hemodynamic parameters | ||||||||
| Systolic blood pressure | 0.030 | 0.833 | 0.116 | 0.426 | −0.269 | 0.061 | 0.239 | 0.097 |
| Diastolic blood pressure | 0.013 | 0.925 | 0.051 | 0.725 | −0.212 | 0.141 | 0.183 | 0.206 |
| Aortic pulse pressure | −0.088 | 0.543 | 0.126 | 0.386 | −0.132 | 0.361 | 0.183 | 0.206 |
| Mean blood pressure | 0.020 | 0.888 | 0.132 | 0.361 | −0.266 | 0.064 | 0.162 | 0.261 |
| Demographics and anthropometric parameters | ||||||||
| Body mass index | 0.153 | 0.283 | −0.097 | 0.498 | −0.124 | 0.387 | 0.093 | 0.513 |
| Abdominal circumference | 0.181 | 0.214 | −0.124 | 0.398 | 0.003 | 0.981 | 0.203 | 0.160 |
| Waist to hip ratio | −0.060 | 0.674 | −0.138 | 0.337 | 0.170 | 0.233 | 0.193 | 0.176 |
| Central obesity index | 0.213 | 0.135 | −0.252 | 0.079 | −0.017 | 0.907 | 0.247 | 0.084 |
| Age | −0.115 | 0.426 | 0.337 | 0.020 | −0.249 | 0.087 | 0.061 | 0.673 |
| Arterial stiffness parameters | ||||||||
| AIx brachial | −0.013 | 0.926 | 0.326 | 0.023 | −0.043 | 0.761 | 0.180 | 0.207 |
| SBP aortic | 0.100 | 0.483 | 0.185 | 0.198 | −0.311 | 0.030 | 0.220 | 0.123 |
| PP aortic | −0.033 | 0.815 | 0.216 | 0.134 | −0.281 | 0.050 | 0.230 | 0.107 |
| AIx aortic | −0.033 | 0.515 | 0.326 | 0.023 | −0.043 | 0.761 | 0.180 | 0.207 |
| DRA | 0.037 | 0.797 | −0.292 | 0.044 | 0.261 | 0.071 | −0.068 | 0.639 |
| SAI | 0.03 | 0.761 | 0.054 | 0.708 | −0.242 | 0.092 | 0.117 | 0.413 |
| DAI | −0.050 | 0.726 | −0.094 | 0.512 | 0.259 | 0.072 | −0.141 | 0.326 |
| SEVR | −0.070 | 0.624 | −0.067 | 0.640 | 0.248 | 0.084 | −0.124 | 0.387 |
| PWVAo | 0.003 | 0.981 | 0.175 | 0.224 | −0.151 | 0.293 | 0.054 | 0.708 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Onofrei, V.A.; Anisie, E.; Zamfir, C.L.; Ceasovschih, A.; Constantin, M.; Mitu, F.; Grigorescu, E.-D.; Petroaie, A.D.; Timofte, D.V. Role of Chemerin and Perivascular Adipose Tissue Characteristics on Cardiovascular Risk Assessment by Arterial Stiffness Markers in Patients with Morbid Obesity. J. Clin. Med. 2023, 12, 2885. https://doi.org/10.3390/jcm12082885
Onofrei VA, Anisie E, Zamfir CL, Ceasovschih A, Constantin M, Mitu F, Grigorescu E-D, Petroaie AD, Timofte DV. Role of Chemerin and Perivascular Adipose Tissue Characteristics on Cardiovascular Risk Assessment by Arterial Stiffness Markers in Patients with Morbid Obesity. Journal of Clinical Medicine. 2023; 12(8):2885. https://doi.org/10.3390/jcm12082885
Chicago/Turabian StyleOnofrei, Viviana Aursulesei, Ecaterina Anisie, Carmen Lacramioara Zamfir, Alexandr Ceasovschih, Mihai Constantin, Florin Mitu, Elena-Daniela Grigorescu, Antoneta Dacia Petroaie, and Daniel Vasile Timofte. 2023. "Role of Chemerin and Perivascular Adipose Tissue Characteristics on Cardiovascular Risk Assessment by Arterial Stiffness Markers in Patients with Morbid Obesity" Journal of Clinical Medicine 12, no. 8: 2885. https://doi.org/10.3390/jcm12082885
APA StyleOnofrei, V. A., Anisie, E., Zamfir, C. L., Ceasovschih, A., Constantin, M., Mitu, F., Grigorescu, E.-D., Petroaie, A. D., & Timofte, D. V. (2023). Role of Chemerin and Perivascular Adipose Tissue Characteristics on Cardiovascular Risk Assessment by Arterial Stiffness Markers in Patients with Morbid Obesity. Journal of Clinical Medicine, 12(8), 2885. https://doi.org/10.3390/jcm12082885









