Maternal Preconception Body Mass Index Overtakes Age as a Risk Factor for Gestational Diabetes Mellitus
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Statistical Analysis
3. Results
3.1. Characteristics of Pregnant Women and Risk Factors for Gestational Diabetes Mellitus (GDM)
3.2. Effect of Maternal Preconception Body Mass Index and Age on GDM Risk
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chiefari, E.; Arcidiacono, B.; Foti, D.; Brunetti, A. Gestational diabetes mellitus: An updated overview. J. Endocrinol. Investig. 2017, 40, 899–909. [Google Scholar] [CrossRef] [PubMed]
- Mirabelli, M.; Chiefari, E.; Tocci, V.; Greco, E.; Foti, D.; Brunetti, A. Gestational diabetes: Implications for fetal growth, intervention timing, and treatment options. Curr. Opin. Pharmacol. 2021, 60, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Wei, T.; Ni, W.; Zhang, A.; Zhang, J.; Xing, Y.; Xing, Q. Incidence and Risk Factors of Gestational Diabetes Mellitus: A Prospective Cohort Study in Qingdao, China. Front. Endocrinol. 2020, 11, 636. [Google Scholar] [CrossRef] [PubMed]
- International Diabetes Federation. IDF Atlas, 10th ed.; 2021; Available online: https://diabetesatlas.org/atlas/tenth-edition/ (accessed on 11 March 2023).
- Capula, C.; Chiefari, E.; Vero, A.; Iiritano, S.; Arcidiacono, B.; Puccio, L.; Pullano, V.; Foti, D.; Brunetti, A.; Vero, R. Predictors of postpartum glucose tolerance testing in italian women with gestational diabetes mellitus. ISRN Endocrinol. 2013, 2013, 182505. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ren, X.; He, L.; Li, J.; Zhang, S.; Chen, W. Maternal age and the risk of gestational diabetes mellitus: A systematic review and meta-analysis of over 120 million participants. Diabetes Res. Clin. Pract. 2020, 162, 108044. [Google Scholar] [CrossRef]
- Chia, C.W.; Egan, J.M.; Ferrucci, L. Age-Related Changes in Glucose Metabolism, Hyperglycemia, and Cardiovascular Risk. Circ. Res. 2018, 123, 886–904. [Google Scholar] [CrossRef]
- Ferrannini, E.; Vichi, S.; Beck-Nielsen, H.; Laakso, M.; Paolisso, G.; Smith, U. Insulin action and age. European Group for the Study of Insulin Resistance (EGIR). Diabetes 1996, 45, 947–953. [Google Scholar] [CrossRef]
- Arcidiacono, B.; Chiefari, E.; Foryst-Ludwig, A.; Currò, G.; Navarra, G.; Brunetti, F.S.; Mirabelli, M.; Corigliano, D.M.; Kintscher, U.; Britti, D.; et al. Obesity-related hypoxia via miR-128 decreases insulin-receptor expression in human and mouse adipose tissue promoting systemic insulin resistance. EBioMedicine 2020, 59, 102912. [Google Scholar] [CrossRef]
- Guo, X.; Asthana, P.; Gurung, S.; Zhang, S.; Wong, S.K.K.; Fallah, S.; Chow, C.F.W.; Che, S.; Zhai, L.; Wang, Z.; et al. Regulation of age-associated insulin resistance by MT1-MMP-mediated cleavage of insulin receptor. Nat. Commun. 2022, 13, 3749. [Google Scholar] [CrossRef]
- Kim, S.Y.; England, L.; Wilson, H.G.; Bish, C.; Satten, G.A.; Dietz, P. Percentage of gestational diabetes mellitus attributable to overweight and obesity. Am. J. Public Health 2010, 100, 1047–1052. [Google Scholar] [CrossRef]
- Chu, S.Y.; Callaghan, W.M.; Kim, S.Y.; Schmid, C.H.; Lau, J.; England, L.J.; Dietz, P.M. Maternal obesity and risk of gestational diabetes mellitus. Diabetes Care 2007, 30, 2070–2076. [Google Scholar] [CrossRef] [PubMed]
- Mnatzaganian, G.; Woodward, M.; McIntyre, H.D.; Ma, L.; Yuen, N.; He, F.; Nightingale, H.; Xu, T.; Huxley, R.R. Trends in percentages of gestational diabetes mellitus attributable to overweight, obesity, and morbid obesity in regional Victoria: An eight-year population-based panel study. BMC Pregnancy Childbirth 2022, 22, 95. [Google Scholar] [CrossRef]
- Yong, H.Y.; Shariff, Z.M.; Yusof, B.N.M.; Rejali, Z.; Tee, Y.Y.S.; Bindels, J.; van der Beek, E.M. Independent and combined effects of age, body mass index and gestational weight gain on the risk of gestational diabetes mellitus. Sci. Rep. 2020, 10, 8486. [Google Scholar] [CrossRef] [PubMed]
- Mirabelli, M.; Chiefari, E.; Puccio, L.; Foti, D.P.; Brunetti, A. Potential Benefits and Harms of Novel Antidiabetic Drugs During COVID-19 Crisis. Int. J. Environ. Res. Public Health 2020, 17, 3664. [Google Scholar] [CrossRef] [PubMed]
- Linee Guida per la Gravidanza Fisiologica. In Sistema Nazionale per le Linee Guida dell’Istituto Superiore di Sanità. Available online: http://www.salute.gov.it/imgs/C_17_pubblicazioni_1436_allegato.pdf (accessed on 11 March 2023).
- Chiefari, E.; Quaresima, P.; Visconti, F.; Mirabelli, M.; Brunetti, A. Gestational diabetes and fetal overgrowth: Time to rethink screening guidelines. Lancet Diabetes Endocrinol. 2020, 8, 561–562. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, C.; de Gennaro, G.; Romano, M.; Battini, L.; Aragona, M.; Corfini, M.; Del Prato, S.; Bertolotto, A. Italian national guidelines for the screening of gestational diabetes: Time for a critical appraisal? Nutr. Metab. Cardiovasc. Dis. 2017, 27, 717–722. [Google Scholar] [CrossRef]
- Quaresima, P.; Visconti, F.; Chiefari, E.; Mirabelli, M.; Borelli, M.; Caroleo, P.; Foti, D.; Puccio, L.; Venturella, R.; Di Carlo, C.; et al. Appropriate Timing of Gestational Diabetes Mellitus Diagnosis in Medium- and Low-Risk Women: Effectiveness of the Italian NHS Recommendations in Preventing Fetal Macrosomia. J. Diabetes Res. 2020, 2020, 5393952. [Google Scholar] [CrossRef]
- Torlone, E.; Festa, C.; Formoso, G.; Scavini, M.; Sculli, M.; Succurro, E.; Sciacca, L.; Lapolla, A. Raccomandazioni per la diagnosi del diabete gestazionale durante la pandemia COVID-19. JAMD 2020, 23, 2. [Google Scholar]
- Salatino, A.; Mirabelli, M.; Chiefari, E.; Greco, M.; Di Vito, A.; Bonapace, G.; Brunetti, F.S.; Crocerossa, F.; Epstein, A.L.; Foti, D.P.; et al. The anticancer effects of Metformin in the male germ tumor SEM-1 cell line are mediated by HMGA1. Front. Endocrinol. 2022, 13, 1051988. [Google Scholar] [CrossRef]
- Lao, T.T.; Ho, L.F.; Chan, B.C.; Leung, W.C. Maternal age and prevalence of gestational diabetes mellitus. Diabetes Care 2006, 29, 948–949. [Google Scholar] [CrossRef]
- Coustan, D.R. Recurrent GDM and the development of type 2 diabetes have similar risk factors. Endocrine 2016, 53, 624–625. [Google Scholar] [CrossRef] [PubMed]
- Mirabelli, M.; Chiefari, E.; Caroleo, P.; Vero, R.; Brunetti, F.S.; Corigliano, D.M.; Arcidiacono, B.; Foti, D.P.; Puccio, L.; Brunetti, A. Long-Term Effectiveness and Safety of SGLT-2 Inhibitors in an Italian Cohort of Patients with Type 2 Diabetes Mellitus. J. Diabetes Res. 2019, 2019, 3971060. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, M.; Rizzo, M.R.; Manzella, D.; Paolisso, G. Age-related insulin resistance: Is it an obligatory finding? The lesson from healthy centenarians. Diabetes Metab. Res. Rev. 2001, 17, 19–26. [Google Scholar] [CrossRef]
- Barbour, L.A.; McCurdy, C.E.; Hernandez, T.L.; Kirwan, J.P.; Catalano, P.M.; Friedman, J.E. Cellular mechanisms for insulin resistance in normal pregnancy and gestational diabetes [published correction appears in Diabetes Care. 2007;30:3154]. Diabetes Care 2007, 30 (Suppl. 2), S112–S119. [Google Scholar] [CrossRef]
- Szoke, E.; Shrayyef, M.Z.; Messing, S.; Woerle, H.J.; van Haeften, T.W.; Meyer, C.; Mitrakou, A.; Pimenta, W.; Gerich, J.E. Effect of aging on glucose homeostasis: Accelerated deterioration of beta-cell function in individuals with impaired glucose tolerance. Diabetes Care 2008, 31, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Rieck, S.; Kaestner, K.H. Expansion of beta-cell mass in response to pregnancy. Trends Endocrinol. Metab. 2010, 21, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Gautier, J.-F.; Mourier, A.; De Kerviler, E.; Tarentola, A.; Bigard, A.X.; Villette, J.-M.; Guezennec, C.Y.; Cathelineau, G. Evaluation of abdominal fat distribution in noninsulin-dependent diabetes mellitus: Relationship to insulin resistance. J. Clin. Endocrinol. Metab. 1998, 83, 1306–1311. [Google Scholar] [CrossRef]
- Kampmann, U.; Knorr, S.; Fuglsang, J.; Ovesen, P. Determinants of Maternal Insulin Resistance during Pregnancy: An Updated Overview. J. Diabetes Res. 2019, 2019, 5320156. [Google Scholar] [CrossRef]
- Sitoris, G.; Veltri, F.; Ichiche, M.; Kleynen, P.; Praet, J.-P.; Rozenberg, S.; Poppe, K.G. Association between thyroid autoimmunity and gestational diabetes mellitus in euthyroid women. Eur. Thyroid. J. 2022, 11, e210142. [Google Scholar] [CrossRef]
- Sun, H.; Su, X.; Liu, Y.; Li, G.; Liu, X.; Du, Q. Association between Abortion History and Perinatal and Neonatal Outcomes of Singleton Pregnancies after Assisted Reproductive Technology. J. Clin. Med. 2022, 12, 1. [Google Scholar] [CrossRef]
- Wang, H.; Guo, X.; Song, Q.; Su, W.; Meng, M.; Sun, C.; Li, N.; Liang, Q.; Qu, G.; Liang, M.; et al. Association between the history of abortion and gestational diabetes mellitus: A meta-analysis. Endocrine 2023, 80, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, E.; Hedlund, E.; Perin, T.; Lyndrup, J. Is thrombophilia a risk factor for placenta-mediated pregnancy complications? Arch. Gynecol. Obstet. 2012, 286, 585–589. [Google Scholar] [CrossRef] [PubMed]
- Manna, C.; Lacconi, V.; Rizzo, G.; De Lorenzo, A.; Massimiani, M. Placental Dysfunction in Assisted Reproductive Pregnancies: Perinatal, Neonatal and Adult Life Outcomes. Int. J. Mol. Sci. 2022, 23, 659. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.; Yu, H.; Wei, Q.; Zhi, M.; Wu, C.; Zhu, X.; Li, L. The effect of pre-pregnancy body mass index and excessive gestational weight gain on the risk of gestational diabetes in advanced maternal age. Oncotarget 2017, 8, 58364–58371. [Google Scholar] [CrossRef]
- Hedderson, M.M.; Gunderson, E.P.; Ferrara, A. Gestational weight gain and risk of gestational diabetes mellitus. Obstet. Gynecol. 2010, 115, 597–604. [Google Scholar] [CrossRef]
- Institute of Medicine (US) and National Research Council (US) Committee to Reexamine IOM Pregnancy Weight Guidelines; Rasmussen, K.M.; Yaktine, A.L. (Eds.) Weight Gain During Pregnancy: Reexamining the Guidelines; Composition and Components of Gestational Weight Gain: Physiology and Metabolism; National Academies Press: Washington, DC, USA, 2009; p. 3. Available online: https://www.ncbi.nlm.nih.gov/books/NBK32815/ (accessed on 11 March 2023).
- Sparks, J.R.; Ghildayal, N.; Hivert, M.F.; Redman, L.M. Lifestyle interventions in pregnancy targeting GDM prevention: Looking ahead to precision medicine. Diabetologia 2022, 65, 1814–1824. [Google Scholar] [CrossRef]
- Mirabelli, M.; Chiefari, E.; Foti, D.; Brunetti, A. Preventing gestational diabetes mellitus with physical activity: Time to move towards precision medicine. L’Endocrinologo 2023, 24, 22–28. [Google Scholar] [CrossRef]
- Capula, C.; Chiefari, E.; Borelli, M.; Oliverio, R.; Vero, A.; Foti, D.; Puccio, L.; Vero, R.; Brunetti, A. A new predictive tool for the early risk assessment of gestational diabetes mellitus. Prim. Care Diabetes 2016, 10, 315–323. [Google Scholar] [CrossRef]
- Temmesen, C.G.; Faber Frandsen, T.; Svarre-Nielsen, H.; Petersen, K.B.; Clemensen, J.; Andersen, H.L.M. Women’s reflections on timing of motherhood: A meta-synthesis of qualitative evidence. Reprod. Health 2023, 20, 30. [Google Scholar] [CrossRef]
- Zelenytė, V.; Valius, L.; Domeikienė, A.; Gudaitytė, R.; Endzinas, Ž.; Šumskas, L.; Maleckas, A. Body size perception, knowledge about obesity and factors associated with lifestyle change among patients, health care professionals and public health experts. BMC Fam. Pract. 2021, 22, 37. [Google Scholar] [CrossRef]
- Sand, A.S.; Emaus, N.; Lian, O. Overweight and obesity in young adult women: A matter of health or appearance? The Tromsø study: Fit futures. Int. J. Qual. Stud. Health Well-Being 2015, 10, 29026. [Google Scholar] [CrossRef]
- Tajgardoon, M.; Cooper, G.F.; King, A.J.; Clermont, G.; Hochheiser, H.; Hauskrecht, M.; Sittig, D.F.; Visweswaran, S. Modeling physician variability to prioritize relevant medical record information. JAMIA Open 2020, 3, 602–610. [Google Scholar] [CrossRef]
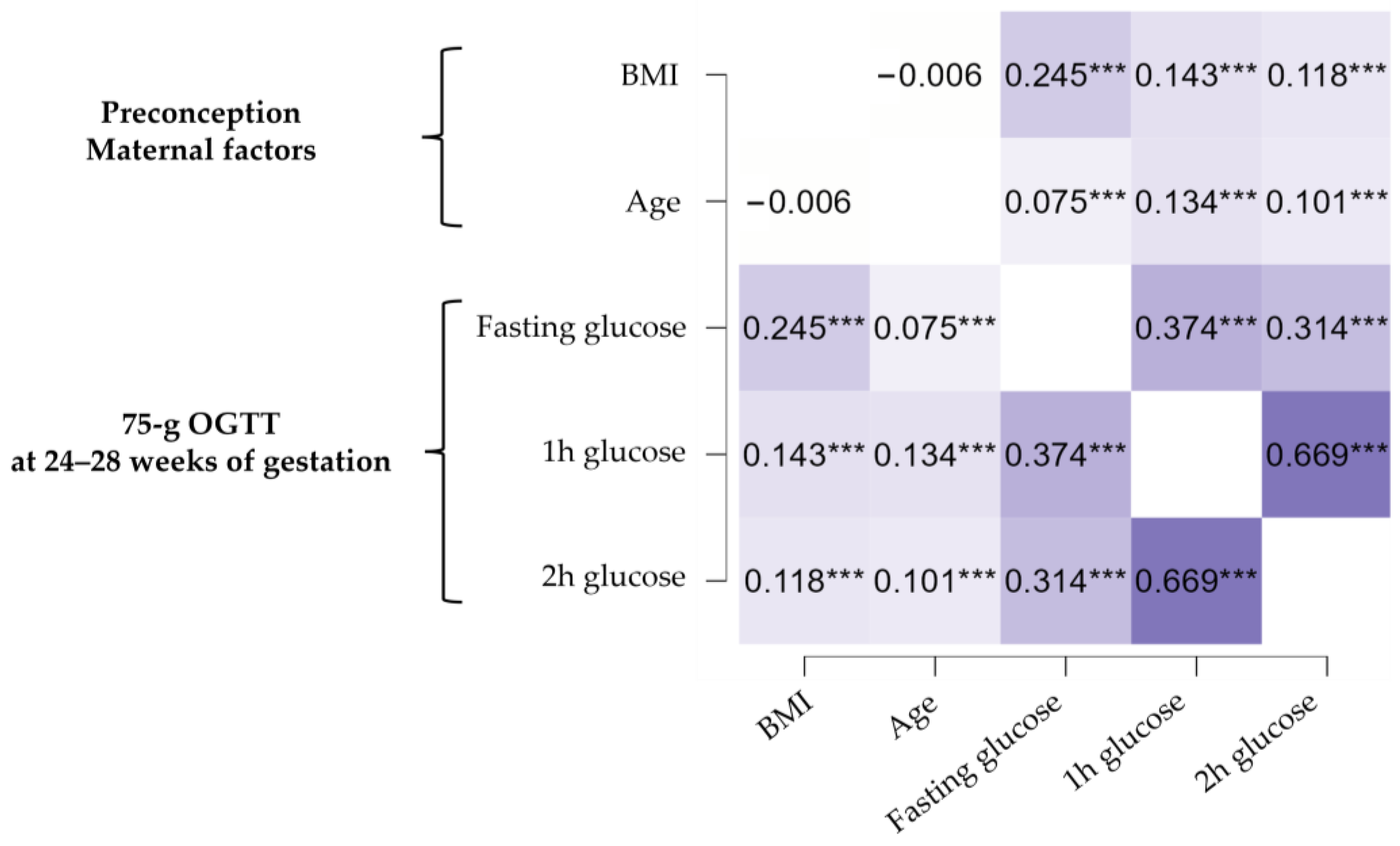
| Characteristics | NGT (n = 2980) | GDM (n = 885) | |
|---|---|---|---|
| Median (IQR) or N (%) | Median (IQR) or N (%) | p Value | |
| Secondary or tertiary level education, N | 1871 (85.8% §) | 496 (83.2% §) | 0.099 |
| Married, N | 1709 (76.1% §) | 446 (78.5% §) | 0.222 |
| Height, m | 1.62 (1.59–1.67) | 1.62 (1.58–1.65) | 0.001 |
| Preconception body weight, kg | 60.0 (54.0–69.0) | 65.0 (57.0–77.8) | <0.001 |
| Preconception BMI, kg/m2 | 22.7 (20.6–25.7) | 25.0 (21.9–29.4) | <0.001 |
| Preconception obesity, N | 241 (8.8% §) | 181 (21.9% §) | <0.001 |
| Preconception overweight, N | 571 (20.7% §) | 224 (27.1% §) | <0.001 |
| Preconception IFG, N | 8 (6.1% §) | 12 (21.1 §) | 0.015 |
| PCOS, N | 20 (0.7%) | 44 (5.0%) | <0.001 |
| Age at menarche, yr | 12 (11–13) | 12 (11–13) | 0.717 |
| Maternal age, yr | 32 (29–36) | 34 (30–37) | <0.001 |
| Advanced maternal age (≥35 yr), N | 1000 (33.6%) | 379 (42.8%) | <0.001 |
| Thyroid disease, N | 94 (3.2% §) | 61 (6.9% §) | <0.001 |
| Thrombophilia, N | 21 (0.7% §) | 28 (3.2% §) | <0.001 |
| Nulliparous, N | 1210 (43.6% §) | 318 (39.0% §) | 0.021 |
| Negative reproductive history of abortion(s), N | 2205 (80.0% §) | 613 (75.2% §) | 0.003 |
| Planned pregnancy, N | 1445 (54.9% §) | 310 (39.8% §) | <0.001 |
| Assisted reproduction, N | 6 (0.2% §) | 12 (1.3% §) | <0.001 |
| Previous GDM, N | 102 (3.4% §) | 153 (17.4% §) | <0.001 |
| Non-smoker, N | 1904 (81.6% §) | 499 (82.3% §) | 0.185 |
| Family history of T2DM, N | 1574 (54.6% §) | 601 (71.1% §) | <0.001 |
| Diagnosis of GDM at early screening, N | _ | 113 (12.8%) | _ |
| Gestational age at 75 g OGTT, wg | 26.0 (26.0–27.0) | 26.0 (25.0–27.0) | 0.541 |
| Fasting glucose *, mg/dL | 80.0 (76.0–84.0) | 92.0 (85.0–96.0) | <0.001 |
| 1 h glucose *, mg/dL | 126.0 (107.0–145.0) | 176.0 (149.0–192.0) | <0.001 |
| 2 h glucose *, mg/dL | 102.0 (89.0–115.0) | 134.0 (115.0–157.0) | <0.001 |
| Fasting glucose ≥ 92 mg/dL, N | _ | 531 (60%) | _ |
| 1 h glucose ≥ 180 mg/dL, N | _ | 401 (45.3%) | _ |
| 2 h glucose ≥ 153 mg/dL, N | _ | 276 (31.2%) | _ |
| Body weight at 75 g OGTT *, kg | 68.0 (61.0–76.0) | 72.0 (64.0–83.0) | <0.001 |
| BMI at 75 g OGTT *, kg/m2 | 25.6 (23.4–28.5) | 27.7 (24.7–31.6) | <0.001 |
| Gestational weight gain at 75 g OGTT *, kg | 7.0 (5.0–9.0) | 7.0 (5.0–10.0) | 0.992 |
| Standardized β | OR | 95% CI | p Value | |
|---|---|---|---|---|
| Preconception BMI | 0.481 | 1.104 | (1.087–1.122) | <0.001 |
| Preconception BMI * | 0.414 | 1.089 | (1.069–1.109) | <0.001 |
| Maternal age | 0.279 | 1.055 | (1.038–1.072) | <0.001 |
| Maternal age * | 0.246 | 1.048 | (1.029–1.068) | <0.001 |
| Standardized β | OR | 95% CI | p Value | |
|---|---|---|---|---|
| Preconception obesity | 0.299 | 2.525 | (1.971–3.236) | <0.001 |
| Maternal age ≥ 35 yr | 0.181 | 1.461 | (1.213–1.759) | <0.001 |
| Standardized β | OR | 95% CI | p Value | |
|---|---|---|---|---|
| Preconception overweight | 0.108 | 1.633 | (1.320–2.019) | <0.001 |
| Maternal age ≥ 35 yr | 0.103 | 1.450 | (1.184–1.776) | <0.001 |
| Preconception Obesity | Preconception Overweight | Preconception Normal Weight | A vs. B | B vs. C | C vs. D | D vs. E | E vs. F | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (A) Maternal Age ≥35 yr | (B) Maternal Age <35 yr | (C) Maternal Age ≥35 yr | (D) Maternal Age <35 yr | (E) Maternal Age ≥35 yr | (F) Maternal Age <35 yr | ||||||||||||
| N | % | N | % | N | % | N | % | N | % | N | % | p Value | |||||
| NGT | 67 | 48.6% | 174 | 61.5% | 182 | 62.8% | 389 | 77.0% | 681 | 79.6% | 1258 | 83.6% | * | ns | *** | ns | * |
| GDM | 71 | 51.4% | 109 | 38.5% | 108 | 37.2% | 116 | 23.0% | 174 | 20.4% | 247 | 16.4% | |||||
| Total | 138 | 283 | 290 | 505 | 855 | 1505 | |||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mirabelli, M.; Tocci, V.; Donnici, A.; Giuliano, S.; Sarnelli, P.; Salatino, A.; Greco, M.; Puccio, L.; Chiefari, E.; Foti, D.P.; et al. Maternal Preconception Body Mass Index Overtakes Age as a Risk Factor for Gestational Diabetes Mellitus. J. Clin. Med. 2023, 12, 2830. https://doi.org/10.3390/jcm12082830
Mirabelli M, Tocci V, Donnici A, Giuliano S, Sarnelli P, Salatino A, Greco M, Puccio L, Chiefari E, Foti DP, et al. Maternal Preconception Body Mass Index Overtakes Age as a Risk Factor for Gestational Diabetes Mellitus. Journal of Clinical Medicine. 2023; 12(8):2830. https://doi.org/10.3390/jcm12082830
Chicago/Turabian StyleMirabelli, Maria, Vera Tocci, Alessandra Donnici, Stefania Giuliano, Paola Sarnelli, Alessandro Salatino, Marta Greco, Luigi Puccio, Eusebio Chiefari, Daniela Patrizia Foti, and et al. 2023. "Maternal Preconception Body Mass Index Overtakes Age as a Risk Factor for Gestational Diabetes Mellitus" Journal of Clinical Medicine 12, no. 8: 2830. https://doi.org/10.3390/jcm12082830
APA StyleMirabelli, M., Tocci, V., Donnici, A., Giuliano, S., Sarnelli, P., Salatino, A., Greco, M., Puccio, L., Chiefari, E., Foti, D. P., & Brunetti, A. (2023). Maternal Preconception Body Mass Index Overtakes Age as a Risk Factor for Gestational Diabetes Mellitus. Journal of Clinical Medicine, 12(8), 2830. https://doi.org/10.3390/jcm12082830













