Deep Learning Algorithms for Estimation of Demographic and Anthropometric Features from Electrocardiograms
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Dataset
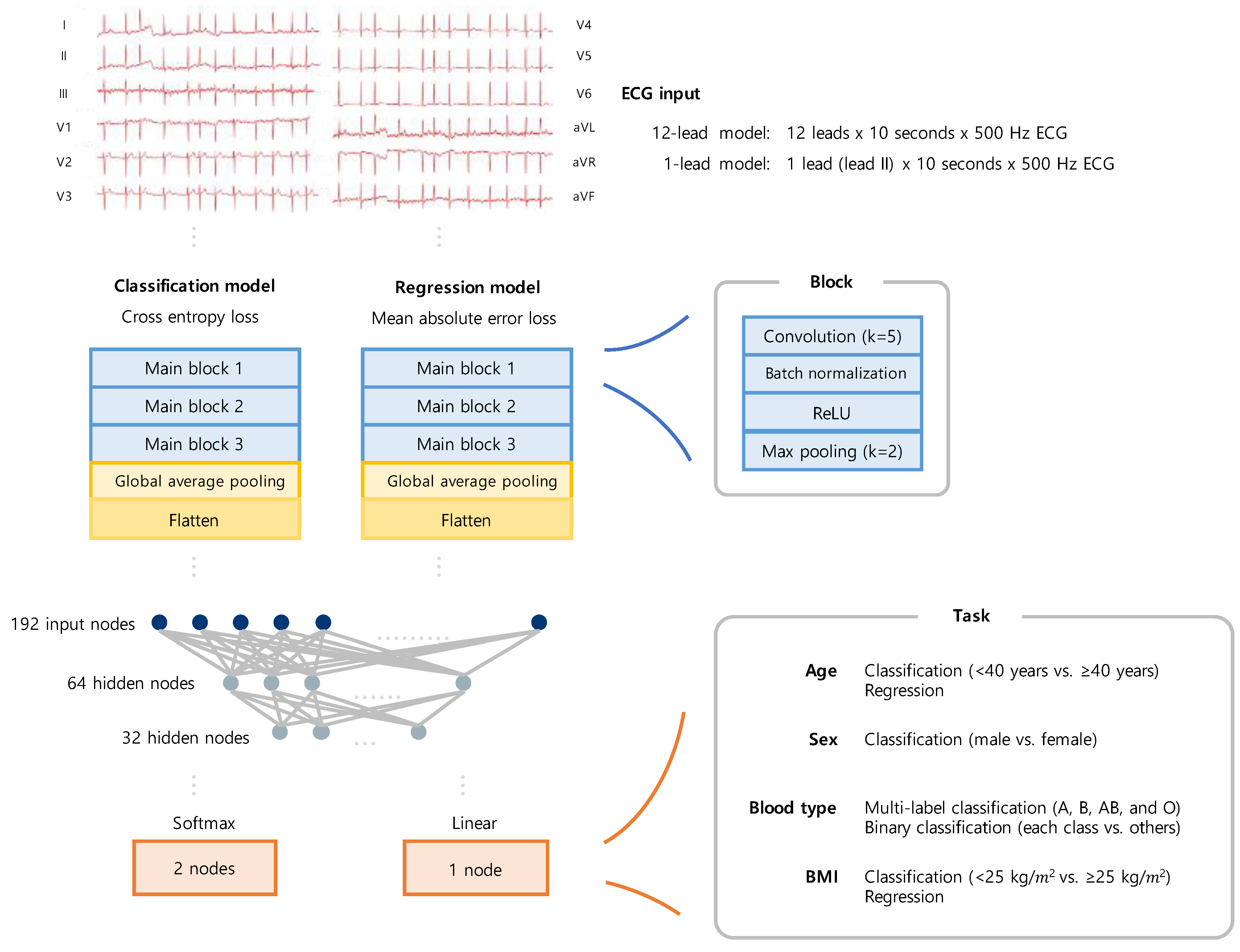
2.3. Classification and Regression Task
2.4. Deep Learning Method
2.5. Scaling a Model
2.6. Statistical Metrics
2.7. Visualization for Model Explanation
2.8. Data and Code Availability
3. Results
3.1. Study Population
3.2. Evaluation Protocol
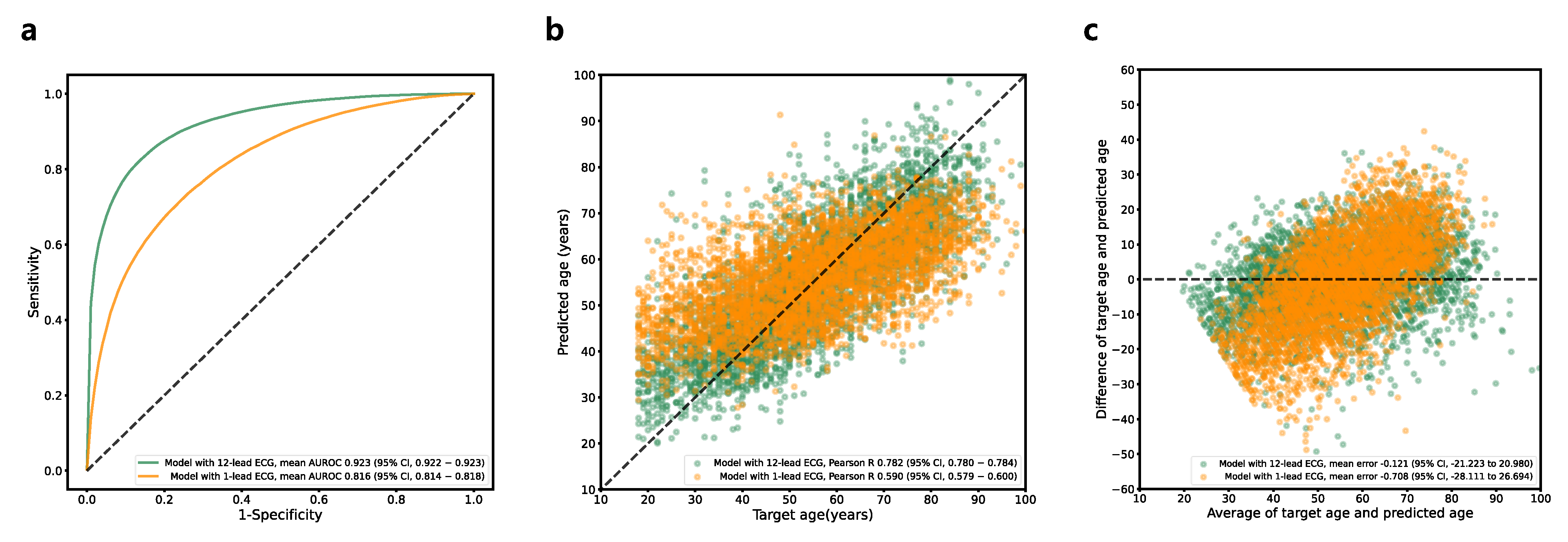
3.3. Age Estimation
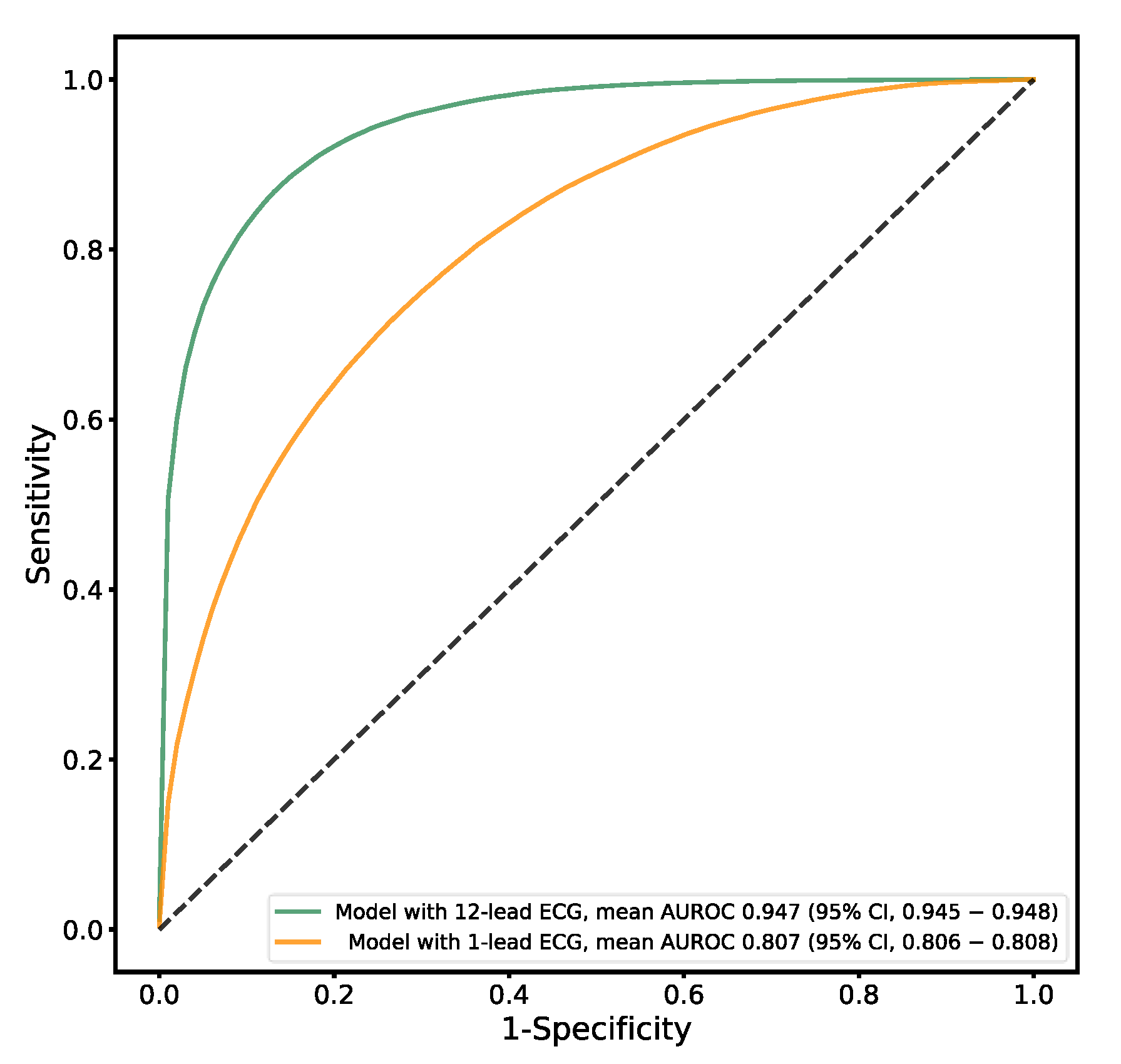
3.4. Sex Estimation
3.5. ABO Blood Type Estimation
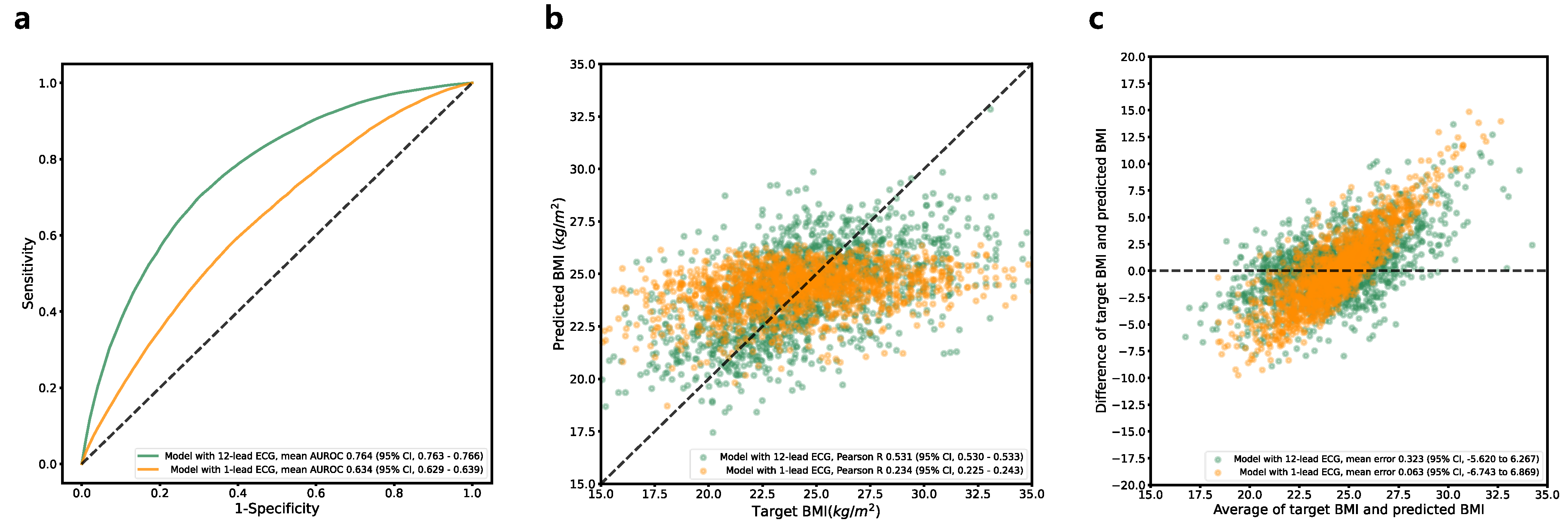
3.6. BMI Estimation
3.7. Visualization for Explainable AI
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Serhani, M.A.; TEl Kassabi, H.; Ismail, H.; Nujum Navaz, A. ECG Monitoring Systems: Review, Architecture, Processes, and Key Challenges. Sensors 2020, 20, 1796. [Google Scholar] [CrossRef] [PubMed]
- Steinhubl, S.R.; Waalen, J.; Edwards, A.M.; Ariniello, L.M.; Mehta, R.R.; Ebner, G.S.; Topol, E.J. Effect of a home-based wearable continuous ECG monitoring patch on detection of undiagnosed atrial fibrillation: The mSToPS randomized clinical trial. JAMA 2018, 320, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Benhamida, A.; Zouaoui, A.; Szócska, G.; Karóczkai, K.; Slimani, G.; Kozlovszky, M. Problems in archiving long-term continuous ECG data—A review. In Proceedings of the 2019 IEEE 17th World Symposium on Applied Machine Intelligence and Informatics (SAMI), Herlany, Slovakia, 24–26 January 2019. [Google Scholar]
- Petrėnas, A.; Marozas, V.; Jaruševičius, G.; Sörnmo, L. A modified Lewis ECG lead system for ambulatory monitoring of atrial arrhythmias. J. Electrocardiol. 2015, 48, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Fung, E.; Jarvelin, M.-R.; Doshi, R.N.; Shinbane, J.S.; Carlson, S.K.; Grazette, L.P.; Chang, P.M.; Sangha, R.S.; Huikuri, H.V.; Peters, N.S. Electrocardiographic patch devices and contemporary wireless cardiac monitoring. Front. Physiol. 2015, 6, 149. [Google Scholar] [CrossRef]
- EsEsteva, A.; Robicquet, A.; Ramsundar, B.; Kuleshov, V.; Depristo, M.; Chou, K.; Cui, C.; Corrado, G.; Thrun, S.; Dean, J. A guide to deep learning in healthcare. Nat. Med. 2019, 25, 24–29. [Google Scholar] [CrossRef]
- Suri, J.S.; Biswas, M.; Kuppili, V.; Saba, L.; Edla, D.R.; Suri, H.S.; Cuadrado-Godia, E.; Laird, J.R.; Marinhoe, R.T.; Sanches, J.M.; et al. State-of-the-art review on deep learning in medical imaging. Front. Biosci. 2019, 24, 392–426. [Google Scholar] [CrossRef]
- Janowczyk, A.; Madabhushi, A. Deep learning for digital pathology image analysis: A comprehensive tutorial with selected use cases. J. Pathol. Inform. 2016, 7, 27. [Google Scholar] [CrossRef]
- Lee, S.; Lee, H.-C.; Chu, Y.S.; Song, S.W.; Ahn, G.J.; Lee, H.; Yang, S.; Koh, S.B. Deep learning models for the prediction of intraoperative hypotension. Br. J. Anaesth. 2021, 126, 808–817. [Google Scholar] [CrossRef]
- Alizadehsani, R.; Khosravi, A.; Roshanzamir, M.; Abdar, M.; Sarrafzadegan, N.; Shafie, D.; Khozeimeh, F.; Shoeibi, A.; Nahavandi, S.; Panahiazar, M.; et al. Coronary artery disease detection using artificial intelligence techniques: A survey of trends, geographical differences and diagnostic features 1991–2020. Comput. Biol. Med. 2021, 128, 104095. [Google Scholar] [CrossRef]
- Yildirim, O.; Talo, M.; Ciaccio, E.J.; San Tan, R.; Acharya, U.R. Accurate deep neural network model to detect cardiac arrhythmia on more than 10,000 individual subject ECG records. Comput. Methods Programs Biomed. 2020, 197, 105740. [Google Scholar] [CrossRef]
- Zhang, X.; Gu, K.; Miao, S.; Zhang, X.; Yin, Y.; Wan, C.; Yu, Y.; Hu, J.; Wang, Z.; Shan, T.; et al. Automated detection of cardiovascular disease by electrocardiogram signal analysis: A deep learning system. Cardiovasc. Diagn. Ther. 2020, 10, 227. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, A.; Mena, L.J.; Felix, V.G. Noise-tolerant neural network approach for electrocardiogram signal classification. In Proceedings of the International Conference on Compute and Data Analysis, Lakeland, FL, USA, 19–23 May 2017. [Google Scholar]
- Poungponsri, S.; Yu, X.-H. An adaptive filtering approach for electrocardiogram (ECG) signal noise reduction using neural networks. Neurocomputing 2013, 117, 206–213. [Google Scholar] [CrossRef]
- Ribeiro, A.H.; Ribeiro, M.H.; Paixão, G.M.M.; Oliveira, D.M.; Gomes, P.R.; Canazart, J.A.; Ferreira, M.P.S.; Andersson, C.R.; Macfarlane, P.W.; Meira, W.; et al. Automatic diagnosis of the 12-lead ECG using a deep neural network. Nat. Commun. 2020, 11, 1760. [Google Scholar] [CrossRef] [PubMed]
- Mateo, J.; Rieta, J. Application of artificial neural networks for versatile preprocessing of electrocardiogram recordings. J. Med. Eng. Technol. 2012, 36, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.M.; Cho, Y.; Jeon, K.H.; Cho, S.; Kim, K.H.; Baek, S.D.; Oh, B.H. A deep learning algorithm to detect anaemia with ECGs: A retrospective, multicentre study. Lancet Digit. Health 2020, 2, e358–e367. [Google Scholar] [CrossRef]
- Wu, J.M.-T.; Tsai, M.-H.; Xiao, S.-H.; Liaw, Y.-P. A deep neural network electrocardiogram analysis framework for left ventricular hypertrophy prediction. J. Ambient. Intell. Humaniz. Comput. 2020, 1–17. [Google Scholar] [CrossRef]
- Galloway, C.D.; Valys, A.V.; Shreibati, J.B.; Treiman, D.L.; Petterson, F.L.; Gundotra, V.P.; Albert, D.E.; Attia, Z.I.; Carter, R.E.; Asirvatham, S.J.; et al. Development and validation of a deep-learning model to screen for hyperkalemia from the electrocardiogram. JAMA Cardiol. 2019, 4, 428–436. [Google Scholar] [CrossRef]
- Toya, T.; Ahmad, A.; Attia, Z.; Cohen-Shelly, M.; Ozcan, I.; A Noseworthy, P.; Lopez-Jimenez, F.; Kapa, S.; O Lerman, L.; A Friedman, P.; et al. Vascular Aging Detected by Peripheral Endothelial Dysfunction Is Associated With ECG-Derived Physiological Aging. J. Am. Heart Assoc. 2021, 10, e018656. [Google Scholar] [CrossRef]
- Schulte, F.; Fry, E. Death by 1000 clicks: Where electronic health records went wrong. Kaiser Health News, 18 March 2019. [Google Scholar]
- Wilcox, A.B.; Chen, Y.-H.; Hripcsak, G. Minimizing electronic health record patient-note mismatches. J. Am. Med. Inform. Assoc. 2011, 18, 511–514. [Google Scholar] [CrossRef]
- Macfarlane, P.; McLaughlin, S.; Devine, B.; Yang, T. Effects of age, sex, and race on ECG interval measurements. J. Electrocardiol. 1994, 27, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Salama, G.; Bett, G.C. Sex differences in the mechanisms underlying long QT syndrome. Am. J. Physiol.-Heart Circ. Physiol. 2014, 307, H640–H648. [Google Scholar] [CrossRef] [PubMed]
- Attia, Z.I.; Friedman, P.A.; Noseworthy, P.A.; Lopez-Jimenez, F.; Ladewig, D.J.; Satam, G.; Pellikka, P.A.; Munger, T.M.; Asirvatham, S.J.; Scott, C.G.; et al. Age and Sex Estimation Using Artificial Intelligence From Standard 12-Lead ECGs. Circ. Arrhythm Electrophysiol. 2019, 12, e007284. [Google Scholar] [CrossRef] [PubMed]
- Fraley, M.; Birchem, J.; Senkottaiyan, N.; Alpert, M. Obesity and the electrocardiogram. Obes. Rev. 2005, 6, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Hassing, G.J.; van der Wall, H.E.C.; van Westen, G.J.P.; Kemme, M.J.B.; Adiyaman, A.; Elvan, A.; Burggraaf, J.; Gal, P. Body mass index related electrocardiographic findings in healthy young individuals with a normal body mass index. Neth. Heart J. 2019, 27, 506–512. [Google Scholar] [CrossRef]
- Webster, J.G. The Physiological Measurement Handbook; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Cho, Y.; Kwon, J.M.; Kim, K.H.; Medina-Inojosa, J.R.; Jeon, K.H.; Cho, S.; Oh, B.H. Artificial intelligence algorithm for detecting myocardial infarction using six-lead electrocardiography. Sci. Rep. 2020, 10, 20495. [Google Scholar] [CrossRef]
- Nam, G.E.; Park, H.S. Perspective on diagnostic criteria for obesity and abdominal obesity in Korean adults. J. Obes. Metab. Syndr. 2018, 27, 134. [Google Scholar] [CrossRef]
- Selvaraju, R.R.; Cogswell, M.; Das, A.; Vedantam, R.; Parikh, D.; Batra, D. Grad-cam: Visual explanations from deep networks via gradient-based localization. In Proceedings of the IEEE international conference on computer vision, Venice, Italy, 27–29 October 2017. [Google Scholar]
- Malik, M.; Hnatkova, K.; Kowalski, D.; Keirns, J.J.; van Gelderen, E.M. QT/RR curvatures in healthy subjects: Sex differences and covariates. Am. J. Physiol.-Heart Circ. Physiol. 2013, 305, H1798–H1806. [Google Scholar] [CrossRef]
- Yamasaki, Y.; Kodama, M.; Matsuhisa, M.; Kishimoto, M.; Ozaki, H.; Tani, A.; Ueda, N.; Ishida, Y.; Kamada, T. Diurnal heart rate variability in healthy subjects: Effects of aging and sex difference. Am. J. Physiol.-Heart Circ. Physiol. 1996, 271, H303–H310. [Google Scholar] [CrossRef]
- Adjei, T.; Xue, J.; Mandic, D.P. The female heart: Sex differences in the dynamics of ECG in response to stress. Front. Physiol. 2018, 9, 1616. [Google Scholar] [CrossRef]
- Bachman, S.; Sparrow, D.; Smith, L.K. Effect of aging on the electrocardiogram. Am. J. Cardiol. 1981, 48, 513–516. [Google Scholar] [CrossRef] [PubMed]
- Surawicz, B.; Parikh, S.R. Prevalence of male and female patterns of early ventricular repolarization in the normal ECG of males and females from childhood to old age. J. Am. Coll. Cardiol. 2002, 40, 1870–1876. [Google Scholar] [CrossRef] [PubMed]
- Mieszczanska, H.; Pietrasik, G.; Piotrowicz, K.; McNitt, S.; Moss, A.J.; Zareba, W. Gender-related differences in electrocardiographic parameters and their association with cardiac events in patients after myocardial infarction. Am. J. Cardiol. 2008, 101, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Lima, E.M.; Ribeiro, A.H.; Paixão, G.M.M.; Ribeiro, M.H.; Pinto-Filho, M.M.; Gomes, P.R.; Oliveira, D.M.; Sabino, E.C.; Duncan, B.B.; Giatti, L.; et al. Deep neural network-estimated electrocardiographic age as a mortality predictor. Nat. Commun. 2021, 12, 5117. [Google Scholar] [CrossRef]
- van der Wall, H.E.; Hassing, G.-J.; Doll, R.-J.; van Westen, G.J.; Cohen, A.F.; Selder, J.L.; Kemme, M.; Burggraaf, J.; Gal, P. Cardiac age detected by machine learning applied to the surface ECG of healthy subjects: Creation of a benchmark. J. Electrocardiol. 2022, 72, 49–55. [Google Scholar] [CrossRef]
- Noseworthy, P.A.; Attia, Z.I.; Brewer, L.C.; Hayes, S.N.; Yao, X.; Kapa, S.; Friedman, P.A.; Lopez-Jimenez, F. Assessing and Mitigating Bias in Medical Artificial Intelligence: The Effects of Race and Ethnicity on a Deep Learning Model for ECG Analysis. Circ. Arrhythm Electrophysiol. 2020, 13, e007988. [Google Scholar] [CrossRef]
- Stritzke, J.; Markus, M.R.P.; Duderstadt, S.; Lieb, W.; Luchner, A.; Döring, A.; Keil, U.; Hense, H.-W.; Schunkert, H. The aging process of the heart: Obesity is the main risk factor for left atrial enlargement during aging: The MONICA/KORA (Monitoring of Trends and Determinations in Cardiovascular Disease/Cooperative Research in the Region of Augsburg) study. J. Am. Coll. Cardiol. 2009, 54, 1982–1989. [Google Scholar] [CrossRef]
- Capuzzo, E.; Bonfanti, C.; Frattini, F.; Montorsi, P.; Turdo, R.; Previdi, M.G.; Turrini, E.; Franchini, M. The relationship between ABO blood group and cardiovascular disease: Results from the Cardiorisk program. Ann. Transl. Med. 2016, 4, 189. [Google Scholar] [CrossRef]
- Fan, Q.; Zhang, W.; Li, B.; Li, D.-J.; Zhang, J.; Zhao, F. Association between ABO blood group system and COVID-19 susceptibility in Wuhan. Front. Cell. Infect. Microbiol. 2020, 10, 404. [Google Scholar] [CrossRef]
- Tsuchimine, S.; Saruwatari, J.; Kaneda, A.; Yasui-Furukori, N. ABO blood type and personality traits in healthy Japanese subjects. PLoS ONE 2015, 10, e0126983. [Google Scholar] [CrossRef]
- Somani, S.; Russak, A.J.; Richter, F.; Zhao, S.; Vaid, A.; Chaudhry, F.; De Freitas, J.K.; Naik, N.; Miotto, R.; Nadkarni, G.N.; et al. Deep learning and the electrocardiogram: Review of the current state-of-the-art. EP Eur. 2021, 23, 1179–1191. [Google Scholar] [CrossRef] [PubMed]
- Attia, Z.I.; Kapa, S.; Lopez-Jimenez, F.; McKie, P.M.; Ladewig, D.J.; Satam, G.; Pellikka, P.A.; Enriquez-Sarano, M.; Noseworthy, P.A.; Munger, T.M.; et al. Screening for cardiac contractile dysfunction using an artificial intelligence–enabled electrocardiogram. Nat. Med. 2019, 25, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Shelly, M.; I Attia, Z.; A Friedman, P.; Ito, S.; A Essayagh, B.; Ko, W.-Y.; Murphree, D.H.; I Michelena, H.; Enriquez-Sarano, M.; E Carter, R.; et al. Electrocardiogram screening for aortic valve stenosis using artificial intelligence. Eur. Heart J. 2021, 42, 2885–2896. [Google Scholar] [CrossRef] [PubMed]
- Ko, W.-Y.; Siontis, K.C.; Attia, Z.I.; Carter, R.E.; Kapa, S.; Ommen, S.R.; Demuth, S.J.; Ackerman, M.J.; Gersh, B.J.; Arruda-Olson, A.M.; et al. Detection of hypertrophic cardiomyopathy using a convolutional neural network-enabled electrocardiogram. J. Am. Coll. Cardiol. 2020, 75, 722–733. [Google Scholar] [CrossRef]
- Choi, W.; Kim, S.-H.; Lee, W.; Kang, S.-H.; Yoon, C.-H.; Youn, T.-J.; Chae, I.-H. Comparison of Continuous ECG Monitoring by Wearable Patch Device and Conventional Telemonitoring Device. J. Korean Med. Sci. 2020, 35, e363. [Google Scholar] [CrossRef]
- Tison, G.; Sanchez, J.M.; Ballinger, B.; Singh, A.; Olgin, J.E.; Pletcher, M.J.; Vittinghoff, E.; Lee, E.S.; Fan, S.M.; Gladstone, R.A.; et al. Passive Detection of Atrial Fibrillation Using a Commercially Available Smartwatch. JAMA Cardiol. 2018, 3, 409–416. [Google Scholar] [CrossRef]




| Training Set | Validation Set | Test Set | |
|---|---|---|---|
| Age (y) | |||
| No. of unique patients | 49,762 | 37,324 | 37,329 |
| Mean (SD) | 55.25 (17.25) | 55.25 (17.24) | 55.23 (17.26) |
| ≥40 y, n (%) | 39,871 (80.12%) | 29,904 (80.12%) | 29,908 (80.12%) |
| <40 y, n (%) | 9891 (19.87%) | 7420 (19.87%) | 7421 (19.87) |
| Sex | |||
| No. of unique patients | 49,766 | 37,324 | 37,325 |
| Male sex, n (%) | 25,432 (51.10%) | 19,074 (51.10%) | 19,074 (51.10%) |
| Female sex, n (%) | 24,334 (48.89%) | 18,250 (48.89%) | 18,251 (48.89%) |
| ABO blood type | |||
| No. of unique patients | 49,760 | 37,322 | 37,324 |
| A, n (%) | 15,913 (31.97%) | 11,935 (31.97%) | 11,935 (31.97%) |
| B, n (%) | 14,362 (28.86%) | 10,772 (28.86%) | 10,773 (28.86%) |
| AB, n (%) | 14,086 (28.30%) | 10,565 (28.30%) | 10,566 (28.30%) |
| O, n (%) | 5399 (10.85%) | 4050 (10.85%) | 4050 (10.85%) |
| BMI | |||
| No. of unique patients | 19,393 | 14,546 | 14,549 |
| Height (cm), mean (SD) | 160.60 (14.94) | 160.46 (9.64) | 160.62 (20.73) |
| Weight (kg), mean (SD) | 63.08 (13.60) | 63.01 (12.14) | 62.93 (12.23) |
| BMI (kg/m2), mean (SD) | 24.38 (4.36) | 24.41 (4.81) | 24.75 (33.36) |
| ≥25 kg/m2, n (%) | 7727 (39.84%) | 5812 (39.95%) | 5788 (39.78%) |
| <25 kg/m2, n (%) | 11,666 (60.15%) | 8734 (60.04%) | 8761 (60.21%) |
| Estimation Target | ||||
|---|---|---|---|---|
| Age | Sex | BMI | ABO Blood Type | |
| Classification model | ||||
| AUROC curve (95% CI) | 0.923 (0.922–0.923) | 0.947 (0.945–0.948) | 0.764 (0.763–0.766) | 0.501 (0.496–0.506) |
| Sensitivity, % (95% CI) | 81.96% (81.55–82.37%) | 87.42% (85.92–88.91%) | 70.00% (67.80–72.20%) | 56.12% (4.96–107.27%) |
| Specificity, % (95% CI) | 86.67% (86.27–87.08%) | 86.25% (85.07–87.42%) | 69.82% (67.30–72.35%) | 44.03% (−7.31–95.37%) |
| PPV, % (95% CI) | 95.73% (95.62–95.83%) | 85.89% (85.05–86.74%) | 60.45% (59.18–61.72%) | 25.08% (9.82–40.33%) |
| NPV, % (95% CI) | 56.87 (56.42–57.32%) | 87.76% (86.65–88.88%) | 77.97% (77.26–78.69%) | 74.93% (59.50–90.37%) |
| Regression model | ||||
| MAE (95% CI) | 8.410 (8.380–8.441) | N/A | 2.332 (2.328–2.336) | N/A |
| Pearson R (95% CI) | 0.782 (0.780–0.784) | N/A | 0.531 (0.530–0.533) | N/A |
| R2 (95% CI) | 0.610 (0.607–0.612) | N/A | 0.279 (0.276–0.282) | N/A |
| ICC (95% CI) | 0.636 (0.628–0.645) | N/A | 0.474 (0.464–0.484) | N/A |
| Estimation Target | |||
|---|---|---|---|
| Age | Sex | BMI | |
| Classification model | |||
| AUROC curve (95% CI) | 0.816 (0.814–0.818) | 0.807 (0.806–0.808) | 0.633 (0.629–0.639) |
| Sensitivity, % (95% CI) | 69.92% (67.41–72.43%) | 72.35% (70.41–74.29%) | 65.24% (61.76–68.72%) |
| Specificity, % (95% CI) | 77.46% (74.98–79.95%) | 72.69% (71.02–74.36%) | 53.60% (49.48–57.72%) |
| PPV, % (95% CI) | 91.88% (91.33–92.44%) | 71.72% (71.01–72.44%) | 48.09% (47.16–49.02%) |
| NPV, % (95% CI) | 41.45% (40.21–42.70%) | 73.33% (72.42–74.25%) | 70.11% (69.45–70.77%) |
| Regression model | |||
| MAE (95% CI) | 11.220 (11.096–11.344) | N/A | 2.684 (2.682–2.687) |
| Pearson R (95% CI) | 0.590 (0.579–0.600) | N/A | 0.234 (0.225–0.243) |
| R2 (95% CI) | 0.345 (0.332–0.358) | N/A | 0.048 (0.040–0.056) |
| ICC (95% CI) | 0.428 (0.417–0.440) | N/A | 0.107 (0.103–0.111) |
| Estimation Target | Method | Training Set | Validation Set | Test Set | AUROC (95% CI) | MAE (95% CI) | R2 (95% CI) | |
|---|---|---|---|---|---|---|---|---|
| Ours | Age classification | DL | 49,762 | 37,324 | 37,329 | 0.923 (0.922–0.923) | - | - |
| Age regression | DL | 49,762 | 37,324 | 37,329 | - | 8.410 (8.380–8.441) | 0.610 (0.607–0.612) | |
| Sex classification | DL | 49,766 | 37,324 | 37,325 | 0.947 (0.945–0.948) | - | - | |
| Z. I. Attia et al. [25] | Age regression | DL | 399,750 | 99,977 | 275,056 | - | 6.9 (1.3–15.5) | 0.7 |
| Sex classification | DL | 399,750 | 99,977 | 275,056 | 0.968 | - | - | |
| E. M. Lima et al. [38] | Age regression | ML | 185,444 | 32,725 | - | 8.38 (1.38–15.38) | 0.71 | |
| Age regression | ML | 185,444 | 14,263 | - | 8.44 (2.25–14.63) | 0.32 | ||
| Age regression | ML | 185,444 | 1631 | - | 10.04 (2.28–17.8) | 0.35 | ||
| H. E. van der Wall et al. [39] | Age regression | ML | 6110 | 118 | - | 6.9 (1.3–12.5) | 0.72 (0.68–0.76) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryu, J.S.; Lee, S.; Chu, Y.; Koh, S.B.; Park, Y.J.; Lee, J.Y.; Yang, S. Deep Learning Algorithms for Estimation of Demographic and Anthropometric Features from Electrocardiograms. J. Clin. Med. 2023, 12, 2828. https://doi.org/10.3390/jcm12082828
Ryu JS, Lee S, Chu Y, Koh SB, Park YJ, Lee JY, Yang S. Deep Learning Algorithms for Estimation of Demographic and Anthropometric Features from Electrocardiograms. Journal of Clinical Medicine. 2023; 12(8):2828. https://doi.org/10.3390/jcm12082828
Chicago/Turabian StyleRyu, Ji Seung, Solam Lee, Yuseong Chu, Sang Baek Koh, Young Jun Park, Ju Yeong Lee, and Sejung Yang. 2023. "Deep Learning Algorithms for Estimation of Demographic and Anthropometric Features from Electrocardiograms" Journal of Clinical Medicine 12, no. 8: 2828. https://doi.org/10.3390/jcm12082828
APA StyleRyu, J. S., Lee, S., Chu, Y., Koh, S. B., Park, Y. J., Lee, J. Y., & Yang, S. (2023). Deep Learning Algorithms for Estimation of Demographic and Anthropometric Features from Electrocardiograms. Journal of Clinical Medicine, 12(8), 2828. https://doi.org/10.3390/jcm12082828







