Abstract
This clinical trial aims to compare hormonal and metabolic changes after a 9-week continuous use of oral or vaginal combined hormonal contraceptives (CHCs) in women with polycystic ovary syndrome (PCOS). We recruited 24 women with PCOS and randomized them to use either combined oral (COC, n = 13) or vaginal (CVC, n = 11) contraception. At baseline and 9 weeks, blood samples were collected and a 2 h glucose tolerance test (OGTT) was performed to evaluate hormonal and metabolic outcomes. After treatment, serum sex hormone binding globulin (SHBG) levels increased (p < 0.001 for both groups) and the free androgen index (FAI) decreased in both study groups (COC p < 0.001; CVC p = 0.007). OGTT glucose levels at 60 min (p = 0.011) and AUCglucose (p = 0.018) increased in the CVC group. Fasting insulin levels (p = 0.037) increased in the COC group, and insulin levels at 120 min increased in both groups (COC p = 0.004; CVC p = 0.042). There was a significant increase in triglyceride (p < 0.001) and hs-CRP (p = 0.032) levels in the CVC group. Both oral and vaginal CHCs decreased androgenicity and tended to promote insulin resistance in PCOS women. Larger and longer studies are needed to compare the metabolic effects of different administration routes of CHCs on women with PCOS.
1. Introduction
Polycystic ovary syndrome (PCOS) is the most common endocrine disorder, affecting 5–18% of women of reproductive age [1,2]. According to the Rotterdam criteria [3] and the international evidence-based PCOS guideline [4], PCOS is defined by at least two of the following three features: (1) polycystic ovaries on gynecological ultrasonography, (2) oligo- or anovulation, and/or (3) clinical and/or biochemical hyperandrogenism (hirsutism or high serum testosterone or androgen levels). Women with PCOS display an increased risk for glucose metabolism disorders [5,6], obesity, hypertension, dyslipidemia, insulin resistance (IR), and metabolic syndrome [5,6,7,8].
Combined hormonal contraceptives (CHCs) are the first-line treatment for the most common PCOS-related clinical manifestations, namely menstrual irregularity and hirsutism [4,9]. However, CHCs are known to induce unfavorable metabolic effects, especially on glucose metabolism, in the general population [10,11,12,13]. Recommendations for CHC use by women with PCOS are generally based on studies of women without PCOS, with a limited number of studies on the use of CHCs in PCOS. Given the metabolic burden related to PCOS, it is plausible that CHCs may worsen already existing metabolic disorders related to the syndrome [14]. Some studies of women without PCOS have indicated that the use of combined vaginal contraception (CVC) causes fewer metabolic effects than combined oral contraception (COC) [12,15], although the findings are inconsistent [13]. Given the widespread use of CVC among women and possibly less adverse systemic effects of vaginal administration due to lower hepatic stress compared with oral administration, there is a necessity for studies to evaluate the metabolic effects of CVC use in women with PCOS.
Although the international evidence-based PCOS guideline recommends COCs in first-line pharmacological management for menstrual irregularity and hyperandrogenism, it does not provide specific recommendations for dosage, mode of administration, or specific preparation [4]. Earlier, the Amsterdam ESHRE/ASRM consensus on women’s health aspects of PCOS concluded that there is a need to perform head-to-head trials comparing different CHC strategies, as well as longitudinal follow-up studies on CHC use in women with PCOS [3].
The aim of this randomized controlled trial was to compare the hormonal and metabolic effects of COC and CVC in women with PCOS after nine weeks.
2. Materials and Methods
This randomized, prospective, open-label, single-centered study was conducted at Oulu University Hospital, Finland, between 2011 and 2016. The study was approved by the Ethics Committee of Oulu University Hospital. All participants signed a written consent document. The study was registered at ClinicalTrials.gov (NCT01588873).
2.1. Study Population (Figure 1)
The participants were selected from the hospital register of Oulu University Hospital according to the ICD10 diagnosis code for PCOS (E 28.2). The women were eligible to participate if they were aged 18–40 years, healthy, and without medical contraindications for the use of CHCs (high blood pressure, migraine with focal aura, severe or multiple risk factors to thromboembolism, acute or chronic hepatocellular disease or hepatic adenomas or carcinomas, unexplained abnormal vaginal bleeding, diagnosed or suspected cancer, or an estrogen-dependent tumor), not using any medication, not smoking, not pregnant or breastfeeding, and had not used any hormonal or cortisone medicines for at least 2 months prior to study entry.

Figure 1.
Flowchart of the study.
The participants were randomized to use either a combined hormonal oral contraceptive pill (COC group: ethinylestradiol, EE, 20 µg and desogestrel, 150 µg; Mercilon®; Organon Ltd., Dublin, Ireland) or a combined hormonal contraceptive vaginal ring (CVC group: EE, 15 µg/day and etonogestrel, an active metabolite of desogestrel, 120 µg/day; NuvaRing®; N.V.Organon, Oss, Netherlands) continuous for 9 weeks. The randomization list (allocation 1:1) was computer-generated. The participants went through two clinical examinations, the first at baseline and the second at 9 weeks of treatment, which included a gynecological examination, a transvaginal ultrasound (endometrium thickness, ovarian volumes, and the number of follicles), blood sampling for hormonal and metabolic parameters, and an oral glucose tolerance test (OGTT). At baseline, the clinical examination was performed between cycle days 1–3 and, at the ninth study week, within 3 days from the beginning of menstruation after discontinuation of the contraceptive preparation.
In all, 24 women with PCOS were recruited, 13 in the COC group and 11 in the CVC group (Figure 1). Diagnosis of PCOS was made according to the Rotterdam criteria. The baseline characteristics of the participants are described in Table 1.

Table 1.
Baseline characteristics of the study population.
2.2. Oral Glucose Tolerance Test
The 75 g 2 h oral OGTT was performed after 12 h of fasting at baseline and 9 weeks. Blood samples were taken at 0, 30, 60, and 120 min. Glucose and insulin areas under the curve (glucose AUC and insulin AUC), the homeostatic model assessment of insulin resistance (HOMA-IR), the homeostatic model assessment of β-cell function (HOMA-2β), and whole-body insulin sensitivity (i.e., Matsuda index) [16] were calculated based on OGTT results to evaluate glucose tolerance, IR, and insulin sensitivity.
Although the hyperinsulinemic-euglycemic glucose clamp is the gold standard for evaluating insulin sensitivity, it is costly, time-consuming, invasive, and requires staff. The calculated indexes, such as HOMA-IR and Matsuda, have been shown to estimate insulin resistance and sensitivity more easily [16,17,18].
HOMA-IR (=insulin (mU/L) × glucose (mmol/L)/22.5) is a calculated index used to quantify IR from basal glucose and insulin levels and was first described in 1985 by Matthews et al. [18]. A strong linear correlation of HOMA-IR with the clamp has been found [17,18]. In women with PCOS, HOMA-IR has been used in various studies of different populations to assess IR [19,20,21,22] and has proven to be a robust clinical and epidemiological tool for assessing IR. HOMA-2β (=20 × fasting insulin (μIU/mL)/fasting glucose (mmol/mL) − 3.5) has been used as a marker of basal insulin secretion by pancreatic β-cells [21].
The Matsuda index (=[10,000/√fasting glucose × fasting insulin) (mean glucose (OGTT) × mean insulin OGTT)]) was described by Matsuda and DeFronzo in 1999. It estimates whole-body physiological insulin sensitivity [16]. In women with PCOS, the Matsuda index correlates well with HOMA-IR and the quantitative insulin-sensitivity check index (QUICKI), which indicates its reliability in the detection of IR [23,24].
2.3. Assays
Serum samples for the assay of total testosterone (T) were conducted by using Agilent triple quadrupole 6410 liquid chromatography–mass spectrometry (LC-MS) equipment with an electrospray ionization source operating in positive-ion mode (Agilent Technologies, Wilmington, DE, USA). Multiple reaction monitoring was used to quantify T by using trideuterated T (d3-T) with the following transitions: m/z 289.2 to 97 and 289.2 to 109 for T and 292.2 to 97 and 292.2 to 109 for d3-T. The intra-assay coefficients of variation (CVs) of the method were 5.3%, 1.6%, and 1.2% for T at 0.6, 6.6, and 27.7 nmol/L, respectively.
Sex hormone binding globulin (SHBG) was analyzed by chemiluminometric immunoassays (Immulite 2000, Siemens Healthcare Diagnostics, Los Angeles, CA, USA) with a sensitivity of 0.02 nmol/L. Serum glucose, total cholesterol, low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and triglycerides were assayed using an automatic chemical analyzer (Advia, 1800; Siemens Healthcare Diagnostics, Tarrytown, NY, USA), insulin by using an automated a chemiluminescence system (Advia Centaur; Siemens Healthcare Diagnostics, Tarrytown, NY, USA), and high-sensitivity C-reactive protein (hs-CRP) by using an immunonephelometry (BN ProSpec; Siemens Healthcare Diagnostics, Marburg, Germany). All samples (baseline and 9 weeks) from the same subject were analyzed in the same assay.
2.4. Statistical Analyses
Power calculation was based on our previous study comparing the metabolic effects of the same preparations (Mercilon® and Nuvaring®) in young healthy women [13]. That study showed a significant increase of 0.44 mmol in serum triglyceride levels at 9 weeks of treatment with both preparations. The power analysis indicated that 17 women would have been needed in both study groups to reveal a similar increase in the serum level of triglycerides. To allow for dropouts, the planned sample size was 40 women (20 women in each group). Unfortunately, because of the strong criticism at the time of the recruitment raised in the media toward the thromboembolic risks linked to the use of hormonal contraception, the recruitment was extremely slow, and we managed eventually to recruit 24 women, 13 in the COC group and 11 in the CVC group.
All variables are present as means with standard deviation (SD) in Table 2. Paired samples t-tests were performed for normally distributed variables and Wilcoxon’s tests were used for variables with a skewed distribution to explore changes in hormonal and metabolic levels within the same study group at the baseline and during the treatment. To analyze the differences between the study groups and the change from baseline to the 9th study week, we used a linear mixed model (repeated measures) with a random intercept. All results were adjusted with the BMI and age of the participants.

Table 2.
Differences in the parameters of androgen secretion, glucose metabolism, lipid profile, and inflammation between the study groups. Analyses were performed with a linear mixed model with a random intercept.
Statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) software (version 28.0 for Windows, SPSS Inc., Chicago, IL, USA). The statistical significance level was set at p ≤ 0.05.
3. Results
3.1. Anthropometric Parameters
At baseline, women in the COC group were older (32.4 vs. 30.8 years, p = 0.051), and their BMI tended to be higher (25.4 vs. 23.5, p = 0.068) compared to the CVC group (Table 1). At baseline and 9 weeks of treatment, there were no significant differences between the two study groups regarding BMI (p = 0.245), waist circumference (WC, p = 0.812), diastolic blood pressure (dBP, p = 0.550), or systolic blood pressure (sBP, p = 0.613) (Table 2).
3.2. Serum Levels of Androgens and SHBG
There were no significant differences in serum levels of testosterone at 9 weeks between the groups. However, SHBG levels increased significantly in both groups (COC p < 0.001, CVC p < 0.001) between baseline and week 9, and FAI was decreased in both groups (COC p < 0.001, CVC p = 0.007) (Table 3). The changes in SHBG and FAI between baseline and week 9 were similar within the groups (Table 2).

Table 3.
Parameters (mean ± standard deviation, SD) related to androgen secretion, glucose metabolism, lipid profile, and inflammation in the study groups.
3.3. Oral Glucose Tolerance Test (OGTT)
In the OGTT, glucose levels at 60 min were higher after 9 weeks of treatment compared to baseline (p = 0.008, adjusted p = 0.011) in the CVC group. Further, glucose AUC was increased significantly in the CVC group (p = 0.018, adjusted p = 0.034) (Table 3, Figure 2).
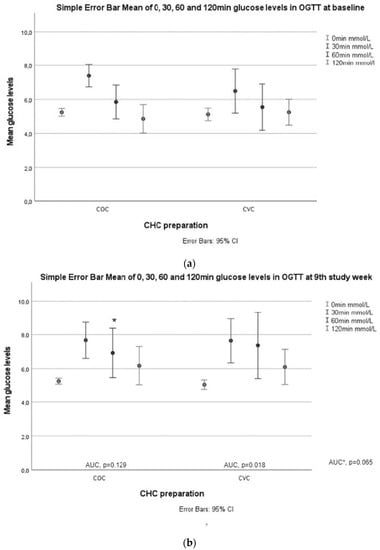
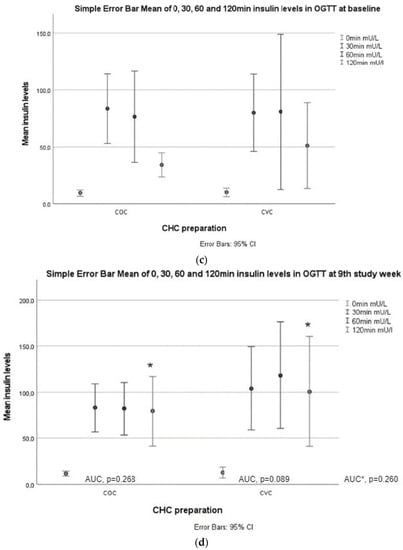
Figure 2.
Glucose and insulin responses during OGTT. (a) Glucose values at 0, 30, 60, and 120 min in both study groups at baseline; (b) glucose values at 0, 30, 60, and 120 min in both study groups week 9. AUCglucose values between baseline and the ninth week in the COC and CVC groups. AUC * is AUCglucose between the study groups. An asterisk (*) marks a significant increase in 60 min glucose value in the CVC group compared to baseline; (c) insulin values at 0, 30, 60, and 120 min in both study groups at baseline.; (d) insulin values at 0, 30, 60, and 120 min in both study groups at baseline. AUCinsulin values between baseline and the ninth week in the COC and CVC groups. AUC * is AUCinsulin between the study groups at week 9. An asterisk (*) marks a significant increase in 120 min insulin values in both groups compared to baseline.
Fasting insulin levels (p = 0.037, adjusted p = 0.023) increased after 9 weeks of treatment in the COC group, and insulin levels at 120 min increased in both groups (COC p = 0.005, adjusted p = 0.004; CVC p = 0.028, adjusted p = 0.042) (Table 3, Figure 2).
HOMA-2β levels increased significantly in the COC group (p = 0.011), but the change became nonsignificant after adjustments (adjusted p = 0.10) (Table 3). HOMA-2β differed significantly between the two groups (p = 0.037, adjusted p = 0.030), but the increase in HOMA-2β levels was similar in both groups (Table 2).
3.4. Serum Lipids and hs-CRP
Serum levels of triglycerides (p < 0.001, adjusted p < 0.001) increased significantly at 9 weeks of treatment in the CVC group (Table 3). There were differences in triglyceride levels (p = 0.030, adjusted p = 0.103) between baseline and 9 weeks; the levels increased, and the change differed between the groups (p = 0.542, adjusted p = 0.046) (Table 2).
4. Discussion
In this study, both COC and CVC decreased androgenicity but displayed only mild effects on glucose metabolism, IR, lipid profile, and chronic inflammation. Though the study failed to recruit the targeted number of women, the data contrasted with our hypothesis, as CVC did not seem to have a more beneficial hormonal or metabolic profile than COC in PCOS.
CHC preparations are the most used medical treatment for PCOS, as they reduce menstrual abnormalities and relieve manifestations of clinical hyperandrogenism (acne and hirsutism). In the present study, a 9-week use of 20 μg EE + DSG or 15 μg/d EE/EGS caused an approximately 200–270% increase in SHBG levels and a subsequent 74–84% decrease in FAI levels, in line with the results of previous studies [13,15,25]. Some studies have reported a greater increase in SHBG during oral CHC use [13,26], whereas others have found that SHBG increased more during CVC treatment [15,25]. The present results suggest that the routes are comparably efficient in improving hyperandrogenemia in PCOS.
In some studies, CVC was considered to cause fewer adverse changes in glucose metabolism and insulin sensitivity in general female populations [15]. In the present study, after 9 weeks of treatment, AUCglucose increased in the CVC group. A small compensatory increase in insulin secretion was observed in both groups. In the COC group, fasting insulin and 120 min insulin levels increased and, in the CVC group, there was an increase in 120 min insulin levels. These results are partly in line with those of our previous study, which demonstrated a reduction in insulin sensitivity and an increase in AUCglucose levels among 54 healthy young women who were given oral, vaginal, or transdermal CHCs continuously for 9 weeks [13]. However, not all studies agree on the detrimental effect of CHCs on glucose metabolism. In one study, CVC users did not experience any changes in carbohydrate metabolism compared to COC users over five cycles [15]. Furthermore, CVC for 24 months was found to be safe in women with type 1 diabetes when estimated by glycosylated hemoglobin levels [27]. Moreover, in the only randomized study performed with women with PCOS (n = 37), CVC improved insulin sensitivity and glucose tolerance, whereas COC (containing EE + drospirenone) worsened IR and insulin secretion at 6 months of treatment [28]. Comparisons with previous studies are challenging because studies differ regarding CHC preparations and the methods used to evaluate glucose metabolism and IR. The gold standard for assessing IR is the hyperinsulinemic-euglycemic glucose clamp technique. However, several measurements based on serum glucose and insulin response to glucose intake, such as HOMA-IR, HOMA-2β, and the Matsuda index, have been shown to be useful. These indexes have been shown to reliably reflect IR in several previous studies in women with PCOS [19,20,21,22]. On the other hand, it is unclear whether patients presenting mild IR (i.e., an altered response to a clamp but a normal HOMA-IR and a normal response to an oral glucose test) display any additional clinical problems when compared to patients with a normal response to the clamp. Indeed, there are also concerns that the evaluation of IR in women with PCOS is method-dependent, and there may be discrepancies between markers [29]. In addition, lean women with PCOS may be more easily misclassified as insulin sensitive [30]. Nonetheless, our results raise concerns that CVC may not be safer than COC in women with PCOS regarding its effects on glucose metabolism.
In the present study, changes in lipid profile showed an increase in triglyceride levels in the CVC group, and the increase was slightly greater in the CVC group. These results align with those of previous studies among women with PCOS, showing that COC use is associated with increased levels of triglycerides but also HDL and total cholesterol levels [14,31]. Similar results were generated in a study performed on healthy women treated with oral, transdermal, or vaginal CHCs for 9 weeks, showing an increase in triglycerides and HDL in all study groups [13]. Comparably, oral EE administration has been shown to result in increased total cholesterol, mainly due to increased HDL cholesterol and triglycerides [32,33,34]. A similar effect during the use of CVC has also been described [35]. High triglyceride levels seem to be an independent predictor of the future risk of myocardial infarction [36] and have been associated with elevated cardiovascular risks [37], specifically in women [37]. A meta-analysis of CHC effects on the lipid profile in PCOS women concluded that desogestrel containing CHCs increased triglyceride levels after 6 months of treatment [33]. In the present study, triglyceride levels e increased significantly in the CVC group. A 9-week study is too short for solid conclusions regarding lipid profile changes with COC or CVC use. However, our results do not support the recommendation that vaginal CHC be preferred to oral preparations to decrease the cardiovascular risks linked to PCOS. As an abnormal lipid profile is typically present in women with PCOS [38], larger, long-lasting follow-up studies and real-world register data analyses are needed to clarify whether the use of CHCs (either oral or vaginal) will increase the risk of cardiovascular morbidity and mortality in women with PCOS. It is possible that the alleviation of hyperandrogenemia with CHCs overcomes mild impairments in metabolic parameters.
Serum levels of hs-CRP increased in the CVC group after 9 weeks of treatment. Despite differences in CRP levels, the change in hs-CRP levels was similar between the two groups. This result is in line with studies performed with oral and vaginal preparations [39,40] and with our recently published data showing EE to be a strong promoter of chronic low-grade inflammation [13,41]. This is an important finding, as women with PCOS have been shown to display chronic inflammation [42,43], which, in turn, is associated with an increased risk of cardiovascular diseases and events, as well as overall mortality [44]. Of note, the findings of a recent study comparing estradiol valerate (EV) with EE among healthy women suggest that EV could display a more neutral effect on inflammation and lipids [41]. Further randomized studies are needed to clarify whether preparations containing EV instead of EE could be safer regarding cardiovascular risks in women with PCOS.
Strengths and Limitations
The main strengths of the present study are that PCOS diagnosis was made by a gynecologist and the participants were homogeneous concerning ethnicity, as all were Caucasians. All in all, the study provides important data for future meta-analyses. The weaknesses of the study are the failure to recruit enough participants to meet the power calculation criteria for a sufficient sample size and to engage participants to finish the study, which underscores the challenge of running a randomized clinical trial. Additionally, a slightly higher BMI (nonsignificant) and age at baseline in the COC group may have influenced the results. Further, the power calculation was based on changes in triglycerides, not on glucose metabolism parameters or inflammation markers, which must be taken into consideration when interpreting the results. Additionally, as discussed earlier, the hyperinsulinemic-euglycemic glucose clamp technique is the gold standard for IR measurement, but it is costly, time-consuming, invasive, and requires staff. We used basal and OGTT-derived calculated indexes (HOMA-IR, HOMA-2β, and the Matsuda index) to evaluate IR in women with PCOS. Lastly, the short follow-up period does not permit conclusions to be drawn on the long-term consequences of the use of COC or CVC, warranting future studies.
5. Conclusions
Contrary to our hypothesis, CVC did not seem to be metabolically safer than COC based on this short clinical study. As there are a limited number of studies assessing different administration routes of CHCs for women with PCOS, the results show some new data and underline the need for larger and longer studies comparing the metabolic effects of CHC administration routes in PCOS.
Author Contributions
Conceptualization, T.P., J.S.T. and L.M.-P.; methodology, T.P., J.S.T. and L.M.-P.; software, T.P. and L.M.-P.; validation, T.P., J.S.T. and L.M.-P.; formal analysis, M.-E.M., R.B. and E.K.; investigation, A.S.R., M.-E.M., T.P. and L.M.-P.; resources, T.P., J.S.T. and L.M.-P.; data curation, M.-E.M. and R.B.; writing—original draft preparation, M.-E.M.; writing—review and editing, M.-E.M., T.P., M.K., J.S.T. and L.M.-P.; visualization, M.-E.M., J.S.T. and L.M.-P.; supervision, J.S.T. and L.M.-P.; project administration, J.S.T. and L.M.-P.; funding acquisition, T.P., J.S.T. and L.M.-P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by grants from the Sigrid Juselius Foundation, the Academy of Finland (grant number 295760), and Oulu University Medical Research Center, and open access funding provided by University of Helsinki.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Regional Ethics Committee of Northern Ostrobothnia Hospital District (number 106/2011, approval date 23 January 2012).
Informed Consent Statement
Written consent was obtained from all subjects involved in the study.
Data Availability Statement
Data cannot be shared openly but are available on request from authors upon reasonable request.
Acknowledgments
Open access funding provided by University of Helsinki.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Franks, S. Polycystic ovary syndrome. N. Engl. J. Med. 1995, 333, 853–861. [Google Scholar] [CrossRef] [PubMed]
- March, W.A.; Moore, V.M.; Willson, K.J.; Phillips, D.I.W.; Norman, R.J.; Davies, M.J. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum. Reprod. 2010, 25, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil. Steril. 2004, 81, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Teede, H.J.; Misso, M.L.; Costello, M.F.; Dokras, A.; Laven, J.; Moran, L.; Piltonen, T.; Norman, R.J.; Internatiol PCOS Network. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Fertil. Steril. 2018, 110, 364–379. [Google Scholar] [CrossRef] [PubMed]
- Kakoly, N.S.; Khomami, M.B.; Joham, A.E.; Cooray, S.D.; Misso, M.L.; Norman, R.J.; Harrison, C.L.; Ranasinha, S.; Teede, H.J.; Moran, L.J. Ethnicity, obesity and the prevalence of impaired glucose tolerance and type 2 diabetes in PCOS: A systematic review and meta-regression. Hum. Reprod. Update 2018, 24, 455–467. [Google Scholar] [CrossRef]
- Teede, H.; Deeks, A.; Moran, L. Open Access REVIEW Polycystic ovary syndrome: A complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC Med. 2010, 8, 41. [Google Scholar] [CrossRef]
- Ollila, M.M.E.; West, S.; Keinänen-Kiukaanniemi, S.; Jokelainen, J.; Auvinen, J.; Puukka, K.; Ruokonen, A.; Järvelin, M.-R.; Tapanainen, J.S.; Franks, S.; et al. Overweight and obese but not normal weight women with PCOS are at increased risk of Type 2 diabetes mellitus-a prospective, population-based cohort study. Hum. Reprod. 2017, 32, 423–431. [Google Scholar] [CrossRef]
- Fauser, B.C.J.M.; Tarlatzis, B.C.; Rebar, R.W.; Legro, R.S.; Balen, A.H.; Lobo, R.; Carmina, E.; Chang, J.; Yildiz, B.O.; Laven, J.S.E.; et al. Consensus on women’s health aspects of polycystic ovary syndrome (PCOS): The Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Fertil. Steril. 2012, 97, 28–38. [Google Scholar] [CrossRef]
- Sanches De Melo, A.; Maria, R.; Rui, R.; Vieira, C.S. Hormonal contraception in women with polycystic ovary syndrome: Choices, challenges, and noncontraceptive benefits. Open Access J. Contracept. 2017, 8, 13–23. [Google Scholar] [CrossRef]
- Chasan-Taber, L.; Stampfer, M.J. Epidemiology of oral contraceptives and cardiovascular disease. Ann. Intern. Med. 1998, 128, 467–477. [Google Scholar] [CrossRef]
- Morin-Papunen, L.; Martikainen, H.; McCarthy, M.I.; Franks, S.; Soivio, U.; Hartikainen, A.-L.; Ruokonen, A.; Leinonen, M.; Laitinen, J.; Järvelin, M.R.; et al. Comparison of metabolic and inflammatory outcomes in women who used oral contraceptives and the levonorgestrel-releasing intrauterine device in a general population. Am. J. Obs. Gynecol. 2008, 199, 529.e1–529.e10. [Google Scholar] [CrossRef] [PubMed]
- Cagnacci, A.; Ferrari, S.; Tirelli, A.; Zanin, R.; Volpe, A. Route of administration of contraceptives containing desogestrel/etonorgestrel and insulin sensitivity: A prospective randomized study. Contraception 2009, 80, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Piltonen, T.; Puurunen, J.; Hedberg, P.; Ruokonen, A.; Mutt, S.J.; Herzig, K.H.; Nissinen, A.; Morin-Papunen, L.J.; Tapanainen, J.S. Oral, transdermal and vaginal combined contraceptives induce an increase in markers of chronic inflammation and impair insulin sensitivity in young healthy normal-weight women: A randomized study. Hum. Reprod. 2012, 27, 3046–3056. [Google Scholar] [CrossRef]
- Halperin, I.J.; Sujana Kumar, S.; Stroup, D.F.; Laredo, S.E. The association between the combined oral contraceptive pill and insulin resistance, dysglycemia and dyslipidemia in women with polycystic ovary syndrome: A systematic review and meta-analysis of observational studies. META-ANALYSIS Reproductive endocrinology. Hum. Reprod. 2011, 26, 191–201. [Google Scholar] [PubMed]
- Elkind-Hirsch, K.E.; Darensbourg, C.; Ogden, B.; Ogden, L.F.; Hindelang, P. Contraceptive vaginal ring use for women has less adverse metabolic effects than an oral contraceptive. Contraception 2017, 76, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, M.; DeFronzo, R.A. Insulin sensitivity indices obtained from oral glucose tolerance testing: Comparison with the euglycemic insulin clamp. Diabetes Care 1999, 22, 1462–1470. [Google Scholar] [CrossRef]
- Bonora, E.; Targher, G.; Alberiche, M.; Bonadonna, R.C.; Saggiani, F.; Zenere, M.B.; Monauni, T.; Muggero, M. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: Studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care 2000, 23, 57–63. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; NAylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Amisi, C.; Mputu, L.; Mboloko, E.; Pozzili, P. Biological insulin resistance in Congolese woman with polycystic ovary syndrome (PCOS). Gynecol. Obs. 2013, 41, 707–710. [Google Scholar]
- Amisi, C.A.; Ciccozzi, M.; Pozzili, P. Wrist circumference: A new marker for insulin resistance in African women with polycystic ovary syndrome. World J. Diabetes 2020, 11, 42–51. [Google Scholar] [CrossRef]
- DeUgarte, C.M.; Bartolucci, A.A.; Azziz, R. Prevalence of insulin resistance in the polycystic ovary syndrome using the homeostasis model assessment. Fertil. Steril. 2005, 83, 1454–1460. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.J.; Young, R.; Kuo, I.L.; Liaw, C.M.; Chiang, H.S.; Yeh, C.Y. Prevalence of insulin resistance and determination of risk factors for glucose intolerance in Polycystic ovary syndrome: A cross-sectional study of Chinese infertility patients. Fertil. Steril. 2009, 91, 1864–1868. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, M.; Berneis, K.; Spinas, G.; Rini, G.B.; Carmina, E. Long-term consequences of polycystic ovary syndrome on cardiovascular risk. Fertil. Steril. 2009, 91, 1563–1567. [Google Scholar] [CrossRef] [PubMed]
- Ciampelli, M.; Leoni, F.; Cucinelli, F.; Mancuso, S.; Panunzi, S.; De Gaetano, A.; Lanzone, A. Assessment of insulin sensitivity from measurements in the fasting state and during an oral glucose tolerance test in polycystic ovary syndrome and menopausal patients. J. Clin. Endocrinol. Metab. 2005, 90, 1398–1406. [Google Scholar] [CrossRef]
- Tuppurainen, M.; Klimscheffskij, R.; Venhola, M.; Dieben, T.O.M. The combined contraceptive vaginal ring (NuvaRing) and lipid metabolism: A comparative study. Contraception 2004, 69, 389–394. [Google Scholar] [CrossRef]
- van den Heuvel, M.W.; van Bragt, A.J.M.; Alnabawy, A.K.M.; Kaptein, M.C.J. Comparison of ethinylestradiol pharmacokinetics in three hormonal contraceptive formulations: The vaginal ring, the transdermal patch and an oral contraceptive. Contraception 2005, 72, 168–174. [Google Scholar] [CrossRef]
- Duijkers, I.; Killick, S.; Bigrigg, A.; Diebem, T. A comparative study on effects of a contraceptive vaginal ring Nuvaring and an oral contraceptive on carbohydrate metabolism and adrenal and thyroid function. Eur. J. Contracept. Reprod. Health Care 2004, 9, 131–140. [Google Scholar] [CrossRef]
- Grodnitskaya, E.E.; Grigoryan, O.R.; Klinyshkova, E.V.; Andreeva, E.N.; Melnichenko, G.A.; Dedov, I.I. Effect on carbohydrate metabolism and analysis of acceptability (menstrual cycle control) of extended regimens of the vaginally inserted hormone-releasing system “NuvaRing” as compared with the standard 21/7 regime in reproductive-age women with type 1 d. Gynecol. Endocrinol. 2010, 26, 663–668. [Google Scholar] [CrossRef]
- Battaglia, C.; Mancini, F.; Cianciosi, A.; Busacchi, P.; Facchinetti, F.; Marchesini, G.R.; Marzocchi, R.; de Aloysio, D. Vascular risk in young women with polycystic ovary and polycystic ovary syndrome. Obstet. Gynecol. 2008, 111, 385–395. [Google Scholar] [CrossRef]
- Battaglia, C.; Mancini, F.; Fabbri, R.; Persico, N.; Busacchi, P.; Facchinetti, F.; Venturoli, S. Polycystic ovary syndrome and cardiovascular risk in young patients treated with drospirenone-ethinylestradiol or contraceptive vaginal ring. A prospective, randomized, pilot study. Fertil. Steril. 2010, 94, 1417–1425. [Google Scholar] [CrossRef]
- Lewandowski, K.C.; Skowrońska-Jóźwiak, E.; Łukasiak, K.; Gałuszko, K.; Dukowicz, A.; Cedro, M.; Lewiński, A. How much insulin resistance in polycystic ovary syndrome? Comparison of HOMA-IR and insulin resistance (Belfiore) index models. Arch. Med. Sci. 2019, 15, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Tosi, F.; Bonora, E.; Moghetti, P. Insulin resistance in a large cohort of women with polycystic ovary syndrome: A comparison between euglycaemic-hyperinsulinaemic clamp and surrogate indexes. Hum. Reprod. 2017, 1, 2515–2521. [Google Scholar] [CrossRef] [PubMed]
- Amiri, M.; Ramezani Tehrani, F.; Nahidi, F.; Kabir, A.; Azizi, F.; Carmina, E. Effects of oral contraceptives on metabolic profile in women with polycystic ovary syndrome: A meta-analysis comparing products containing cyproterone acetate with third generation progestins. Metabolism 2017, 73, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Åkerlund, M.; Almström, E.; Högstedt, S.; Nabrink, M. Oral contraceptive tablets containing 20 and 30 micrograms of ethinyl estradiol with 150 micrograms desogestrel. Their influence on lipids, lipoproteins, sex hormone binding globulin and testosterone. Acta Obstet. Gynecol. Scand. 1994, 73, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Guazzelli, C.A.; Barreiros, F.A.; Torloni, M.R.; Barbieri, M. Effects of extended regimens of the contraceptive vaginal ring on carbohydrate metabolism. Contraception 2012, 85, 253–256. [Google Scholar] [CrossRef] [PubMed]
- Wiegratz, I.; Stahlberg, S.; Manthey, T.; Sänger, N.; Mittmann, K.; Palombo-Kinne, E.; Mellinger, U.; Lange, E.; Kuhl, H. Effects of an oral contraceptive containing 30 mcg ethinyl estradiol and 2 mg dienogest on lipid metabolism during 1 year of conventional or extended-cycle use. Contraception 2010, 81, 57–61. [Google Scholar] [CrossRef]
- Toth, P.P.; Fazio, S.; Wong, N.D.; Hull, M.; Nichols, G.A. Risk of cardiovascular events in patients with hypertriglyceridaemia: A review of real-world evidence. Diabetes Obes. Metab. 2020, 22, 279–289. [Google Scholar] [CrossRef]
- Contreras-Manzano, A.; Villalpando, S.; García-Diaz, C.; Flores-Aldana, M. Cardiovascular risk factors and their association with vitamin D deficiency in Mexican women of reproductive age. Nutrients 2019, 11, 1211. [Google Scholar] [CrossRef]
- Cauci, S.; Di Santolo, M.; Culhane, J.F.; Stel, G.; Gonano, F.; Guaschino, S. Effects of third-generation oral contraceptives on high-sensitivity C-reactive protein and homocysteine in young women. Obs. Gynecol. 2008, 111, 857–864. [Google Scholar] [CrossRef]
- Divani, A.A.; Luo, X.; Datta, Y.H.; Flaherty, J.D.; Panoskaltsis-Mortari, A. Effect of oral and vaginal hormonal contraceptives on inflammatory blood biomarkers. Mediat. Inflamm. 2015, 2015, 379501. [Google Scholar] [CrossRef]
- Kangasniemi, M.H.; Haverinen, A.; Luiro, K.; Hiltunen, K.; Komsi, E.K.; Arffman, R.K.; Heikinheimo, O.; Tapanainen, J.S.; Piltonen, T.T. Estradiol Valerate in COC Has More Favorable Inflammatory Profile Than Synthetic Ethinyl Estradiol: A Randomized Trial. J. Clin. Endocrinol. Metab. 2020, 105, e2483–e2490. [Google Scholar] [CrossRef] [PubMed]
- Dehdashtihaghighat, S.; Mehdizadehkashi, A.; Arbabi, A.; Pishgahroudsari, M.; Chaichian, S. Assessment of C-reactive Protein and C3 as Inflammatory Markers of Insulin Resistance in Women with Polycystic Ovary Syndrome: A Case-Control Study. J. Reprod. Infertil. 2013, 14, 197–201. [Google Scholar] [PubMed]
- Güdücü, N.; İşçi, H.; Yiğiter, A.B.; Dünder, I. C-reactive protein and lipoprotein-a as markers of coronary heart disease in polycystic ovary syndrome. J. Turk.-German Gynecol. Assoc. 2012, 13, 227–259. [Google Scholar] [CrossRef] [PubMed]
- The Emerging Risk Factors Collaboration. C-reactive protein concentration and risk of coronary heart disease, stroke and mortality: An individual participant meta-analysis. Lancet 2010, 375, 132–140. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).