Evaluation of the Reduction of Skin Hyperpigmentation Changes under the Influence of a Preparation Containing Kojic Acid Using Hyperspectral Imaging—Preliminary Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Methods
2.2.1. The Procedure of the Applied Treatment
2.2.2. ROI Images in Visible Light
2.2.3. Hyperspectral Imaging
2.2.4. GLCM
- i is the brightness of the tested pixel;
- j is the neighboring pixel brightness.
- i is the brightness of the tested pixel;
- j is the neighboring pixel brightness.
2.2.5. Statistical Analysis
3. Results
3.1. Imaging
3.2. Quantitative Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nautiyal, A.; Wairkar, S. Management of hyperpigmentation: Current treatments and emerging therapies. Pigment. Cell Melanoma Res. 2021, 34, 1000–1014. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Park, J.H.; Kim, S.J.; Kwon, J.E.; Kang, H.Y.; Lee, E.S.; Kim, Y.C. Two histopathological patterns of postinflammatory hyperpigmentation: Epidermal and dermal. J. Cutan. Pathol. 2017, 44, 118–124. [Google Scholar] [CrossRef]
- Nieuweboer-Krobotova, L. Hyperpigmentation: Types, diagnostics and targeted treatment options. J. Eur. Acad. Dermatol. Venereol. 2013, 27 (Suppl. 1), 2–4. [Google Scholar] [CrossRef]
- Vashi, N.A.; Wirya, S.A.; Inyang, M.; Kundu, R.V. Facial Hyperpigmentation in Skin of Color: Special Considerations and Treatment. Am. J. Clin. Dermatol. 2017, 18, 215–230. [Google Scholar] [CrossRef] [PubMed]
- Rzepka, Z.; Buszman, E.; Beberok, A.; Wrześniok, D. From tyrosine to melanin: Signaling pathways and factors regulating melanogenesis. Postep. Hig. Med. Dosw. 2016, 70, 695–708. [Google Scholar] [CrossRef] [PubMed]
- Hill, H.Z.; Hill, G.J. UVA, pheomelanin and the carcinogenesis of melanoma. Pigment Cell Res. 2000, 13 (Suppl. 8), 140–144. [Google Scholar] [CrossRef]
- Kadekaro, A.L.; Kavanagh, R.J.; Wakamatsu, K.; Ito, S.; Pipitone, M.A.; Abdel-Malek, Z.A. Cutaneous photobiology. The melanocyte vs. the sun: Who will win the final round? Pigment Cell Res. 2003, 16, 434–447. [Google Scholar] [CrossRef]
- Napolitano, A.; Panzella, L.; Monfrecola, G.; D’Ischia, M. Pheomelanin-induced oxidative stress: Bright and dark chemistry bridging red hair phenotype and melanoma. Pigment Cell Melanoma Res. 2014, 27, 721–733. [Google Scholar] [CrossRef]
- Lizak, M.; Załęska, I.; Matuła, A.; Morawiec, M.; Wasylewski, M. Evaluation of the effectiveness of cosmetic preparations and treatments in people with discolorations of the face skin. Kosmetol. Estet. 2018, 7, 255–262. [Google Scholar]
- Plensdorf, S.; Livieratos, M.; Dada, N. Pigmentation Disorders: Diagnosis and Management. Am. Fam. Physician 2017, 96, 797–804. [Google Scholar]
- Giménez García, R.M.; Carrasco Molina, S. Drug-Induced Hyperpigmentation: Review and Case Series. J. Am. Board. Fam. Med. 2019, 32, 628–638. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, M.; Eslamifar, M.; Khezri, K. Kojic acid applications in cosmetic and pharmaceutical preparations. Biomed. Pharmacother. 2019, 110, 582–593. [Google Scholar] [CrossRef] [PubMed]
- Saraei, M.; Zarrini, G.; Esmati, M.; Ahmadzadeh, L. Novel functionalized monomers based on kojic acid: Snythesis, characterization, polymerization and evalution of antimicrobial activity. Des. Monomers Polym. 2016, 20, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Mohiuddin, A.K. Skin Lightening and Management of Hyperpigmentation. Int. J. Res. Biol. Pharm. 2019, 5, 180020. [Google Scholar]
- Aytemir, D.M.; Karakay, G. Kojic Acid Derivatives. In Medicinal Chemistry and Drug Design; IntechOpen: Rijeka, Croatia, 2012; pp. 1–27. [Google Scholar]
- Van Tran, V.; Loi Nguyen, T.; Moon, J.Y.; Lee, Y.C. Core-shell materials, lipid particles and nanoemulsions, for delivery of active anti-oxidants in cosmetics applications: Challenges and development strategies. Chem. Eng. J. 2018, 368, 88–114. [Google Scholar] [CrossRef]
- Nurunnabi, T. Antimicrobial activity of kojic acid from endophytic fungus Colletotrichum gloeosporioides isolated from Sonneratia apetala, a mangrove plant of the Sundarbans. Asian Pac. J. Trop. Med. 2018, 11, 350–354. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, H.; Chen, M. Kojic acid-mediated damage responses induce mycelial regeneration in the basidiomycete Hypsizygus marmoreus. PLoS ONE 2017, 12, e0187351. [Google Scholar] [CrossRef]
- Brtko, J.; Rondahl, L.; Ficková, M.; Hudecová, D.; Eybl, V.; Uher, M. Kojic acid and its derivatives: History and present state of art. Cent. Eur. J. Public Health 2004, 12 (Suppl. 12), S16–S18. [Google Scholar]
- He, Q.; Wang, R. Hyperspectral imaging enabled by an unmodified smartphone for analyzing skin morphological features and monitoring hemodynamics. Biomed. Opt. Express. 2020, 11, 895–910. [Google Scholar] [CrossRef]
- Vasefi, F.; MacKinnon, N.; Farkas, D.L. Hyperspectral and Multispectral Imaging in Dermatology. In Imaging in Dermatology; Academic Press: Cambridge, MA, USA, 2016; pp. 187–201. [Google Scholar]
- Ortega, S.; Halicek, M.; Fabelo, H.; Callico, G.M.; Fei, B. Hyperspectral and multispectral imaging in digital and computational pathology: A systematic review [Invited]. Biomed. Opt. Express. 2020, 11, 3195–3233. [Google Scholar] [CrossRef]
- Schneider, A.; Feussner, H. Diagnostic Procedures. In Biomedical Engineering in Gastrointestinal Surgery; Academic Press: Cambridge, MA, USA, 2017; pp. 87–220. [Google Scholar] [CrossRef]
- Mäkelä, M.; Geladi, P.; Rissanen, M.; Rautkari, L.; Dahl, O. Hyperspectral near infrared image calibration and regression. Anal. Chim. Acta 2020, 1105, 56–63. [Google Scholar] [CrossRef]
- Rybitwa, D.; Wawrzyk, A.; Łobacz, M.; Machoy, M.; Zeljaś, D.; Wilczyński, S. Hyperspectral imaging and directional reflectance for predicting interaction of laser radiation with biodeteriorated objects threatening human health. Int. Biodeterior. Biodegrad. 2022, 173, 105440. [Google Scholar] [CrossRef]
- Vyas, S.; Meyerle, J.; Burlina, P. Non-invasive estimation of skin thickness from hyperspectral imaging and validation using echography. Comput. Biol. Med. 2015, 57, 173–181. [Google Scholar] [CrossRef]
- D’Mello, S.A.; Finlay, G.J.; Baguley, B.C.; Askarian-Amiri, M.E. Signaling Pathways in Melanogenesis. Int. J. Mol. Sci. 2016, 17, 1144. [Google Scholar] [CrossRef]
- Zhou, J.; Ren, T.; Li, Y.; Cheng, A.; Xie, W.; Xu, L.; Peng, L.; Lin, J.; Lian, L.; Diao, Y.; et al. Oleoylethanolamide inhibits α-melanocyte stimulating hormone-stimulated melanogenesis via ERK, Akt and CREB signaling pathways in B16 melanoma cells. Oncotarget 2017, 8, 56868–56879. [Google Scholar] [CrossRef]
- Hearing, V.J. Biochemical Control of Melanogenesis and Melanosomal Organization. J. Investig. Dermatol. Symp. Proc. 1999, 4, 24–28. [Google Scholar] [CrossRef]
- Schallreuter, K.U. Advances in melanocyte basic science research. Dermatol. Clin. 2007, 25, 283–291. [Google Scholar] [CrossRef]
- Costin, G.E.; Hearing, V.J. Human skin pigmentation: Melanocytes modulate skin color in response to stress. FASEB J. 2007, 21, 976–994. [Google Scholar] [CrossRef]
- Granstein, R.; Sober, A. Drug- and heavy metal-induced hyperpigmentation. J. Am. Acad. Dermatol. 1981, 5, 1–18. [Google Scholar] [CrossRef]
- Saghaie, L.; Pourfarzam, M.; Fassihi, A.; Sartippour, B. Synthesis and tyrosinase inhibitory properties of some novel derivatives of kojic acid. Res. Pharm. Sci. 2013, 8, 233–242. [Google Scholar]
- Engler-Jastrzębska, M.; Kamm, A. Molekularne podstawy pigmentacji skóry: Etiologia i profilaktyka hiperpigmentacji. Kosmetol. Estet. 2019, 8, 275–284. [Google Scholar]
- Burdzy, D.; Ozga, D.; Kosydar-Bochenek, J.; Burdzy, K.; Lewandowski, B. Lasers application in treatment of selected skin problems. Review of available methods. Kosmetol. Estet. 2017, 6, 645–652. [Google Scholar]
- Kaufman, B.P.; Aman, T.; Alexis, A.F. Postinflammatory Hyperpigmentation: Epidemiology, Clinical Presentation, Pathogenesis and Treatment. Am. J. Clin. Dermatol. 2018, 19, 489–503. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.L.; Xu, T.; Chan, D.S.; Man, B.Y.; Fong, W.F.; Leung, C.H. A highly selective, label-free, homogenous luminescent switch-on probe for the detection of nanomolar transcription factor NF-kappaB. Nucleic Acids Res. 2011, 39, e67. [Google Scholar] [CrossRef]
- Moon, K.Y.; Ahn, K.S.; Lee, J.; Kim, Y.S. Kojic acid, a potential inhibitor of NF-kappaB activation in transfectant human HaCaT and SCC-13 cells. Arch. Pharm. Res. 2001, 24, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Owolabi, J.O.; Fabiyi, O.S.; Adelakin, L.A.; Ekwerike, M.C. Effects of Skin Lightening Cream Agents—Hydroquinone and Kojic Acid, on the Skin of Adult Female Experimental Rats. Clin. Cosmet. Investig. Dermatol. 2020, 13, 283–289. [Google Scholar] [CrossRef]
- Tsunoda, K.; Akasaka, K.; Akasaka, T.; Amano, H. Successful treatment of erythematotelangiectatic rosacea with intense pulsed light: Report of 13 cases. J. Dermatol. 2018, 45, 1113–1116. [Google Scholar] [CrossRef]
- Henseler, H. Measurement of the effects of the IMAGE MD® skin care regimen on skin surface features via modern imaging technology with the Visia® complexion analysis camera system. GMS Interdiscip. Plast. Reconstr. Surg. DGPW 2022, 11. [Google Scholar] [CrossRef]
- Xu, Y.; Ma, R.; Juliandri, J.; Wang, X.; Xu, B.; Wang, D.; Lu, Y.; Zhou, B.; Luo, D. Efficacy of functional microarray of microneedles combined with topical tranexamic acid for melasma: A randomized, self-controlled, split-face study. Medicine 2017, 96, 6897. [Google Scholar] [CrossRef]
- An, J.H.; Lee, H.J.; Yoon, M.S.; Kim, D.H. Anti-Wrinkle Efficacy of Cross-Linked Hyaluronic Acid-Based Microneedle Patch with Acetyl Hexapeptide-8 and Epidermal Growth Factor on Korean Skin. Ann. Dermatol. 2019, 31, 263–271. [Google Scholar] [CrossRef]
- Ratanapokasatit, Y.; Sirithanabadeekul, P. The Efficacy and Safety of Epidermal Growth Factor Combined with Fractional Carbon Dioxide Laser for Acne Scar Treatment: A Split-Face Trial. J. Clin. Aesthetic Dermatol. 2022, 15, 44–48. [Google Scholar]
- Bloemen, M.C.; van Gerven, M.S.; van der Wal, M.B.; Verhaegen, P.D.; Middelkoop, E. An objective device for measuring surface roughness of skin and scars. J. Am. Acad. Dermatol. 2011, 64, 706–715. [Google Scholar] [CrossRef]
- Lim, J.T. Treatment of melasma using kojic acid in a gel containing hydroquinone and glycolic acid. Dermatol. Surg. 1999, 25, 282–284. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.; Fulton, J.E., Jr. The combination of glycolic acid and hydroquinone or kojic acid for the treatment of melasma and related conditions. Dermatol. Surg. 1996, 22, 443–447. [Google Scholar] [CrossRef]
- Deo, K.S.; Dash, K.N.; Sharma, Y.K.; Virmani, N.C.; Oberai, C. Kojic Acid vis-a-vis its Combinations with Hydroquinone and Betamethasone Valerate in Melasma: A Randomized, Single Blind, Comparative Study of Efficacy and Safety. Indian J. Dermatol. 2013, 58, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Nkengne, A.; Robic, J.; Seroul, P.; Gueheunneux, S.; Jomier, M.; Vie, K. SpectraCam®: A new polarized hyperspectral imaging system for repeatable and reproducible in vivo skin quantification of melanin, total hemoglobin, and oxygen saturation. Skin Res. Technol. 2018, 24, 99–107. [Google Scholar] [CrossRef]
- Koprowski, R.; Wilczyński, S.; Wróbel, Z.; Błońska-Fajfrowska, B. Calibration and segmentation of skin areas in hyperspectral imaging for the needs of dermatology. Biomed. Eng. Online 2014, 13, 113. [Google Scholar] [CrossRef] [PubMed]
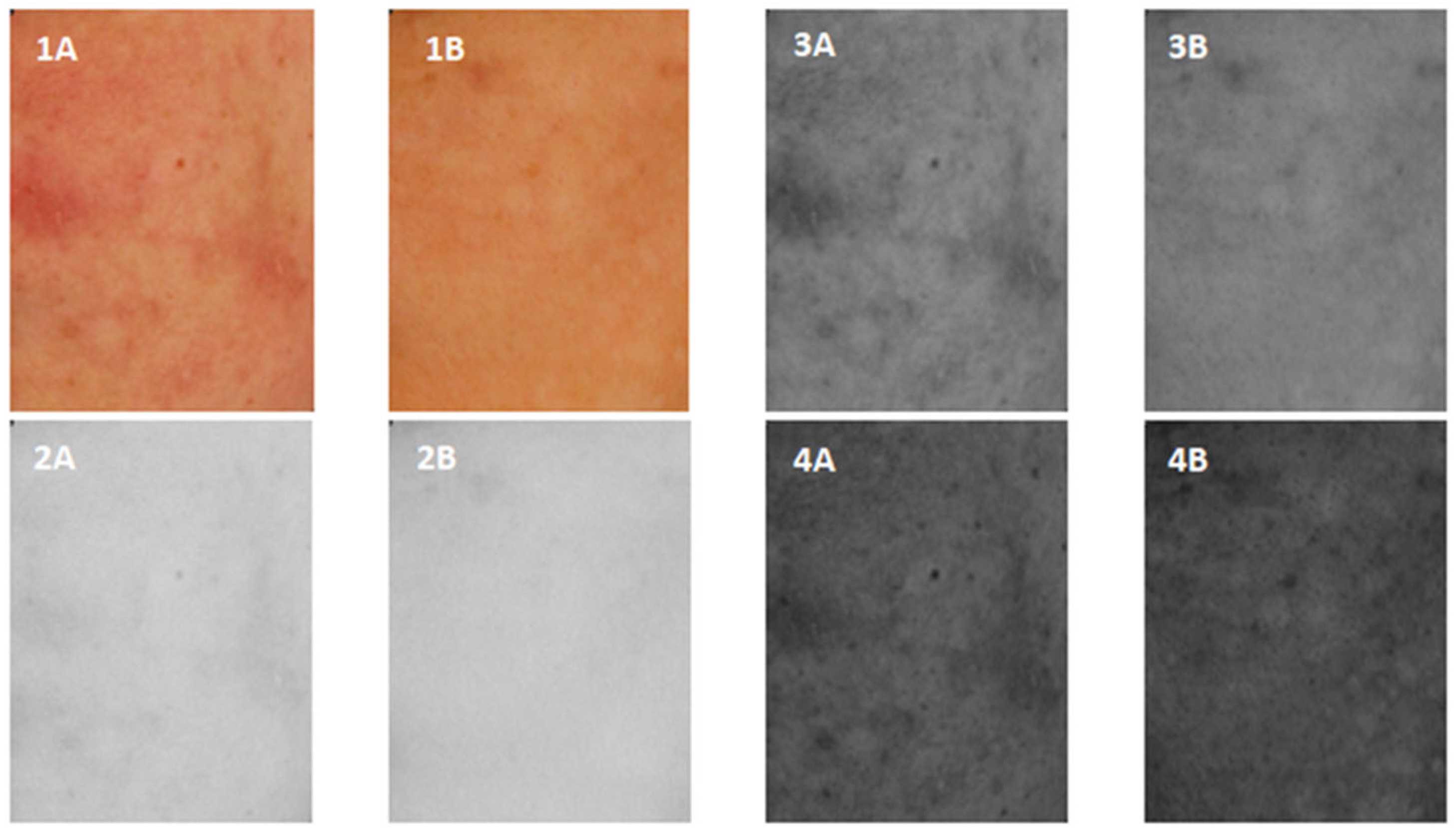
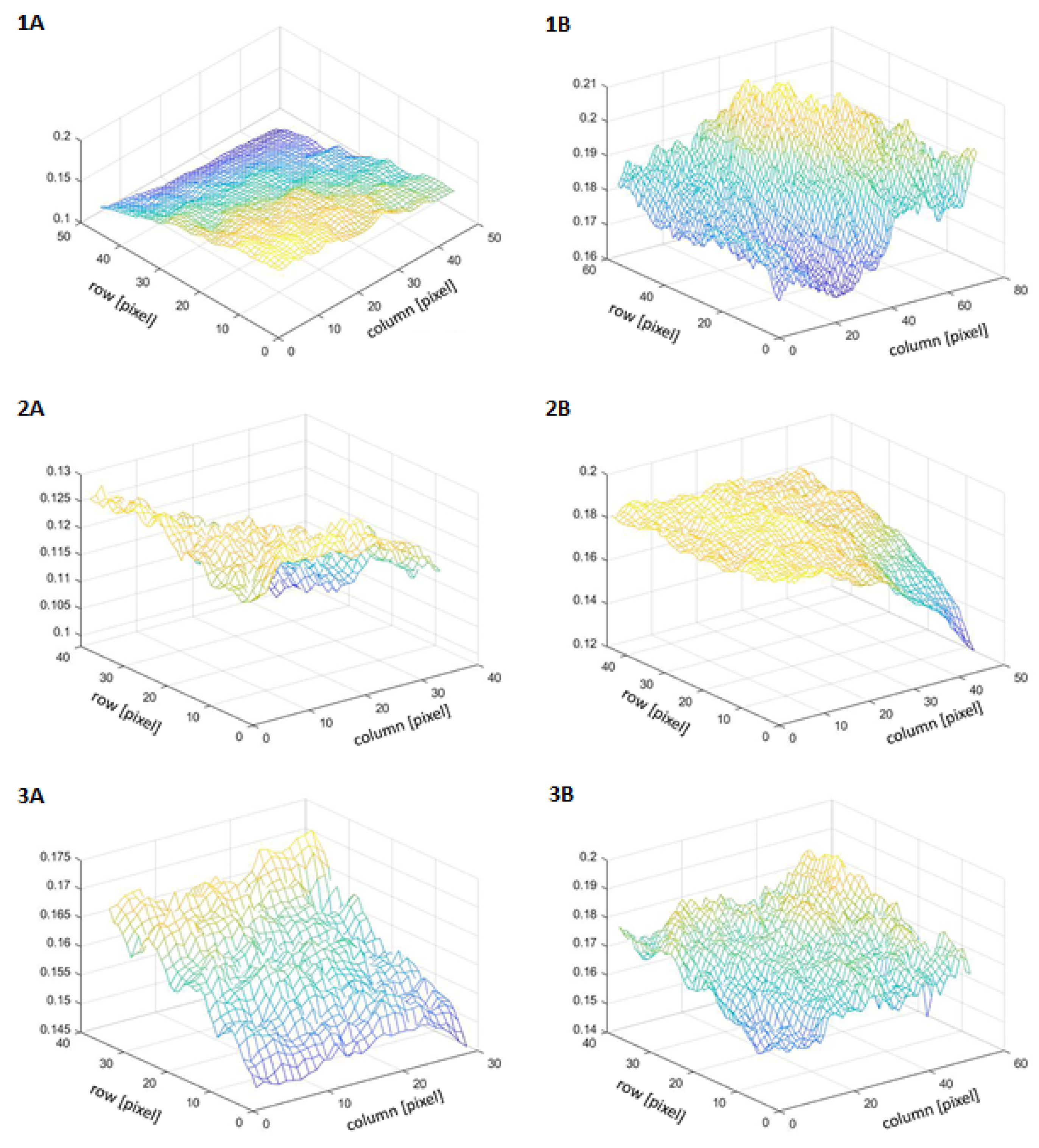
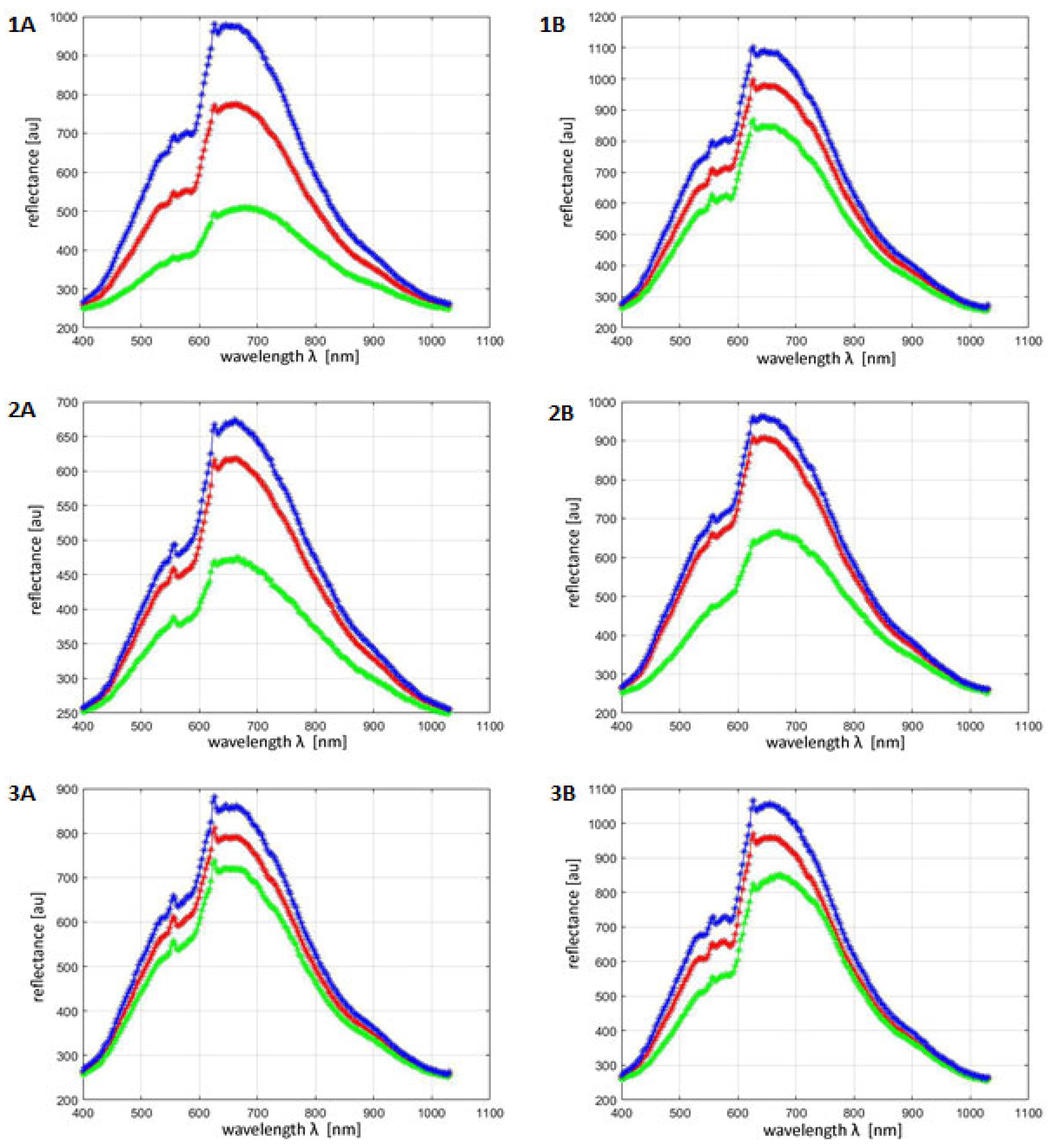
| Skin | Brightness | Contrast | Homogeneity |
|---|---|---|---|
| Before applying kojic acid | 143.33 ± 23.75 | 5.86 ±1.09 | 0.56 ± 0.16 |
| After applying kojic acid | 157.67 ± 19.89 | 5.21 ± 1.06 | 0.64 ± 0.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wawrzyk-Bochenek, I.; Rahnama, M.; Stachura, M.; Wilczyński, S.; Wawrzyk, A. Evaluation of the Reduction of Skin Hyperpigmentation Changes under the Influence of a Preparation Containing Kojic Acid Using Hyperspectral Imaging—Preliminary Study. J. Clin. Med. 2023, 12, 2710. https://doi.org/10.3390/jcm12072710
Wawrzyk-Bochenek I, Rahnama M, Stachura M, Wilczyński S, Wawrzyk A. Evaluation of the Reduction of Skin Hyperpigmentation Changes under the Influence of a Preparation Containing Kojic Acid Using Hyperspectral Imaging—Preliminary Study. Journal of Clinical Medicine. 2023; 12(7):2710. https://doi.org/10.3390/jcm12072710
Chicago/Turabian StyleWawrzyk-Bochenek, Iga, Mansur Rahnama, Martyna Stachura, Sławomir Wilczyński, and Anna Wawrzyk. 2023. "Evaluation of the Reduction of Skin Hyperpigmentation Changes under the Influence of a Preparation Containing Kojic Acid Using Hyperspectral Imaging—Preliminary Study" Journal of Clinical Medicine 12, no. 7: 2710. https://doi.org/10.3390/jcm12072710
APA StyleWawrzyk-Bochenek, I., Rahnama, M., Stachura, M., Wilczyński, S., & Wawrzyk, A. (2023). Evaluation of the Reduction of Skin Hyperpigmentation Changes under the Influence of a Preparation Containing Kojic Acid Using Hyperspectral Imaging—Preliminary Study. Journal of Clinical Medicine, 12(7), 2710. https://doi.org/10.3390/jcm12072710







