Efficacy and Safety of the Genistein Nutraceutical Product Containing Vitamin E, Vitamin B3, and Ceramide on Skin Health in Postmenopausal Women: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Study Participants and Sample Size Determination
2.3. Study Intervention and Comparator
2.4. Randomization, Blinding, and Allocation Concealment
2.5. Study Procedures
2.6. Outcome Assessment
- (1)
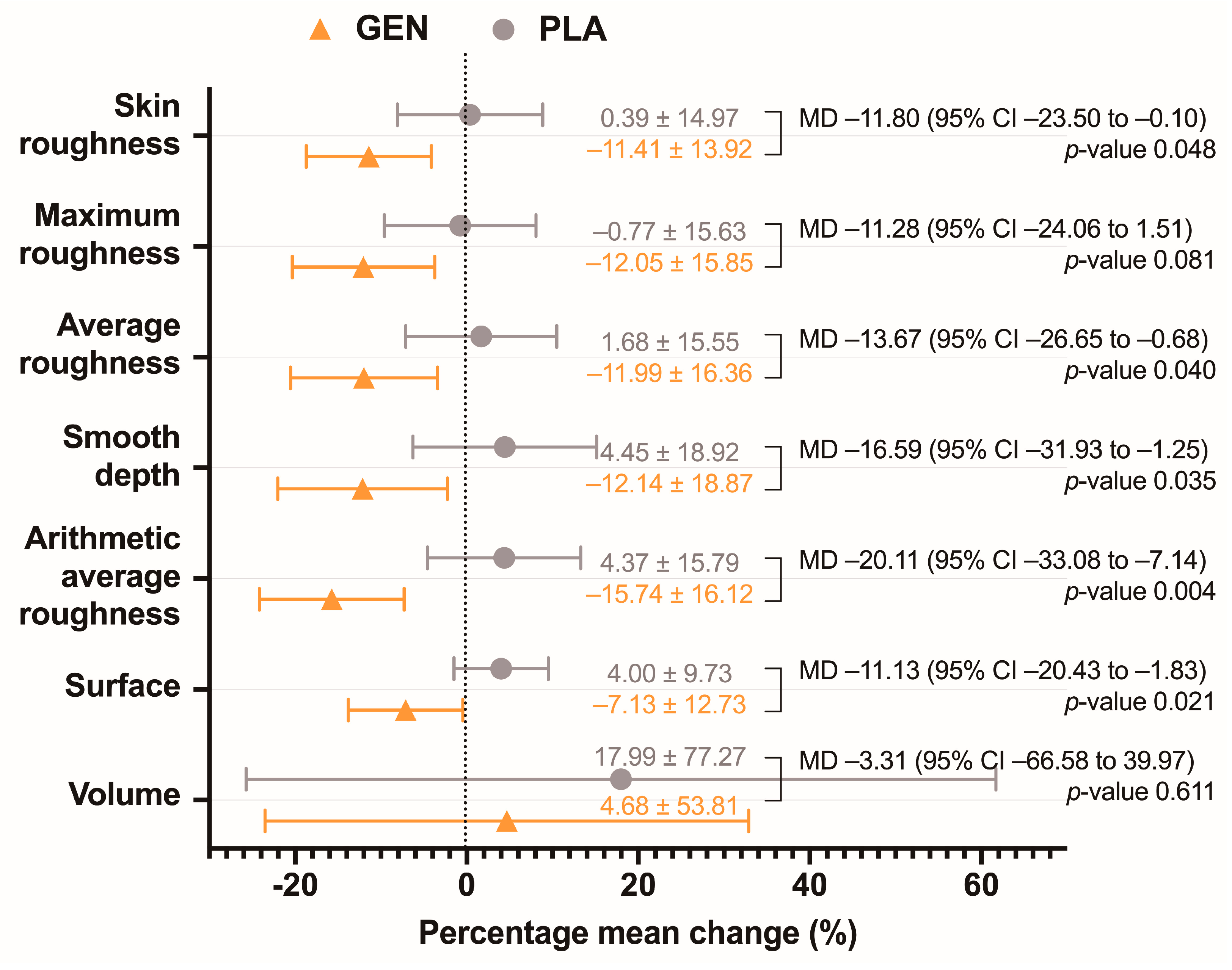
- Skin wrinkling parameters, including depth of skin roughness (R1, the distance between the highest and the lowest value), maximum roughness (R2, the biggest roughness of the different segment roughness values), average roughness (R3, the arithmetic average of the different segment roughness values), smooth depth (R4, the average distance between the real profile and the maximum profile), arithmetic average roughness (R5, the distance between the average line and the average deviation), surface (the size of the wavy surface which was compared to a fully stretched flat surface), and volume (the virtual amount of liquid needed in the calculation area to fill the image until the average height of all mountains), all of which were measured by the Skin Visiometer SV 700 (Courage and Khazaka electronic, Cologne, Germany);
- (2)
- Skin color parameters, including brightness (LAB1, L-skin color), redness (LAB2, A-the dermatologist’s perception of skin redness and erythema), pigmentation (LAB3, B-pigmentation, and tanning), and individual typology angle (ITA, skin color-white-dark whitening), all of which were measured by the Skin-Colorimeter® CL 400 (Courage and Khazaka electronic, Cologne, Germany);
- (3)
- Hydration value, which was measured by the Corneometer (Cutometer® dual MPA 580, Courage and Khazaka electronic, Cologne, Germany);
- (4)
- Facial skin parameters, including fine pores, large pores, and spots, all of which were measured by the Visioface 1000 D (Courage and Khazaka electronic, Cologne, Germany); and
- (5)
- Acne parameters, including size and quantity, were all measured by the Visiopor® PP34 (Courage and Khazaka electronic, Cologne, Germany) (using a specific UV light to visualize the fluorescing acne lesions).
2.7. Statistical Analysis
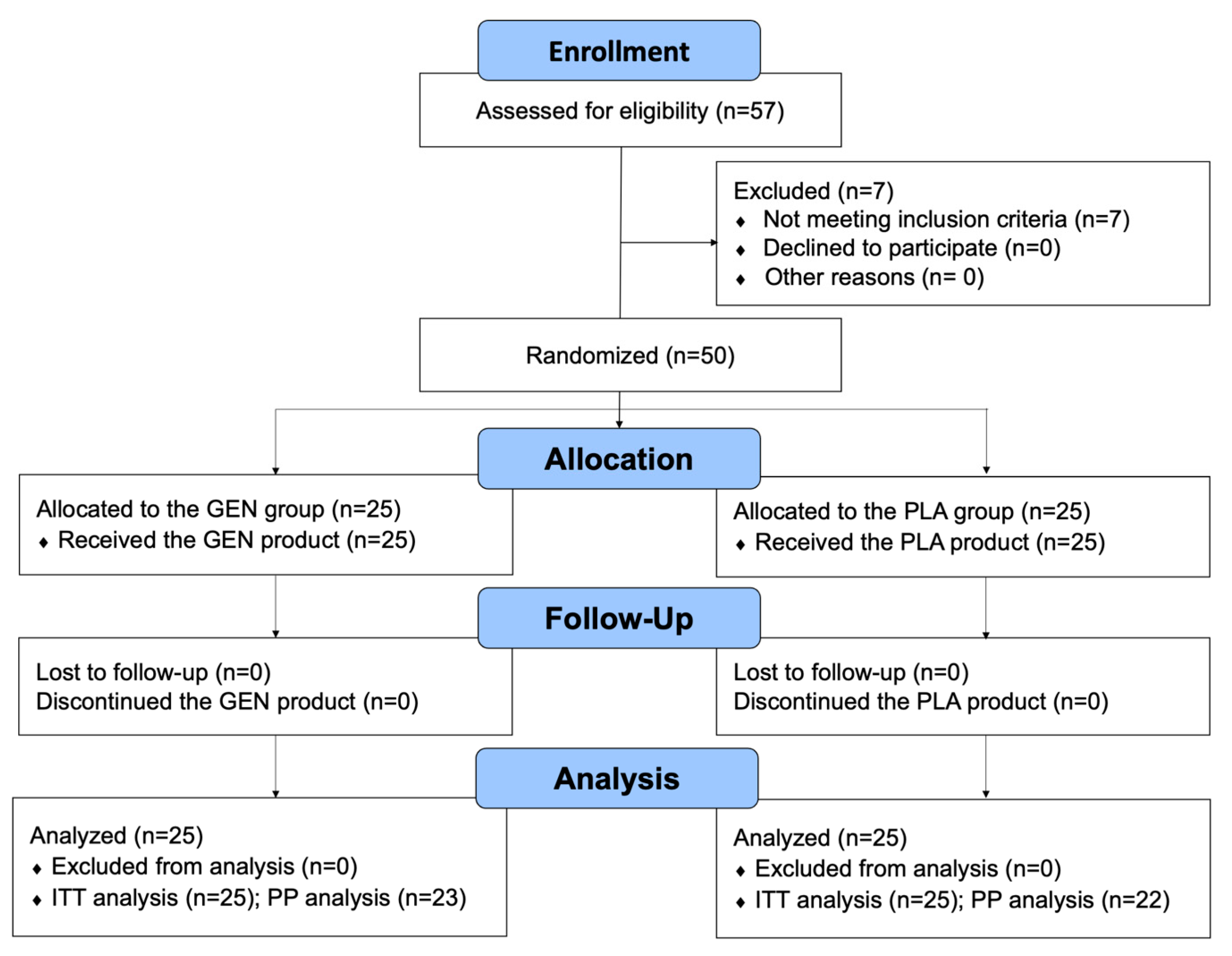
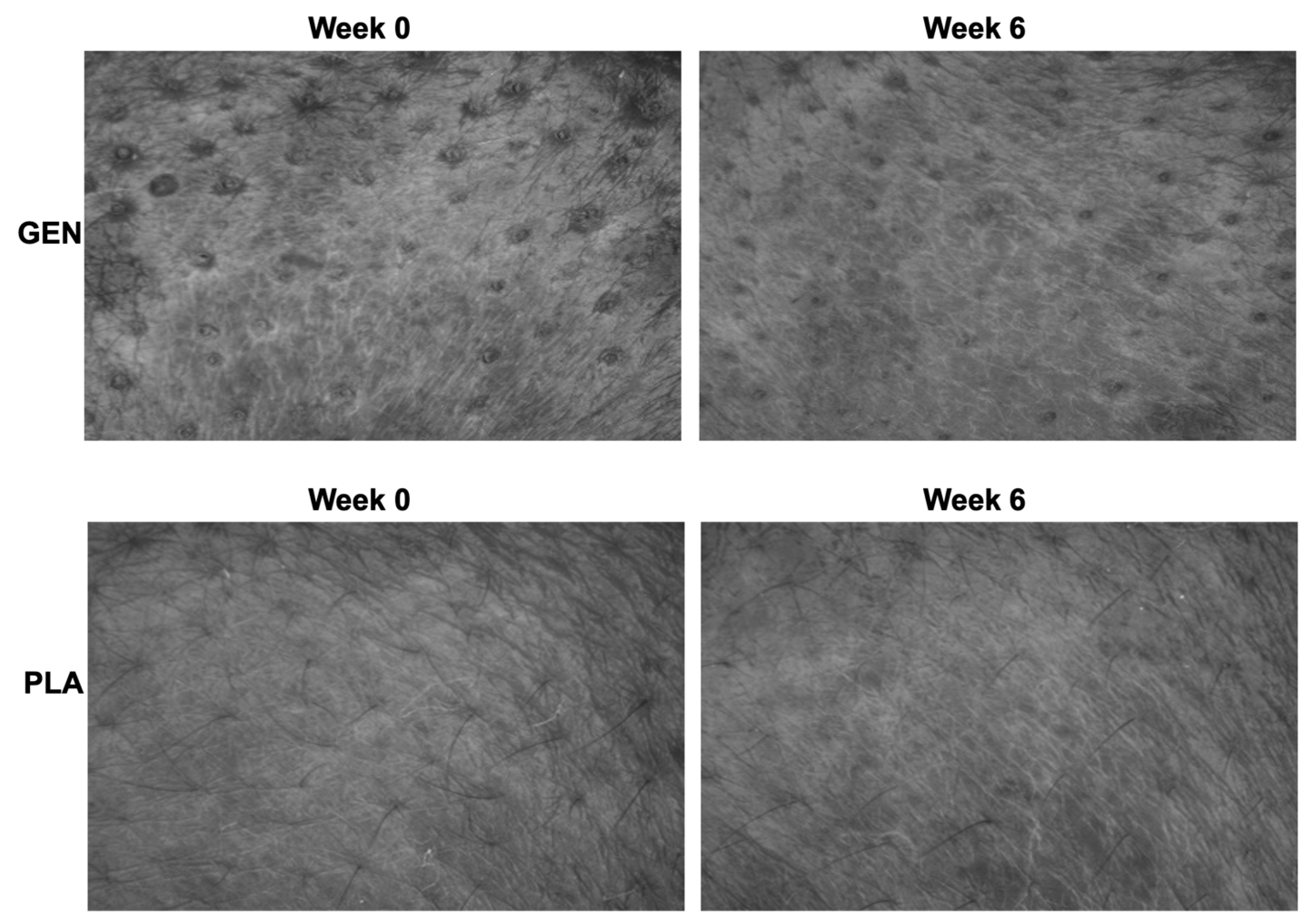
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sowers, M.; Pope, S.; Welch, G.; Sternfeld, B.; Albrecht, G. The Association of Menopause and Physical Functioning in Women at Midlife. J. Am. Geriatr. Soc. 2001, 49, 1485–1492. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Li, N.; Yan, Y.Q.; Liu, Y.; Xiong, K.; Liu, Y.; Xia, Q.M.; Zhang, H.; Liu, Z.D. Recent advances in the anti-aging effects of phytoestrogens on collagen, water content, and oxidative stress. Phytother. Res. 2020, 34, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Thornton, M.J. Estrogens and aging skin. Dermatoendocrinol 2013, 5, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Lephart, E.D.; Naftolin, F. Menopause and the Skin: Old Favorites and New Innovations in Cosmeceuticals for Estrogen-Deficient Skin. Dermatol. Ther. 2021, 11, 53–69. [Google Scholar] [CrossRef] [PubMed]
- Goyal, N.; Jerold, F. Biocosmetics: Technological advances and future outlook. Environ. Sci. Pollut. Res. Int. 2021, 1–22. [Google Scholar] [CrossRef]
- Jackson, R.L.; Greiwe, J.S.; Schwen, R.J. Ageing skin: Oestrogen receptor β agonists offer an approach to change the outcome. Exp. Dermatol. 2011, 20, 879–882. [Google Scholar] [CrossRef]
- Grand View Research, Inc. Menopause Market Size, Share & Trends Analysis Report By Treatment (Dietary Supplements, OTC Pharma Products), By Region (North America, Europe, APAC, Latin America, MEA), And Segment Forecasts, 2022–2030; Market Analysis Report; Grand View Research, Inc.: San Francisco, CA, USA, 2022; Volume GVR-4-68039-434-2, pp. 1–100. [Google Scholar]
- Setchell, K. Phytoestrogens: The biochemistry, physiology, and implications for human health of soy isoflavones. Am. J. Clin. Nutr. 1998, 68, 1333S–1346S. [Google Scholar] [CrossRef]
- McCarty, M.F. Isoflavones made simple—genistein’s agonist activity for the beta-type estrogen receptor mediates their health benefits. Med. Hypotheses 2006, 66, 1093–1114. [Google Scholar] [CrossRef]
- Rzepecki, A.K.; Murase, J.E.; Juran, R.; Fabi, S.G.; McLellan, B.N. Estrogen-deficient skin: The role of topical therapy. Int. J. Womens Dermatol. 2019, 5, 85–90. [Google Scholar] [CrossRef]
- Tanaka, H.; Okada, T.; Konishi, H.; Tsuji, T. The effect of reactive oxygen species on the biosynthesis of collagen and glycosaminoglycans in cultured human dermal fibroblasts. Arch. Dermatol. Res. 1993, 285, 352–355. [Google Scholar] [CrossRef]
- Keen, M.A.; Hassan, I. Vitamin E in dermatology. Indian Dermatol. Online J. 2016, 7, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Gehring, W. Nicotinic acid/niacinamide and the skin. J. Cosmet. Dermatol. 2004, 3, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Tanno, O.; Ota, Y.; Kitamura, N.; Katsube, T.; Inoue, S. Nicotinamide increases biosynthesis of ceramides as well as other stratum corneum lipids to improve the epidermal permeability barrier. Br. J. Dermatol. 2000, 143, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Coderch, L.; López, O.; de la Maza, A.; Parra, J.L. Ceramides and skin function. Am. J. Clin. Dermatol. 2003, 4, 107–129. [Google Scholar] [CrossRef] [PubMed]
- Kono, T.; Miyachi, Y.; Kawashima, M. Clinical significance of the water retention and barrier function-improving capabilities of ceramide-containing formulations: A qualitative review. J. Dermatol. 2021, 48, 1807–1816. [Google Scholar] [CrossRef]
- Silva, L.A.; Ferraz Carbonel, A.A.; de Moraes, A.R.B.; Simões, R.S.; da Silva Sasso, G.R.; Goes, L.; Nunes, W.; Simões, M.J.; Patriarca, M.T. Collagen concentration on the facial skin of postmenopausal women after topical treatment with estradiol and genistein: A randomized double-blind controlled trial. Gynecol. Endocrinol. 2017, 33, 845–848. [Google Scholar] [CrossRef]
- Michalak, M.; Pierzak, M.; Krecisz, B.; Suliga, E. Bioactive compounds for skin health: A review. Nutrients 2021, 13, 203. [Google Scholar] [CrossRef]
- Bissett, D.; Miyamoto, K.; Sun, P.; Li, J.; Berge, C. Topical niacinamide reduces yellowing, wrinkling, red blotchiness, and hyperpigmented spots in aging facial skin 1. Int. J. Cosmet. Sci. 2004, 26, 231–238. [Google Scholar] [CrossRef]
- Sun, Q.; Wu, J.; Qian, G.; Cheng, H. Effectiveness of Dietary Supplement for Skin Moisturizing in Healthy Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Nutr. 2022, 9, 1117. [Google Scholar] [CrossRef]
- Vitale, D.C.; Piazza, C.; Melilli, B.; Drago, F.; Salomone, S. Isoflavones: Estrogenic activity, biological effect and bioavailability. Eur. J. Drug Metab. Pharm. 2013, 38, 15–25. [Google Scholar] [CrossRef]
- Pike, A.C.; Brzozowski, A.M.; Hubbard, R.E.; Bonn, T.; Thorsell, A.G.; Engström, O.; Ljunggren, J.; Gustafsson, J.A.; Carlquist, M. Structure of the ligand-binding domain of oestrogen receptor beta in the presence of a partial agonist and a full antagonist. Embo. J. 1999, 18, 4608–4618. [Google Scholar] [CrossRef] [PubMed]
- Berg, K.A.; Clarke, W.P. Making Sense of Pharmacology: Inverse Agonism and Functional Selectivity. Int. J. Neuropsychopharmacol. 2018, 21, 962–977. [Google Scholar] [CrossRef] [PubMed]
- Hwang, C.S.; Kwak, H.S.; Lim, H.J.; Lee, S.H.; Kang, Y.S.; Choe, T.B.; Hur, H.G.; Han, K.O. Isoflavone metabolites and their in vitro dual functions: They can act as an estrogenic agonist or antagonist depending on the estrogen concentration. J. Steroid Biochem. Mol. Biol. 2006, 101, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.E. Endocrinology of the Menopause. Endocrinol. Metab. Clin. N. Am. 2015, 44, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Moraes, A.B.; Haidar, M.A.; Júnior, J.M.S.; Simões, M.J.; Baracat, E.C.; Patriarca, M.T. The effects of topical isoflavones on postmenopausal skin: Double-blind and randomized clinical trial of efficacy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2009, 146, 188–192. [Google Scholar] [CrossRef]
- Patriarca, M.T.; Barbosa de Moraes, A.R.; Nader, H.B.; Petri, V.; Martins, J.R.; Gomes, R.C.; Soares-Jr, J.M. Hyaluronic acid concentration in postmenopausal facial skin after topical estradiol and genistein treatment: A double-blind, randomized clinical trial of efficacy. Menopause 2013, 20, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Nachbar, F.; Korting, H.C. The role of vitamin E in normal and damaged skin. J. Mol. Med. 1995, 73, 7–17. [Google Scholar] [CrossRef]
- Bissett, D.L.; Oblong, J.E.; Berge, C.A. Niacinamide: A B vitamin that improves aging facial skin appearance. Derm. Surg. 2005, 31, 860–866. [Google Scholar] [CrossRef]
- Lueangarun, S.; Tragulplaingam, P.; Sugkraroek, S.; Tempark, T. The 24-hr, 28-day, and 7-day post-moisturizing efficacy of ceramides 1, 3, 6-II containing moisturizing cream compared with hydrophilic cream on skin dryness and barrier disruption in senile xerosis treatment. Dermatol. Ther. 2019, 32, e13090. [Google Scholar] [CrossRef]
- Rosso, J.D.; Zeichner, J.; Alexis, A.; Cohen, D.; Berson, D. Understanding the Epidermal Barrier in Healthy and Compromised Skin: Clinically Relevant Information for the Dermatology Practitioner: Proceedings of an Expert Panel Roundtable Meeting. J. Clin. Aesthet. Dermatol. 2016, 9, S2–S8. [Google Scholar]
- Ly, B.C.K.; Dyer, E.B.; Feig, J.L.; Chien, A.L.; Del Bino, S. Research Techniques Made Simple: Cutaneous Colorimetry: A Reliable Technique for Objective Skin Color Measurement. J. Investig. Dermatol. 2020, 140, 3–12.e1. [Google Scholar] [CrossRef]
- Fullerton, A.; Fischer, T.; Lahti, A.; Wilhelm, K.P.; Takiwaki, H.; Serup, J. Guidelines for measurement of skin colour and erythema. A report from the Standardization Group of the European Society of Contact Dermatitis. Contact Dermat. 1996, 35, 1–10. [Google Scholar] [CrossRef]
- Fullerton, A.; Serup, J. Site, gender and age variation in normal skin colour on the back and the forearm: Tristimulus colorimeter measurements. Ski. Res. Technol. 1997, 3, 49–52. [Google Scholar] [CrossRef]
- Seitz, J.C.; Whitmore, C.G. Measurement of erythema and tanning responses in human skin using a tri-stimulus colorimeter. Dermatologica 1988, 177, 70–75. [Google Scholar] [CrossRef]
- Clarys, P.; Alewaeters, K.; Lambrecht, R.; Barel, A.O. Skin color measurements: Comparison between three instruments: The Chromameter(R), the DermaSpectrometer(R) and the Mexameter(R). Skin Res. Technol. 2000, 6, 230–238. [Google Scholar] [CrossRef]
- Frew, J.; Penzi, L.; Suarez-Farinas, M.; Garcet, S.; Brunner, P.M.; Czarnowicki, T.; Kim, J.; Bottomley, C.; Finney, R.; Cueto, I.; et al. The erythema Q-score, an imaging biomarker for redness in skin inflammation. Exp. Dermatol. 2021, 30, 377–383. [Google Scholar] [CrossRef]
- Stephen, I.D.; Coetzee, V.; Law Smith, M.; Perrett, D.I. Skin blood perfusion and oxygenation colour affect perceived human health. PLoS ONE 2009, 4, e5083. [Google Scholar] [CrossRef] [PubMed]
- Matias, A.R.; Ferreira, M.; Costa, P.; Neto, P. Skin colour, skin redness and melanin biometric measurements: Comparison study between Antera(®) 3D, Mexameter(®) and Colorimeter(®). Skin Res. Technol. 2015, 21, 346–362. [Google Scholar] [CrossRef]
- Silva, H. The Vascular Effects of Isolated Isoflavones—A Focus on the Determinants of Blood Pressure Regulation. Biology 2021, 10, 49. [Google Scholar] [CrossRef] [PubMed]
- Accorsi-Neto, A.; Haidar, M.; Simões, R.; Simões, M.; Soares, J., Jr.; Baracat, E. Effects of isoflavones on the skin of postmenopausal women: A pilot study. Clinics 2009, 64, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Culp, B.; Scheinfeld, N. Rosacea: A review. Pharm. Ther. 2009, 34, 38–45. [Google Scholar]
- Lodén, M. Role of topical emollients and moisturizers in the treatment of dry skin barrier disorders. Am. J. Clin. Derm. 2003, 4, 771–788. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, K.; Dissanayake, B.; Omotezako, T.; Takemura, M.; Tsuji, G.; Furue, M. Daily Fluctuation of Facial Pore Area, Roughness and Redness among Young Japanese Women; Beneficial Effects of Galactomyces Ferment Filtrate Containing Antioxidative Skin Care Formula. J. Clin. Med. 2021, 10, 2502. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Park, S.R.; Kwon, D.I.; Park, M.S.; Lim, D.H. Depth profiling of epidermal hydration inducing improvement of skin roughness and elasticity: In vivo study by confocal Raman spectroscopy. J. Cosmet. Dermatol. 2022, 21, 4810–4817. [Google Scholar] [CrossRef] [PubMed]
- Bayerl, C.; Keil, D. Isoflavonoide in der Behandlung der Hautalterung postmenopausaler Frauen. Aktuelle Dermatol. 2002, 28, S14–S18. [Google Scholar] [CrossRef]
- Howard, J.J.; Sirotin, Y.B.; Tipton, J.L.; Vemury, A.R. Reliability and Validity of Image-Based and Self-Reported Skin Phenotype Metrics. IEEE Trans. Biom. Behav. Identity Sci. 2021, 3, 550–560. [Google Scholar] [CrossRef]
- Takiddin, A.; Schneider, J.; Yang, Y.; Abd-Alrazaq, A.; Househ, M. Artificial Intelligence for Skin Cancer Detection: Scoping Review. J. Med. Internet Res. 2021, 23, e22934. [Google Scholar] [CrossRef]
| Parameters | GEN Group (n = 25) | PLA Group (n = 25) |
|---|---|---|
| Demographics | ||
| Age (years) | 55.8 ± 3.8 | 55.8 ± 3.0 |
| BMI (kg/m2) | 24.0 ± 2.2 | 23.7 ± 3.1 |
| FSH (IU/L) | 75.2 ± 23.4 | 74.0 ± 18.6 |
| Skin wrinkle parameters | ||
| Skin roughness (arb. unit) | 59.01 ± 9.65 | 54.55 ± 8.46 |
| Maximum roughness (arb. unit) | 49.76 ± 9.12 | 45.08 ± 8.44 |
| Average roughness (arb. unit) | 38.56 ± 6.98 | 34.85 ± 6.57 |
| Smooth depth (arb. unit) | 30.12 ± 5.96 | 27.39 ± 4.98 |
| Arithmetic average roughness (arb. unit) | 8.76 ± 1.69 | 7.96 ± 1.56 |
| Surface (%) | 402.15 ± 52.07 | 374.36 ± 45.04 |
| Volume (mm2) | 55.08 ± 20.00 | 53.91 ± 17.90 |
| Skin color parameters | ||
| Brightness (arb. unit) | 60.33 ± 3.97 | 59.56 ± 2.17 |
| Redness (arb. unit) | 13.33 ± 1.67 | 13.44 ± 1.76 |
| Pigmentation (arb. unit) | 16.41 ± 1.92 | 17.03 ± 1.65 |
| Individual typology angle (arb. unit) | 31.48 ± 10.91 | 29.29 ± 6.47 |
| Skin hydration parameters | ||
| Hydration value (arb. unit) | 78.10 ± 10.21 | 78.87 ± 7.43 |
| Facial skin parameters | ||
| Fine pore (arb. unit) | 22.48 ± 15.59 | 20.96 ± 11.85 |
| Fine pore area (%) | 2.32 ± 1.69 | 2.10 ± 1.21 |
| Large pore (arb. unit) | 6.88 ± 9.55 | 7.76 ± 7.50 |
| Pore area (%) | 1.62 ± 2.03 | 1.87 ± 1.75 |
| Spot (arb. unit) | 0.80 ± 1.04 | 1.08 ± 0.91 |
| Spot area (%) | 0.79 ± 1.17 | 1.30 ± 1.39 |
| Acne parameters | ||
| Size (%) | 0.55 ± 1.69 | 0.42 ± 0.75 |
| Quantity (arb. unit) | 5.87 ± 10.59 | 6.93 ± 9.31 |
| Parameters | Intention-to-Treat Analysis | Per-Protocol Analysis | ||||
|---|---|---|---|---|---|---|
| GEN Group (n = 25) | PLA Group (n = 25) | p-Value | GEN Group (n = 23) | PLA Group (n = 22) | p-Value | |
| Skin wrinkle parameters | ||||||
| Skin roughness | −4.80 ± 16.82 | −3.13 ± 14.14 | 0.704 | −5.46 ± 17.03 | −2.99 ± 15.08 | 0.610 |
| Maximum roughness | −4.78 ± 17.48 | −2.86 ± 14.34 | 0.673 | −5.69 ± 17.51 | −3.25 ± 15.22 | 0.621 |
| Average roughness | −4.00 ± 18.85 | −1.06 ± 13.67 | 0.531 | −4.97 ± 19.15 | −1.17 ± 14.51 | 0.459 |
| Smooth depth | −2.29 ± 22.82 | −1.25 ± 18.44 | 0.549 | −4.20 ± 20.79 | 1.75 ± 19.61 | 0.329 |
| Arithmetic average roughness | −3.17 ± 22.18 | −0.12 ± 16.46 | 0.584 | −4.44 ± 21.35 | 0.51 ± 17.31 | 0.399 |
| Surface | −1.74 ± 16.03 | 2.16 ± 8.55 | 0.291 | −2.66 ± 16.40 | 2.30 ± 9.05 | 0.215 |
| Volume | 17.41 ± 59.50 | 15.59 ± 70.35 | 0.922 | 17.23 ± 61.74 | 20.44 ± 73.64 | 0.875 |
| Skin color parameters | ||||||
| Brightness | −0.14 ± 10.12 | 2.31 ± 4.62 | 0.280 | −1.35 ± 9.59 | 2.52 ± 4.74 | 0.094 |
| Redness | 6.89 ± 20.94 | −2.97 ± 11.02 | 0.044 | 9.35 ± 19.74 | −3.90 ± 11.37 | 0.009 |
| Pigmentation | −3.78 ± 19.34 | −5.40 ± 11.13 | 0.718 | −4.56 ± 17.88 | −5.34 ± 11.62 | 0.863 |
| Individual typology angle | 35.68 ± 141.42 | 19.81 ± 28.92 | 0.587 | 27.60 ± 142.14 | 21.46 ± 29.81 | 0.844 |
| Skin hydration parameters | ||||||
| Hydration value | 12.30 ± 15.66 | 7.90 ± 15.54 | 0.323 | 13.23 ± 16.01 | 7.39 ± 16.06 | 0.229 |
| Parameters | Intention-to-Treat Analysis | Per-Protocol Analysis | ||||
|---|---|---|---|---|---|---|
| GEN Group (n = 25) | PLA Group (n = 25) | p-Value | GEN Group (n = 23) | PLA Group (n = 22) | p-Value | |
| Facial skin parameters | ||||||
| Fine pore | −2.92 ± 7.19 | −0.48 ± 7.57 | 0.248 | −3.39 ± 7.18 | −0.82 ± 7.10 | 0.234 |
| Fine pore area | −0.41 ± 0.79 | −0.03 ± 0.78 | 0.093 | −0.47 ± 0.79 | −0.06 ± 0.69 | 0.075 |
| Large pore | −0.84 ± 6.42 | −0.04 ± 3.62 | 0.590 | −1.00 ± 6.67 | 0.00 ± 3.82 | 0.543 |
| Large pore area | −0.18 ± 1.42 | 0.13 ± 0.98 | 0.368 | −0.21 ± 1.48 | 0.14 ± 1.02 | 0.356 |
| Spot | −0.20 ± 0.82 | −0.04 ± 0.68 | 0.454 | −0.26 ± 0.69 | −0.14 ± 0.64 | 0.534 |
| Spot area | −0.28 ± 1.12 | −0.11 ± 0.71 | 0.534 | −0.38 ± 1.01 | −0.16 ± 0.72 | 0.395 |
| Acne parameters | ||||||
| Size | −0.13 ± 0.46 | −0.17 ± 0.52 | 0.786 | −0.14 ± 0.48 | −0.16 ± 0.55 | 0.927 |
| Quantity | −0.97 ± 5.19 | −1.93 ± 5.10 | 0.512 | −1.51 ± 4.75 | −1.91 ± 5.43 | 0.792 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Na Takuathung, M.; Klinjan, P.; Sakuludomkan, W.; Dukaew, N.; Inpan, R.; Kongta, R.; Chaiyana, W.; Teekachunhatean, S.; Koonrungsesomboon, N. Efficacy and Safety of the Genistein Nutraceutical Product Containing Vitamin E, Vitamin B3, and Ceramide on Skin Health in Postmenopausal Women: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. J. Clin. Med. 2023, 12, 1326. https://doi.org/10.3390/jcm12041326
Na Takuathung M, Klinjan P, Sakuludomkan W, Dukaew N, Inpan R, Kongta R, Chaiyana W, Teekachunhatean S, Koonrungsesomboon N. Efficacy and Safety of the Genistein Nutraceutical Product Containing Vitamin E, Vitamin B3, and Ceramide on Skin Health in Postmenopausal Women: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Journal of Clinical Medicine. 2023; 12(4):1326. https://doi.org/10.3390/jcm12041326
Chicago/Turabian StyleNa Takuathung, Mingkwan, Preeyaporn Klinjan, Wannachai Sakuludomkan, Nahathai Dukaew, Ratchanon Inpan, Rattana Kongta, Wantida Chaiyana, Supanimit Teekachunhatean, and Nut Koonrungsesomboon. 2023. "Efficacy and Safety of the Genistein Nutraceutical Product Containing Vitamin E, Vitamin B3, and Ceramide on Skin Health in Postmenopausal Women: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial" Journal of Clinical Medicine 12, no. 4: 1326. https://doi.org/10.3390/jcm12041326
APA StyleNa Takuathung, M., Klinjan, P., Sakuludomkan, W., Dukaew, N., Inpan, R., Kongta, R., Chaiyana, W., Teekachunhatean, S., & Koonrungsesomboon, N. (2023). Efficacy and Safety of the Genistein Nutraceutical Product Containing Vitamin E, Vitamin B3, and Ceramide on Skin Health in Postmenopausal Women: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Journal of Clinical Medicine, 12(4), 1326. https://doi.org/10.3390/jcm12041326







