Transcranial Direct Current Stimulation for Chronic Stroke: Is Neuroimaging the Answer to the Next Leap Forward?
Abstract
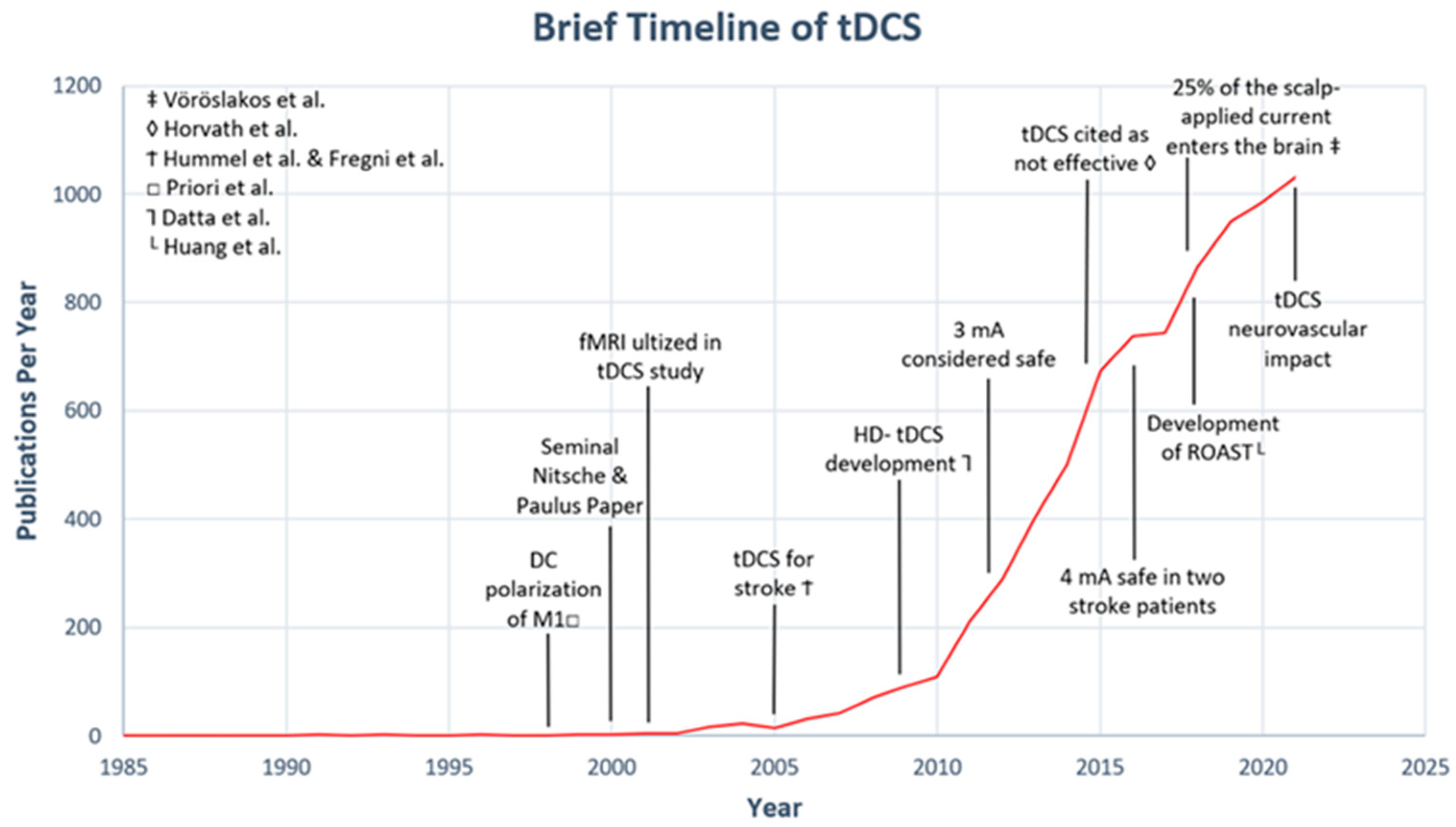
1. Introduction
- Cortical activation via direct or peripheral stimulation in addition to neuroimaging must have been included in the animal studies.
- tDCS for chronic stroke survivors (defined as greater than or equal to six months from the time of stroke) in addition to neuroimaging must have been included in the human studies.
2. Preclinical Studies
3. Early Human Studies Using tDCS for Stroke
4. The Interhemispheric Inhibition Model Is Introduced Then Challenged
5. Inter-Subject Variability: Methodological or Biological?
6. Randomized Control Trials (RCTs) for tDCS and Stroke Motor Recovery Are Still Evolving
7. Discussion
8. New and Unanswered Questions
9. Future Considerations in Neuroimaging
10. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feng, W.; Kautz, S.A.; Schlaug, G.; Meinzer, C.; George, M.S.; Chhatbar, P.Y. Transcranial Direct Current Stimulation for Poststroke Motor Recovery: Challenges and Opportunities. PMR 2018, 10, S157–S164. [Google Scholar] [CrossRef] [PubMed]
- Rudroff, T.; Workman, C.D.; Fietsam, A.C.; Kamholz, J. Response Variability in Transcranial Direct Current Stimulation: Why Sex Matters. Front. Psychiatry 2020, 11, 585. [Google Scholar] [CrossRef] [PubMed]
- López-Alonso, V.; Fernández-Del-Olmo, M.; Costantini, A.; Gonzalez-Henriquez, J.J.; Cheeran, B. Intra-individual variability in the response to anodal transcranial direct current stimulation. Clin. Neurophysiol. 2015, 126, 2342–2347. [Google Scholar] [CrossRef] [PubMed]
- Chew, T.; Ho, K.-A.; Loo, C.K. Inter- and Intra-individual Variability in Response to Transcranial Direct Current Stimulation (tDCS) at Varying Current Intensities. Brain Stimul. 2015, 8, 1130–1137. [Google Scholar] [CrossRef]
- Li, L.M.; Uehara, K.; Hanakawa, T. The contribution of interindividual factors to variability of response in transcranial direct current stimulation studies. Front. Cell. Neurosci. 2015, 9, 181. [Google Scholar] [CrossRef]
- López-Alonso, V.; Cheeran, B.; Río-Rodríguez, D.; Fernández-Del-Olmo, M. Inter-individual variability in response to non-invasive brain stimulation paradigms. Brain Stimul. 2014, 7, 372–380. [Google Scholar] [CrossRef]
- Wiethoff, S.; Hamada, M.; Rothwell, J.C. Variability in response to transcranial direct current stimulation of the motor cortex. Brain Stimul. 2014, 7, 468–475. [Google Scholar] [CrossRef]
- Krause, B.; Kadosh, R.C. Not all brains are created equal: The relevance of individual differences in responsiveness to transcranial electrical stimulation. Front. Syst. Neurosci. 2014, 8, 25. [Google Scholar] [CrossRef]
- Datta, A.; Truong, D.; Minhas, P.; Parra, L.C.; Bikson, M. Inter-Individual Variation during Transcranial Direct Current Stimulation and Normalization of Dose Using MRI-Derived Computational Models. Front. Psychiatry 2012, 3, 91. [Google Scholar] [CrossRef]
- Esmaeilpour, Z.; Shereen, A.D.; Ghobadi-Azbari, P.; Datta, A.; Woods, A.J.; Ironside, M.; O’Shea, J.; Kirk, U.; Bikson, M.; Ekhtiari, H. Methodology for tDCS integration with fMRI. Hum. Brain Mapp. 2020, 41, 1950–1967. [Google Scholar] [CrossRef]
- Dijkhuizen, R.M.; Ren, J.; Mandeville, J.B.; Wu, O.; Ozdag, F.M.; Moskowitz, M.A.; Rosen, B.R.; Finklestein, S.P. Functional magnetic resonance imaging of reorganization in rat brain after stroke. Proc. Natl. Acad. Sci. USA 2001, 98, 12766–12771. [Google Scholar] [CrossRef]
- Dijkhuizen, R.M.; Singhal, A.B.; Mandeville, J.B.; Wu, O.; Halpern, E.F.; Finklestein, S.P.; Rosen, B.R.; Lo, E.H. Correlation between brain reorganization, ischemic damage, and neurologic status after transient focal cerebral ischemia in rats: A functional magnetic resonance imaging study. J. Neurosci. 2003, 23, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Braun, R.; Klein, R.; Walter, H.L.; Ohren, M.; Freudenmacher, L.; Getachew, K.; Ladwig, A.; Luelling, J.; Neumaier, B.; Endepols, H.; et al. Transcranial direct current stimulation accelerates recovery of function, induces neurogenesis and recruits oligodendrocyte precursors in a rat model of stroke. Exp. Neurol. 2016, 279, 127–136. [Google Scholar] [CrossRef]
- Yoon, K.J.; Oh, B.M.; Kim, D.Y. Functional improvement and neuroplastic effects of anodal transcranial direct current stimulation (tDCS) delivered 1 day vs. 1 week after cerebral ischemia in rats. Brain Res. 2012, 1452, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Nitsche, M.A.; Paulus, W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology 2001, 57, 1899–1901. [Google Scholar] [CrossRef]
- Hummel, F.; Celnik, P.; Giraux, P.; Floel, A.; Wu, W.-H.; Gerloff, C.; Cohen, L.G. Effects of non-invasive cortical stimulation on skilled motor function in chronic stroke. Brain 2005, 128, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Fregni, F.; Boggio, P.S.; Mansur, C.G.; Wagner, T.; Ferreira, M.J.L.; Lima, M.C.; Rigonatti, S.P.; Marcolin, M.A.; Freedman, S.D.; Nitsche, M.A.; et al. Transcranial direct current stimulation of the unaffected hemisphere in stroke patients. Neuroreport 2005, 16, 1551–1555. [Google Scholar] [CrossRef]
- Hummel, F.; Cohen, L.G. Improvement of motor function with noninvasive cortical stimulation in a patient with chronic stroke. Neurorehabilit. Neural Repair 2005, 19, 14–19. [Google Scholar] [CrossRef]
- Vöröslakos, M.; Takeuchi, Y.; Brinyiczki, K.; Zombori, T.; Oliva, A.; Fernández-Ruiz, A.; Kozák, G.; Tamás Kincses, Z.; Iványi, B.; Buzsáki, G.; et al. Direct effects of transcranial electric stimulation on brain circuits in rats and humans. Nat Commun 2018, 9, 483. [Google Scholar] [CrossRef]
- Forte, J.D.; Carter, O. Evidence that transcranial direct current stimulation (tDCS) generates little-to-no reliable neurophysiologic effect beyond MEP amplitude modulation in healthy human subjects: A systematic review. Neuropsychologia 2015, 66, 213–236. [Google Scholar] [CrossRef]
- Priori, A. Brain polarization in humans: A reappraisal of an old tool for prolonged non-invasive modulation of brain excitability. Clin. Neurophysiol. 2003, 114, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Datta, A.; Bikson, M.; Parra, L.C. ROAST: An Open-Source, Fully-Automated, Realistic Volumetric-Approach-Based Simulator For TES. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018; pp. 3072–3075. [Google Scholar] [CrossRef]
- Bradnam, L.V.; Stinear, C.M.; Barber, P.A.; Byblow, W.D. Contralesional hemisphere control of the proximal paretic upper limb following stroke. Cereb. Cortex 2012, 22, 2662–2671. [Google Scholar] [CrossRef] [PubMed]
- Lindenberg, R.; Zhu, L.L.; Rüber, T.; Schlaug, G. Predicting functional motor potential in chronic stroke patients using diffusion tensor imaging. Hum. Brain Mapp. 2012, 33, 1040–1051. [Google Scholar] [CrossRef] [PubMed]
- Stagg, C.J.; Bachtiar, V.; O’Shea, J.; Allman, C.; Bosnell, R.A.; Kischka, U.; Matthews, P.M.; Johansen-Berg, H. Cortical activation changes underlying stimulation-induced behavioural gains in chronic stroke. Brain 2012, 135, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Dmochowski, J.F.; Datta, A.; Huang, Y.; Richardson, J.D.; Bikson, M.; Fridriksson, J.; Parra, L.C. Targeted transcranial direct current stimulation for rehabilitation after stroke. Neuroimage 2013, 75, 12–19. [Google Scholar] [CrossRef]
- Gillick, B.T.; Kirton, A.; Carmel, J.B.; Minhas, P.; Bikson, M. Pediatric stroke and transcranial direct current stimulation: Methods for rational individualized dose optimization. Front. Hum. Neurosci. 2014, 8, 739. [Google Scholar] [CrossRef]
- Rosso, C.; Perlbarg, V.; Valabregue, R.; Arbizu, C.; Ferrieux, S.; Alshawan, B.; Vargas, P.; Leger, A.; Zavanone, C.; Corvol, J.; et al. Broca’s area damage is necessary but not sufficient to induce after-effects of cathodal tdcs on the unaffected hemisphere in post-stroke aphasia. Brain Stimul. 2014, 7, 627–635. [Google Scholar] [CrossRef]
- Jindal, U.; Sood, M.; Dutta, A.; Chowdhury, S.R. Development of Point of Care Testing Device for Neurovascular Coupling from Simultaneous Recording of EEG and NIRS During Anodal Transcranial Direct Current Stimulation. IEEE J. Transl. Eng. Health Med. 2015, 3, 2000112. [Google Scholar] [CrossRef]
- Lefebvre, S.; Dricot, L.; Laloux, P.; Gradkowski, W.; Desfontaines, P.; Evrard, F.; Peeters, A.; Jamart, J.; Vandermeeren, Y. Neural substrates underlying stimulation-enhanced motor skill learning after stroke. Brain 2015, 138, 149–163. [Google Scholar] [CrossRef]
- Zheng, X.; Schlaug, G. Structural white matter changes in descending motor tracts correlate with improvements in motor impairment after undergoing a treatment course of tDCS and physical therapy. Front. Hum. Neurosci. 2015, 9, 229. [Google Scholar] [CrossRef]
- Chen, J.L.; Schlaug, G. Increased resting state connectivity between ipsilesional motor cortex and contralesional premotor cortex after transcranial direct current stimulation with physical therapy. Sci. Rep. 2016, 6, 23271. [Google Scholar] [CrossRef]
- Sebastian, R.; Saxena, S.; Tsapkini, K.; Faria, A.V.; Long, C.; Wright, A.; Davis, C.; Tippett, D.C.; Mourdoukoutas, A.P.; Bikson, M.; et al. Cerebellar tDCS: A Novel Approach to Augment Language Treatment Post-stroke. Front. Hum. Neurosci. 2017, 10, 695. [Google Scholar] [CrossRef] [PubMed]
- Hordacre, B.; Moezzi, B.; Ridding, M.C. Neuroplasticity and network connectivity of the motor cortex following stroke: A transcranial direct current stimulation study. Hum. Brain Mapp. 2018, 39, 3326–3339. [Google Scholar] [CrossRef] [PubMed]
- Larcombe, S.J.; Kulyomina, Y.; Antonova, N.; Ajina, S.; Stagg, C.J.; Clatworthy, P.L.; Bridge, H. Visual training in hemianopia alters neural activity in the absence of behavioural improvement: A pilot study. Ophthalmic Physiol. Opt. 2018, 38, 538–549. [Google Scholar] [CrossRef]
- Sánchez-Kuhn, A.; Medina, Y.; García-Pérez, M.; De Haro, P.; Flores, P.; Sánchez-Santed, F. Transcranial direct current stimulation treatment in chronic after-stroke dysphagia: A clinical case. Psicothema 2019, 31, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Iyer, P.C.; Rosenberg, A.; Baynard, T.; Madhavan, S. Influence of neurovascular mechanisms on response to tDCS: An exploratory study. Exp. Brain Res. 2019, 237, 2829–2840. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, A.; Kim, H.; Shin, M.; Yun, S.M.; Jung, Y.; Chang, W.H.; Kim, Y.-H. Different Brain Connectivity between Responders and Nonresponders to Dual-Mode Noninvasive Brain Stimulation over Bilateral Primary Motor Cortices in Stroke Patients. Neural Plast. 2019, 2019, 3826495. [Google Scholar] [CrossRef]
- Abualait, T.S. Effects of transcranial direct current stimulation of primary motor cortex on cortical sensory deficits and hand dexterity in a patient with stroke: A case study. J. Int. Med. Res. 2020, 48, 300060519894137. [Google Scholar] [CrossRef] [PubMed]
- Kuo, I.J.; Tang, C.W.; Tsai, Y.A.; Tang, S.C.; Lin, C.J.; Hsu, S.P.; Liang, W.K.; Juan, C.H.; Zich, C.; Stagg, C.J.; et al. Neurophysiological signatures of hand motor response to dual-transcranial direct current stimulation in subacute stroke: A TMS and MEG study. J. Neuroeng. Rehabil. 2020, 17, 72. [Google Scholar] [CrossRef]
- Richard, G.; Kolskår, K.; Ulrichsen, K.M.; Kaufmann, T.; Alnæs, D.; Sanders, A.-M.; Dørum, E.S.; Sánchez, J.M.; Petersen, A.; Ihle-Hansen, H.; et al. Brain age prediction in stroke patients: Highly reliable but limited sensitivity to cognitive performance and response to cognitive training. NeuroImage Clin. 2020, 25, 102159. [Google Scholar] [CrossRef]
- Rezaee, Z.; Ranjan, S.; Solanki, D.; Bhattacharya, M.; Srivastava, M.V.P.; Lahiri, U.; Dutta, A. Feasibility of combining functional near-infrared spectroscopy with electroencephalography to identify chronic stroke responders to cerebellar transcranial direct current stimulation—A computational modeling and portable neuroimaging methodological study. Cerebellum 2021, 20, 853–871. [Google Scholar] [CrossRef]
- Lee, G.; Lee, J.; Kim, J.; Kim, H.; Chang, W.H.; Kim, Y.-H. Whole Brain Hemodynamic Response Based on Synchrony Analysis of Brain Signals for Effective Application of HD-tDCS in Stroke Patients: An fNIRS Study. J. Pers. Med. 2022, 12, 432. [Google Scholar] [CrossRef]
- Kalloch, B.; Weise, K.; Lampe, L.; Bazin, P.-L.; Villringer, A.; Hlawitschka, M.; Sehm, B. The influence of white matter lesions on the electric field in transcranial electric stimulation. NeuroImage Clin. 2022, 35, 103071. [Google Scholar] [CrossRef]
- Hua, H.; Zhang, B.; Wang, X.; He, Y.; Lai, M.; Chen, N.; Liu, J. Diffusion Tensor Imaging Observation of Frontal Lobe Multidirectional Transcranial Direct Current Stimulation in Stroke Patients with Memory Impairment. J. Health Eng. 2022, 2022, 2545762. [Google Scholar] [CrossRef]
- Yuan, K.; Chen, C.; Lou, W.-T.; Khan, A.; Ti, E.C.-H.; Lau, C.C.-Y.; Wang, X.; Chu, W.C.-W.; Tong, R.K.-Y. Differential Effects of 10 and 20 Hz Brain Stimulation in Chronic Stroke: A tACS-fMRI Study. IEEE Trans. Neural Syst. Rehabil. Eng. 2022, 30, 455–464. [Google Scholar] [CrossRef]
- Morya, E.; Monte-Silva, K.; Bikson, M.; Esmaeilpour, Z.; Biazoli, C.E.; Fonseca, A.; Bocci, T.; Farzan, F.; Chatterjee, R.; Hausdorff, J.M.; et al. Beyond the target area: An integrative view of tDCS-induced motor cortex modulation in patients and athletes. J. Neuroeng. Rehabil. 2019, 16, 141. [Google Scholar] [CrossRef]
- Feng, W.; Wang, J.; Chhatbar, P.Y.; Doughty, C.; Landsittel, D.; Lioutas, V.-A.; Kautz, S.A.; Schlaug, G. Corticospinal tract lesion load: An imaging biomarker for stroke motor outcomes. Ann. Neurol. 2015, 78, 860–870. [Google Scholar] [CrossRef]
- Stinear, C.; Petoe, M.; Byblow, W.D. Primary Motor Cortex Excitability During Recovery After Stroke: Implications for Neuromodulation. Brain Stimul. 2015, 8, 1183–1190. [Google Scholar] [CrossRef]
- McCambridge, A.B.; Stinear, J.W.; Byblow, W.D. Revisiting interhemispheric imbalance in chronic stroke: A tDCS study. Clin. Neurophysiol. 2018, 129, 42–50. [Google Scholar] [CrossRef]
- Cunningham, D.A.; Machado, A.; Janini, D.; Varnerin, N.; Bonnett, C.; Yue, G.; Jones, S.; Lowe, M.; Beall, E.; Sakaie, K.; et al. Assessment of inter-hemispheric imbalance using imaging and noninvasive brain stimulation in patients with chronic stroke. Arch. Phys. Med. Rehabil. 2015, 96, S94–S103. [Google Scholar] [CrossRef]
- Guerra, A.; Lopez-Alonso, V.; Cheeran, B.; Suppa, A. Variability in non-invasive brain stimulation studies: Reasons and results. Neurosci. Lett. 2020, 719, 133330. [Google Scholar] [CrossRef]
- Pestalozzi, M.I.; Di Pietro, M.; Gaytanidis, C.M.; Spierer, L.; Schnider, A.; Chouiter, L.; Colombo, F.; Annoni, J.-M.; Jost, L.B. Effects of Prefrontal Transcranial Direct Current Stimulation on Lexical Access in Chronic Poststroke Aphasia. Neurorehabilit. Neural Repair 2018, 32, 913–923. [Google Scholar] [CrossRef]
- Hodics, T.; Cohen, L.G.; Pezzullo, J.C.; Kowalske, K.; Dromerick, A.W. Barriers to Enrollment in Post-Stroke Brain Stimulation in a Racially and Ethnically Diverse Population. Neurorehabilit. Neural Repair 2022, 36, 596–602. [Google Scholar] [CrossRef]
- Li, L.M.; Violante, I.R.; Leech, R.; Ross, E.; Hampshire, A.; Opitz, A.; Rothwell, J.C.; Carmichael, D.W.; Sharp, D.J. Brain state and polarity dependent modulation of brain networks by transcranial direct current stimulation. Hum. Brain Mapp. 2019, 40, 904–915. [Google Scholar] [CrossRef]
- Grazzi, L.; Usai, S.; Bolognini, N.; Grignani, E.; Sansone, E.; Tramacere, I.; Maravita, A.; Lauria, G. No efficacy of transcranial direct current stimulation on chronic migraine with medication overuse: A double blind, randomised clinical trial. Cephalalgia 2020, 40, 1202–1211. [Google Scholar] [CrossRef]
- Caulfield, K.A.; Badran, B.W.; DeVries, W.H.; Summers, P.M.; Kofmehl, E.; Li, X.; Borckardt, J.J.; Bikson, M.; George, M.S. Transcranial electrical stimulation motor threshold can estimate individualized tDCS dosage from reverse-calculation electric-field modeling. Brain Stimul. 2020, 13, 961–969. [Google Scholar] [CrossRef]
- Caulfield, K.A.; Badran, B.W.; Li, X.; Bikson, M.; George, M.S. Can transcranial electrical stimulation motor threshold estimate individualized tDCS doses over the prefrontal cortex? Evidence from reverse-calculation electric field modeling. Brain Stimul. 2020, 13, 1150–1152. [Google Scholar] [CrossRef]
- Evans, C.; Bachmann, C.; Lee, J.S.; Gregoriou, E.; Ward, N.; Bestmann, S. Dose-controlled tDCS reduces electric field intensity variability at a cortical target site. Brain Stimul. 2020, 13, 125–136. [Google Scholar] [CrossRef]
- Van Hoornweder, S.; Nuyts, M.; Frieske, J.; Verstraelen, S.; Meesen, R.L.J.; Caulfield, K.A. A Systematic Review and Large-Scale tES and TMS Electric Field Modeling Study Reveals How Outcome Measure Selection Alters Results in a Person- and Montage-Specific Manner. Preprint. bioRxiv 2023. [Google Scholar] [CrossRef]
- Laakso, I.; Tanaka, S.; Koyama, S.; De Santis, V.; Hirata, A. Inter-subject Variability in Electric Fields of Motor Cortical tDCS. Brain Stimul. 2015, 8, 906–913. [Google Scholar] [CrossRef]
- Zanto, T.F.; Jones, K.T.; Ostrand, A.E.; Hsu, W.Y.; Campusano, R.; Gazzaley, A. Individual differences in neuroanatomy and neurophysiology predict effects of transcranial alternating current stimulation. Brain Stimul. 2021, 14, 1317–1329. [Google Scholar] [CrossRef]
- Lefebvre, S.; Liew, S.-L. Anatomical Parameters of tDCS to Modulate the Motor System after Stroke: A Review. Front. Neurol. 2017, 8, 29. [Google Scholar] [CrossRef]
- Caulfield, K.A.; Indahlastari, A.; Nissim, N.R.; Bs, J.W.L.; Bs, H.H.F.; Woods, A.J.; George, M.S. Electric Field Strength from Prefrontal Transcranial Direct Current Stimulation Determines Degree of Working Memory Response: A Potential Application of Reverse-Calculation Modeling? Neuromodulation 2022, 25, 578–587. [Google Scholar] [CrossRef]
- Klaus, J.; Schutter, D.J.L.G. Electrode montage-dependent intracranial variability in electric fields induced by cerebellar transcranial direct current stimulation. Sci. Rep. 2021, 11, 22183. [Google Scholar] [CrossRef]
- Pisano, F.; Manfredini, A.; Castellano, A.; Caltagirone, C.; Marangolo, P. Does Executive Function Training Impact on Communication? A Randomized Controlled tDCS Study on Post-Stroke Aphasia. Brain Sci. 2022, 12, 1265. [Google Scholar] [CrossRef]
- Lefaucheur, J.F.; Antal, A.; Ayache, S.S.; Benninger, D.H.; Brunelin, J.; Cogiamanian, F.; Cotelli, M.; De Ridder, D.; Ferrucci, R.; Langguth, B.; et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin. Neurophysiol. 2017, 128, 56–92. [Google Scholar] [CrossRef]
- Monti, A.; Ferrucci, R.; Fumagalli, M.; Mameli, F.; Cogiamanian, F.; Ardolino, G.; Priori, A. Transcranial direct current stimulation (tDCS) and language. J. Neurol. Neurosurg. Psychiatry 2013, 84, 832–842. [Google Scholar] [CrossRef]
- Kunaratnam, N.; Saumer, T.M.; Kuan, G.; Holmes, Z.; Swarbrick, D.; Kiss, A.; Mochizuki, G.; Chen, J.L. Transcranial direct current stimulation leads to faster acquisition of motor skills, but effects are not maintained at retention. PLoS ONE 2022, 17, e0269851. [Google Scholar] [CrossRef]
- Veldema, J.; Gharabaghi, A. Non-invasive brain stimulation for improving gait, balance, and lower limbs motor function in stroke. J. Neuroeng. Rehabil. 2022, 19, 84. [Google Scholar] [CrossRef]
- Darkow, R.; Martin, A.; Würtz, A.; Flöel, A.; Meinzer, M. Transcranial direct current stimulation effects on neural processing in post-stroke aphasia. Hum. Brain Mapp. 2017, 38, 1518–1531. [Google Scholar] [CrossRef]
- Lefebvre, S.; Dricot, L.; Laloux, P.; Desfontaines, P.; Evrard, F.; Peeters, A.; Jamart, J.; Vandermeeren, Y. Increased functional connectivity one week after motor learning and tDCS in stroke patients. Neuroscience 2017, 340, 424–435. [Google Scholar] [CrossRef]
- Welsby, E.; Ridding, M.; Hillier, S.; Hordacre, B. Connectivity as a Predictor of Responsiveness to Transcranial Direct Current Stimulation in People with Stroke: Protocol for a Double-Blind Randomized Controlled Trial. JMIR Res. Protoc. 2018, 7, e10848. [Google Scholar] [CrossRef]
- Carlson, H.L.; Ciechanski, P.; Harris, A.D.; MacMaster, F.P.; Kirton, A. Changes in spectroscopic biomarkers after transcranial direct current stimulation in children with perinatal stroke. Brain Stimul. 2018, 11, 94–103. [Google Scholar] [CrossRef]
- Pruvost-Robieux, E.; Benzakoun, J.; Turc, G.; Marchi, A.; Mancusi, R.L.; Lamy, C.; Domigo, V.; Oppenheim, C.; Calvet, D.; Baron, J.-C.; et al. Cathodal Transcranial Direct Current Stimulation in Acute Ischemic Stroke: Pilot Randomized Controlled Trial. Stroke 2021, 52, 1951–1960. [Google Scholar] [CrossRef]
- Kolskår, K.K.; Richard, G.; Alnæs, D.; Dørum, E.S.; Sanders, A.; Ulrichsen, K.M.; Sánchez, J.M.; Ihle-Hansen, H.; Nordvik, J.E.; Westlye, L.T. Reliability, sensitivity, and predictive value of fMRI during multiple object tracking as a marker of cognitive training gain in combination with tDCS in stroke survivors. Hum. Brain Mapp. 2021, 42, 1167–1181. [Google Scholar] [CrossRef]
- Räty, S.; Ruuth, R.; Silvennoinen, K.; Sabel, B.A.; Tatlisumak, T.; Vanni, S. Resting-state Functional Connectivity After Occipital Stroke. Neurorehabilit. Neural Repair 2022, 36, 151–163. [Google Scholar] [CrossRef]
- Khadka, N.; Borges, H.; Paneri, B.; Kaufman, T.; Nassis, E.; Zannou, A.L.; Shin, Y.; Choi, H.; Kim, S.; Lee, K.; et al. Adaptive current tDCS up to 4 mA. Brain Stimul. 2020, 13, 69–79. [Google Scholar] [CrossRef]
- Chhatbar, P.Y.; Chen, R.; Deardorff, R.; Dellenbach, B.; Kautz, S.A.; George, M.S.; Feng, W. Safety and tolerability of transcranial direct current stimulation to stroke patients—A phase I current escalation study. Brain Stimul. 2017, 10, 553–559. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Paulus, W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J. Physiol. 2000, 527 Pt 3, 633–639. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Paulus, W.; Frahm, J. Regional modulation of BOLD MRI responses to human sensorimotor activation by transcranial direct current stimulation. Magn. Reson. Med. 2001, 45, 196–201. [Google Scholar]
- Spampinato, M.V.; Chan, C.; Jensen, J.H.; Helpern, J.A.; Bonilha, L.; Kautz, S.A.; Nietert, P.J.; Feng, W. Diffusional Kurtosis Imaging and Motor Outcome in Acute Ischemic Stroke. AJNR Am. J. Neuroradiol. 2017, 38, 1328–1334. [Google Scholar] [CrossRef]
- Lewis, A.F.; Stewart, J.C. Comparison of corticospinal tract integrity measures extracted from standard versus native space in chronic stroke. J. Neurosci. Methods 2021, 359, 109216. [Google Scholar] [CrossRef] [PubMed]
- Schilling, K.; Gao, Y.; Janve, V.; Stepniewska, I.; Landman, B.A.; Anderson, A.W. Can increased spatial resolution solve the crossing fiber problem for diffusion MRI? NMR Biomed. 2017, 30, e3787. [Google Scholar] [CrossRef] [PubMed]
- Behrens, T.E.; Johansen-Berg, H.; Jbabdi, S.; Rushworth, M.F.; Woolrich, M.W. Probabilistic diffusion tractography with multiple fibre orientations: What can we gain? Neuroimage 2007, 34, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Alsop, D.C.; Schlaug, G. Effects of transcranial direct current stimulation (tDCS) on human regional cerebral blood flow. Neuroimage 2011, 58, 26–33. [Google Scholar] [CrossRef]
| Authors | Sample | Region(s) of Interest | Experimental Design | Key Finding(s) |
|---|---|---|---|---|
| Dijkhuizen et al. [11] (2001) ** | Unilateral MCA stroke in male Sprague-Dawley rats; N = 6 | S1fl, M1 | Contrast-enhanced fMRIs were administered 3 days and 14 days post-stroke. 4–5 V for 0.5 ms at 3 Hz for 1 min. Stimulation occurred in the right and then the left forelimb. | Stimulation resulted in a significant increase in neuronal activation-induced rCBV in the S1fl and M1. Contrast-enhanced (CBV-weighted) fMRIs with MINO contrast agent enables high temporal spatial resolution when imaging brain activation patterns. Limb dysfunction is related with loss of neural activation in the ipsilesional sensorimotor cortex. |
| Dijkhuizen et al. [12] (2003) ** | Unilateral MCA stroke in male Sprague-Dawley rats; N = 21 | S1fl, M1 | Contrast-enhanced fMRIs were administered 1 day, 3 days, and 14 days post-stroke. 5 V for 0.5 ms at 3 Hz for 40 s in the right and then the left forelimb. | The change in activation balance toward the contralesional hemisphere increases with the amount of infarct injury. Recovery is related mostly with preservation of activation in the ipsilesional hemisphere. |
| Yoon et al. [14] (2012) | MCA territory ischemic rats; N = 30 | N/A | 3 study groups: sham, early tDCS, and late tDCS. | tDCS was applied in acute stroke rats. tDCS can alter neuronal plasticity surrounding the penumbra without aggravating infarct volume. |
| Braun et al. [13] (2016) | Left MCA territory ischemic male Wistar rats; N = 28 | M1 | Rats were assessed for baseline values a day before ischemia. MRI was performed 2 days after ischemia. Rats were trained daily on a motor task. tDCS was administered 3 days after ischemia. tDCS was repeated daily for 5 consecutive days, followed by no stim for 2 days, ending with 5 additional days of stim. There were 3 study groups (ctDCS, atDCS, and sham). | tDCS during acute stroke increases neurogenesis and functional recovery post-stroke. |
| Authors | Sample | Type of Study | Region(s) /Parameter of Interest | Experimental Design | Neuroimaging Modality | Electrode Montage | Key Finding(s) |
|---|---|---|---|---|---|---|---|
| Bradnam et al. [23] (2012) | At least six weeks post subcortical stroke (N = 12) | Cross-over, double-blind design | M1 | Participants attended two experimental sessions in which they completed motor tasks, the NIHSS, FMA, and ASH. One week later, they received either ctDCS or sham tDCS. | sMRI and DWI scans | Contralesional ctDCS was applied over the M1 region with a constant current of 1 mA for 20 min. Anode was placed over the contralateral forehead. | First study to show that ctDCS on the contralesional M1 varies among patients and depends on white matter tract integrity from the ipsilesional hemisphere. ctDCS improved motor function in mildly impaired patients and worsened function for moderately to severely impaired patients. |
| Lindenberg et al. [24] (2012) | Chronic stroke patients (N = 15) | Comparative study | M1 | Participants received bihemispheric tDCS and simultaneous physical/occupational therapy for five consecutive days. Participants underwent DTI at baseline. | DTI | The stimulation consisted of 30 min of 1.5 mA direct current with the anode placed over the ipsilesional and the cathode over the contralesional motor cortex. | DTI measures can be used to predict functional potential for motor recovery. |
| Stagg et al. [25] (2012) | Chronic ischemic and haemorrhagic stroke (N = 17) | Single-blinded | M1, SMA, PMd | Motor task was administered before, during, and after tDCS. | fMRI | atDCS was placed over the M1 region and the ctDCS was placed over the contralateral supraorbital ridge; 1 mA for 10–20 min. | tDCS-induced brain activation changes using fMRI were reported. atDCS improved response times and increased activation in ipsilesional M1, premotor cortex, and SMA. |
| Dmochowski et al. [26] (2013) | Chronic stroke (N = 8) | Pilot study | Varied among participants | Participants received MRIs, stimulation, and completed word-naming tasks afterwards. | sMRI, fMRI | hdtDCS was used at 2 mA. Cathodes were placed over the right supraorbital region. Anodes were placed over the target region which varied from patient to patient. | This work individualized hdtDCS montage by utilizing MRI-based modeling of tDCS current flow. Optimizing the electrode montage will result in a 64% increase in EF magnitude at the target. Task performance increased by 38% following optimized montage. |
| Gillick et al. [27] (2014) | Perinatal ischemic stroke in a 10-year-old | Case report | Bihemispheric M1 | MRI was acquired for tDCS montage personalization. | sMRI | ctDCS was placed over C3 and atDCS was placed over C4 at 0.7 mA for 10 min. | Study demonstrated the ability to adapt tDCS mA to specific patient anatomy based on computational modeling analyses |
| Rosso et al. [28] (2014) | MCA stroke participants with aphasia N = 25 | Cross-over, single blind design | Broca’s area (BA) | Participants over three months post-stroke received neuroimaging, followed by a naming task, ending with cathodal stimulation. | Functional, structural, and diffusion MRIs | The electrode center was placed over the ascendant ramus of the lateral sulcus. Reference electrode was placed over the contralateral supraorbital region; 1 mA for 15 min. | tDCS can reduce inhibition of the right BA and reinstate normal interhemispheric inhibition when the left BA is damaged. |
| Jindal et al. [29] (2015) | Chronic stroke in MCA territory (N = 5) | Joint-imaging and tDCS study | CST | NIRS-EEG/tDCS were placed on the patient’s scalp. Fifteen rounds of tDCS were repeated with 30 s “off” periods in between stim session. | NIRS-EEG/tDCS | ctDCS was placed over the F3 region and atDCS was placed over the Cz region, in accordance to the international 10–20 EEG system. tDCS was repeated 15× with 30 s “off” periods at 0.5A/m2. | Variability in CST excitability changes to tDCS are highlighted. |
| Lefebvre et al. [30] (2015) | Chronic stroke participants (N = 19) | Double-blind, cross-over randomized, sham-controlled experiment | M1 | Each subject had two sessions: intervention session during which dual tDCS or sham was applied during motor skill learning with the paretic upper limb; and an imaging session one week later, during which participants performed a task. | fMRI | The anode was positioned over the ipsilesional M1 and the cathode over the contralesional M1; 1 mA for 30 min. | In the dual-transcranial DCS series, the enhanced retention of the motor skill learned one week prior was associated with lesser activation in both hemispheres compared to the sham series, especially in the premotor/motor areas of the ipsilesional hemisphere. |
| Zheng et al. [31] (2015) | Chronic stroke participants with uni-hemispheric stroke (N = 10) | Pilot study | CST; FA; internal capsule, pons | Participants received 10 days of PT/OT while simultaneously receiving tDCS for 30 min. | DTI | The stimulation consisted of 30 min of 1.5 mA direct current with the anode placed over the ipsilesional motor cortex and the cathode over the contralesional motor cortex. | Chronic stroke survivors who participated in the treatment group (tDCS + PT/OT) showed significant increases in FMA-UE. Furthermore, the treatment group displayed significant increases in FA values for the ipsilesional descending motor fibers. |
| Chen et al. [32] (2016) | First-time MCA ischemic stroke over three months post-stroke (N = 5) | Proof-of-principle pilot study | Precuneus, M1, premotor cortex | Ten sessions of tDCS combined with PT/OT. PT/OT sessions were 60 min with tDCS for 30 min. | rsfMRI, fMRI | atDCS was placed over the C3 or C4 landmark of the 10-20 EEG system depending on the infarcted hemisphere. ctDCS was placed in the opposite C3 or C4 region. tDCS was applied for 30 min at 1.5 mA. | After treatment, there was a reduction in motor impairment. There was an improvement in the ipsilesional M1 and contralesional premotor cortex’s resting-state connectivity. |
| Sebastian et al. [33] (2017) | Bilateral MCA ischemic stroke (N = 1) | Double-blind, within-subject crossover trial design | RC, LC, SFG, SFG_PFC, MFG_DLPC, MTG_pole, ITG, FG | There were two conditions: “RC tDCS + behavioral treatment (spelling task)” and “sham tDCS + behavioral treatment”. Each condition consisted of 15 consecutive training sessions, 3–5 per week, two months apart. | sMRI, rs-fMRI were acquired at start of study and two months after completion of study (six-month interval between scans) | tDCS was administered for 20 min at 2 mA. atDCS was placed over the RC and ctDCS was placed over the right deltoid muscle. | Stim and sham treatments resulted in improved spelling. However, there was a trend for greater improvement for the stim treatment. Improvements in spelling coincided with increased connectivity in the cerebro–cerebellar network. |
| Hordacre et al. [34] (2018) | Chronic stroke (N = 10) | Randomized, cross-over trial | M1 | EEG was acquired at the first 3 min of the session, EMG was used throughout session. TMS was used to find hand-knob region in M1, some participants received stim and others sham. | sMRI | atDCS over the lesioned M1 and ctDCS over the contralateral orbit at 1 mA for 20 min. | atDCS did not increase corticospinal excitability measured using resting motor threshold and motor-evoked potentials. |
| Larcombe et al. [35] (2018) | Stroke survivors with lesion to the primary visual cortex (N = 7) | Pilot study | Visual training | Each participant had a visual performance assessment and an fMRI before and after training. Three participants received anodal tDCS and one had no stimulation. | fMRI | Participants received five 20 min sessions. The stimulation group received 1 mA for 20 min. | No participants showed improvement in visual function, and application of tDCS had no effect on visual performance. |
| Sánchez-Kuhn et al. [36] (2019) * in Spanish | Cerebellar stroke in 64-year-old man | Case report | Cerebellum | The treatment session comprised of 16 sessions of tDCS with neuroimaging and swallowing therapy for dysphagia for a total of four weeks. | sMRI, dMRI | atDCS was placed over the left M1 and ctDCS was placed over the right trapeze at 1mA for 20 min (16 total sessions). | After the treatment session, there was an increase in white matter fibers and connectivity in the left cerebellar peduncle. |
| Iyer et al. [37] (2019) | Chronic stroke survivors with a single episode of stroke (N = 20) | Exploratory study with a cross-over design | M1, CBv changes in relation to CME | The first session included clinical measures and TMS measurements before and after anodal tDCS. Participants were block randomized into anodal and sham stimulation for sessions 2 and 3. | Transcranial Doppler (TCD) ultrasound | Anode was placed over the lower limb M1 hotspot on the lesioned hemisphere. Cathode was placed over the supraorbital region; 1 mA for 15 min. | Explored neurovascular changes after tDCS of the lower limb M1 in individuals with stroke. They observed no change in CME or CBv parameters due to anodal tDCS in any of the participants. |
| Lee et al. [38] (2019) | Subacute stroke survivors (N = 21) & age-matched healthy controls (N = 12) | Randomized study | M1 | 1 Hz rTMS on the contralesional M1 and anodal tDCS on the ipsilesional M1. Participants were classified into responders and non-responders based on the functional improvement of the affected upper extremity after applying NBS. | fMRI | Anode was placed over the ipsilesional M1 and the cathode was placed over the supraorbital area. 2 mA of current was applied for 20 min. | The imbalanced M1 interhemispheric connectivity between affected and unaffected hemispheres in responders was significantly restored. |
| Abualait et al. [39] (2020) | Stroke patient exhibiting cortical sensation deficits | Double-blind, sham-controlled, single-case study | M1 | The participant underwent sham and stimulation. Following that, the patient completed functional measures. Structural and diffusion tensor imaging data were acquired before and after stimulation. | sMRI, DTI | The patient underwent 20 sessions of sham tDCS followed by 30 sessions of tDCS over both M1 cortices. Each session involved 20 min of 2 mA stimulation. | A positive correlation was observed between improved recovery of fine motor skills and higher FA of the CST as well as increased density of gray matter in specific brain regions. Furthermore, the patient with stroke showed functional improvement and structural changes following tDCS. |
| Kuo et al. [40] (2020) | First-time, unilateral subcortical ischemic stroke survivors (N = 18) | Randomized sham-controlled crossover study | M1 | All participants participated in four experimental sessions on separate days: two real and two sham dual-tDCS sessions, which were combined with either TMS or MEG recordings (i.e., TMS + real tDCS, TMS + sham tDCS, MEG + real tDCS, MEG + sham tDCS). | MEG | The anode was placed over the ipsilesional M1, and the cathode over the contralesional M1. Impedance was kept below 5 kΩ. For true stimulation, a 2 mA current was applied for 20 min. | Stroke survivors had decreased excitability in ipsilesional M1 with excessive transcallosal inhibition from the contralesional to ipsilesional hemisphere at baseline compared with controls. |
| Richard et al. [41] (2020) | Stroke survivors (N = 54) | Randomized double-blind study | Working memory training and age prediction | Participants were randomized to sham or tDCS stimulation. Participants underwent CCT and MRI before and after the intervention. | MRI | tDCS current was 1 mA for a total of 120 min. Anode was placed over F3 and the cathode was placed over O2. | Utilizing brain morphometry for longitudinal brain age prediction is possible for stroke participants. However, there was no notable correlation between brain age and cognitive training outcomes. |
| Rezaee et al. [42] (2021) | Male chronic ischemic stroke (N = 12) | Methodological report | Cerebellum | Participants were fitted with an fNIRS-EEG/tDCS cap. Two min of functional connectivity data was collected. Participants then performed a VR task. | fNIRS | atDCS was placed at the contralesional side and ctDCS was placed in the ipsilesional side of the dentate nuclei at 2 mA for 15 min. | Feasibility of fNIRS-EEG joint-imaging of ctDCS was established. However, ctDCS effects on the cerebellum were non-significant. |
| Lee et al. [43] (2022) | Chronic cerebro–vascular disease (N = 26) | Randomized study | Subcortical areas | Design aimed to observe hemodynamic responses based on tDCS. Participants were asked to sit still and stare at a black screen with a plus sign in the middle. | fNIRS with 66 channels | HD-tDCS device was used. The atDCS and ctDCS were located on C3 and C4 of the 10–20 system at 1 mA. | Cortical activity and synchronization were present each in tDCS trial, followed by a sudden decrease in cortical activity and synchrony. |
| Kalloch et al. [44] (2022) | Stroke participants (N = 88) | Simulation study | Electric Field | Participants were assigned to four groups of increasing lesion load. They aimed to quantify the change of electrical properties of white matter lesions. | T1 & T2W FLAIR | All simulations were conducted using a bihemispheric electrode. Setup at 2 mA over the 10–20 coordinates C3 & C4 and a frontal–occipital setup over the coordinates FPZ & OZ. | White matter lesions do not perturb the electric field and can be omitted when modeling participants with low to medium lesion load. |
| Hua et al. [45] (2022) | Participants with poststroke memory impairment (N = 60) | Randomized study | White matter tract FA | Lesion location and memory severity were assessed. | sMRI, DTI | Anodal tDCS of the frontal lobe; parameters were not mentioned. | FA values of the infarct foci and frontal lobe can be used to identify the degree of memory impairment. |
| Yuan et al. [46] (2022) | Chronic stroke participants with unilateral infarcts (N = 13) | Randomized study | Brain activity in sensori-motor region | Participants completed a motor task in the MRI. After that, rs-MRI analysis was performed before, during, and after. Graph theory analysis of the whole brain was conducted. | tACS-fMRI | Electrodes were placed over the ipsilesional M1 and contralesional supraorbital ridge. One mA was delivered for 20 min by an MRI-compatible DC stimulator. | Functional interaction between the brain regions involved in executive control and SMN regions is facilitated by 20 Hz tACS. |
| Authors | Sample | Time from Stroke Onset | Type of Study | Region(s) /Parameter of Interest | Experimental Design | Neuroimaging Technique | Electrode Montage | Key Finding(s) |
|---|---|---|---|---|---|---|---|---|
| Lefebvre et al. [30] (2015) | Chronic stroke (N = 19) | Over six months post-stroke | Cross-over, double-blind, randomized design with two sessions | SMA, PMd | The series consisted of two sessions: dual-tDCS or sham during motor skill learning, and an imaging session one week later during motor skill task. | sMRI, fMRI | atDCS was positioned over the ipsilesional M1 and ctDCS was positioned over the contralesional M1 at 1 mA for 30 min. | tDCS enhanced motor learning. Revealed fMRI activation supporting long-term retention of motor skill in stim group. |
| Darkow et al. [71] (2017) | Chronic stroke (N = 16) | >12 months | Cross-over, sham-tDCS, RCT | Left M1 | Naming task and tDCS during MRI. | sMRI, fMRI | atDCS was placed over the left representation of the hand M1. ctDCS was placed over the right supraorbital region. Stim at 1 mA for 20 min and sham. | tDCS modulated neural processing. Stim group displayed decrease in activation in the ACC, left insula, and right lingual gyrus. |
| Lefebvre et al. [72] (2017) | Chronic hemiparetic stroke (N = 22) | Variable | Randomized, placebo-controlled, double-blind, crossover design | M1, SMA, PMd, SMN, Somato-motor network, salience network | Baseline rs-fMRI, a week later bilateral tDCS and sham, after two weeks from baseline a second tDCS session occurred. | rs-fMRI | Anode over the M1 ipsilesional hemisphere and cathode over M1 in the undamaged hemisphere. | No differences in FC in the ROIs. FC increased in the somatomotor network in the stim group. |
| Welsby et al. [73] (2018) preprint | First-time ischemic stroke (N = 68) | Over six months post-stroke | Double-blind RCT | Ipsilesional M1 | Participants were randomized to sham or stim. MRIs were collected, followed by administration of the FMA, EEG, TMS, ARAT, tDCS, and motor task. | sMRI, fMRI, dMRI | Preprogrammed at-home tDCS for 20 min at 1 mA daily for 2 weeks. atDCS over the ipsilesional M1 and ctDCS over the contralateral supraorbital region. | Results are pending and have not been published yet |
| Carlson et al. [74] (2018) | Children with perinatal stroke (N = 15) | Variable | Double-blind, sham-controlled, RCT | M1 | Ten days of customized, goal-directed therapy was paired with cathodal tDCS over contralesional primary motor cortex. Neuronal metabolites in both M1s were measured before and after intervention using fMRI-guided short-echo 3T MRS. | Proton MRS | Cathode over contralesional M1 at 1 mA for 20 min and sham. | Motor performance improvedin both groups and tDCS was associated with greater goal achievement. |
| Pruvost-Robieux et al. [75] (2021) | Non-lacunar acute ischemic stroke in the MCA territory (N = 45) | Variable | Proof-of-principle; Single-center, prospective, double-blind, sham-controlled RCT | M1 | Participants received imaging and were randomized to ctDCS or sham. | MRI, MRA, DWI | ctDCS electrode was placed in ipsilesional M1, and atDCS was placed in the contralateral supraorbital area. Stimulation current was 1.5 mA for 20 min delivered every hr over 6 h. | ctDCS did not result in a significant reduction of infarct growth volume, although there was an apparent trend towards smaller infarct growth in the stim group. |
| Kolskår et al. [76] (2021) | Chronic stroke participants (N = 48) | Over six months post-stroke | Prospective double-blind RCT | Feasibility of combining CCT and tDCS on working memory | Participants completed an fMRI at three timepoints. They performed a computerized working memory training program. Each participant completed two weekly tDCS stimulation sessions at the hospital, with a total of six tDCS sessions. | fMRI | Participants were randomized to one of two groups, receiving CCT and either (a) tDCS targeting left dorsolateral prefrontal cortex (1 mA), or (b) sham tDCS, with 40s active stimulation (1 mA) before fading out of the current. Stimulation current was 1 mA. | Results revealed increased performance across all trained tasks, with no additional benefit of tDCS. Brain activation prior to the training was not predictive for training outcome, nor was training gains reflected in altered brain activation. |
| Räty et al. [77] (2022) | Chronic occipital stroke survivors (N = 16) & healthy controls (N = 12) | Over six months post-stroke | Randomized, sham-controlled RCT | 74 cortical ROIs | Participants underwent rsfMRI at baseline, after two weeks of rtACS or sham treatment, and two months of treatment-free follow-up. | rtACS and rsfMRI | Electrodes placed supraorbitally while a ctDCS electrode was on the right forearm. Stimulation frequency alternated between 5 and 15 Hz. | rtACS treatment in the given setting did not affect FC. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salazar, C.A.; Feng, W.; Bonilha, L.; Kautz, S.; Jensen, J.H.; George, M.S.; Rowland, N.C. Transcranial Direct Current Stimulation for Chronic Stroke: Is Neuroimaging the Answer to the Next Leap Forward? J. Clin. Med. 2023, 12, 2601. https://doi.org/10.3390/jcm12072601
Salazar CA, Feng W, Bonilha L, Kautz S, Jensen JH, George MS, Rowland NC. Transcranial Direct Current Stimulation for Chronic Stroke: Is Neuroimaging the Answer to the Next Leap Forward? Journal of Clinical Medicine. 2023; 12(7):2601. https://doi.org/10.3390/jcm12072601
Chicago/Turabian StyleSalazar, Claudia A., Wuwei Feng, Leonardo Bonilha, Steven Kautz, Jens H. Jensen, Mark S. George, and Nathan C. Rowland. 2023. "Transcranial Direct Current Stimulation for Chronic Stroke: Is Neuroimaging the Answer to the Next Leap Forward?" Journal of Clinical Medicine 12, no. 7: 2601. https://doi.org/10.3390/jcm12072601
APA StyleSalazar, C. A., Feng, W., Bonilha, L., Kautz, S., Jensen, J. H., George, M. S., & Rowland, N. C. (2023). Transcranial Direct Current Stimulation for Chronic Stroke: Is Neuroimaging the Answer to the Next Leap Forward? Journal of Clinical Medicine, 12(7), 2601. https://doi.org/10.3390/jcm12072601





