Consensus Statement on Animals’ Relationship with Pediatric Oncohematological Patients, on Behalf of Infectious Diseases and Nurse Working Groups of the Italian Association of Pediatric Hematology-Oncology
Abstract
1. Introduction
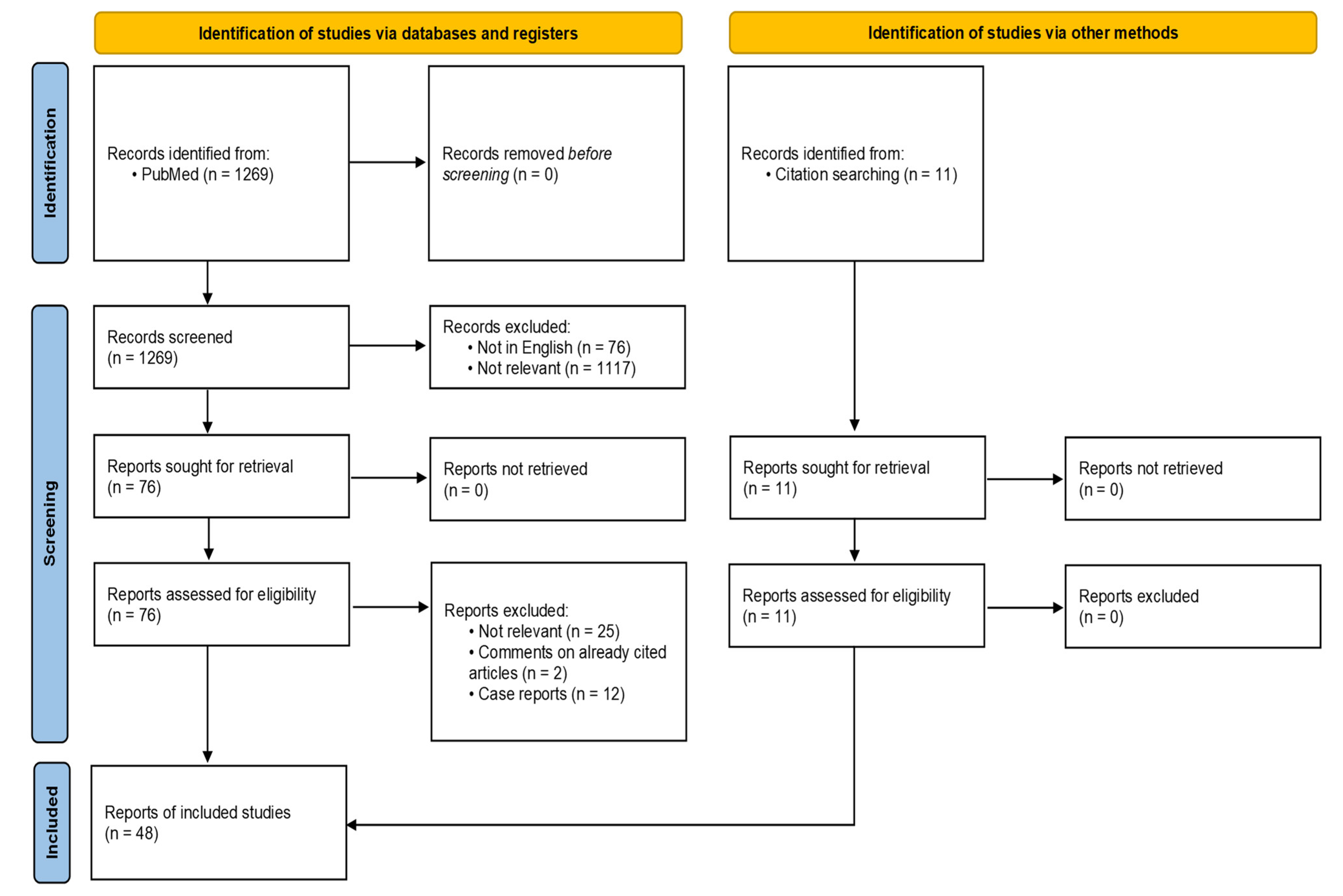
2. Materials and Methods
3. Statements and Discussion
3.1. What Is the Role of Contact between Children with Oncohematologic Diseases and Animals?
3.2. What Are the Risks Associated with Contact with Animals?
3.3. When and How Is This Topic to Be Discussed with Families?
3.4. When Families Decide to Adopt an Animal, Are There Any Recommended Species and Others That Are Better to Avoid?
3.5. Are There Specific Indications for Those Asking to Adopt Animals?
3.6. Is Here Any Hygiene Advice for Those Who Already Own Animals?
3.7. Is Contact with Animals Outside the Home Allowed?
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ssenyonga, N.; Stiller, C.; Nakata, K.; Shalkow, J.; Redmond, S.; Bulliard, J.-L.; Girardi, F.; Fowler, C.; Marcos-Gragera, R.; Bonaventure, A.; et al. Worldwide Trends in Population-Based Survival for Children, Adolescents, and Young Adults Diagnosed with Leukaemia, by Subtype, during 2000-14 (CONCORD-3): Analysis of Individual Data from 258 Cancer Registries in 61 Countries. Lancet Child Adolesc. Health 2022, 6, 409–431. [Google Scholar] [CrossRef] [PubMed]
- Hemsworth, S.; Pizer, B. Pet Ownership in Immunocompromised Children—A Review of the Literature and Survey of Existing Guidelines. Eur. J. Oncol. Nurs. 2006, 10, 117–127. [Google Scholar] [CrossRef] [PubMed]
- McCullough, A.; Ruehrdanz, A.; Jenkins, M.A.; Gilmer, M.J.; Olson, J.; Pawar, A.; Holley, L.; Sierra-Rivera, S.; Linder, D.E.; Pichette, D.; et al. Measuring the Effects of an Animal-Assisted Intervention for Pediatric Oncology Patients and Their Parents: A Multisite Randomized Controlled Trial. J. Pediatr. Oncol. Nurs. 2018, 35, 159–177. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.; Hunter, D. Consensus Methods for Medical and Health Services Research. BMJ 1995, 311, 376–380. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, T.P. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLOS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Elad, D. Immunocompromised Patients and Their Pets: Still Best Friends? Vet. J. 2013, 197, 662–669. [Google Scholar] [CrossRef]
- Chan, M.M.; Tapia Rico, G. The “Pet Effect” in Cancer Patients: Risks and Benefits of Human-Pet Interaction. Crit. Rev. Oncol. Hematol. 2019, 143, 56–61. [Google Scholar] [CrossRef]
- Menna, L.F.; Santaniello, A.; Todisco, M.; Amato, A.; Borrelli, L.; Scandurra, C.; Fioretti, A. The Human-Animal Relationship as the Focus of Animal-Assisted Interventions: A One Health Approach. Int. J. Environ. Res. Public Health 2019, 16, 3660. [Google Scholar] [CrossRef]
- Diniz Pinto, K.; Vieira de Souza, C.T.; Benamor Teixeira, M.d.L.; Fragoso da Silveira Gouvêa, M.I. Animal Assisted Intervention for Oncology and Palliative Care Patients: A Systematic Review. Complement. Ther. Clin. Pract. 2021, 43, 101347. [Google Scholar] [CrossRef]
- Bouchard, F.; Landry, M.; Belles-Isles, M.; Gagnon, J. A Magical Dream: A Pilot Project in Animal-Assisted Therapy in Pediatric Oncology. Can. Oncol. Nurs. J. 2004, 14, 14–17. [Google Scholar] [CrossRef]
- Gagnon, J.; Bouchard, F.; Landry, M.; Belles-Isles, M.; Fortier, M.; Fillion, L. Implementing a Hospital-Based Animal Therapy Program for Children with Cancer: A Descriptive Study. Can. Oncol. Nurs. J. 2004, 14, 217–222. [Google Scholar] [CrossRef]
- Orlandi, M.; Trangeled, K.; Mambrini, A.; Tagliani, M.; Ferrarini, A.; Zanetti, L.; Tartarini, R.; Pacetti, P.; Cantore, M. Pet Therapy Effects on Oncological Day Hospital Patients Undergoing Chemotherapy Treatment. Anticancer Res. 2007, 27, 4301–4303. [Google Scholar]
- Johnson, R.A.; Meadows, R.L.; Haubner, J.S.; Sevedge, K. Animal-Assisted Activity among Patients with Cancer: Effects on Mood, Fatigue, Self-Perceived Health, and Sense of Coherence. Oncol. Nurs. Forum 2008, 35, 225–232. [Google Scholar] [CrossRef]
- Braun, C.; Stangler, T.; Narveson, J.; Pettingell, S. Animal-Assisted Therapy as a Pain Relief Intervention for Children. Complement. Ther. Clin. Pract. 2009, 15, 105–109. [Google Scholar] [CrossRef]
- Urbanski, B.L.; Lazenby, M. Distress among Hospitalized Pediatric Cancer Patients Modified by Pet-Therapy Intervention to Improve Quality of Life. J. Pediatr. Oncol. Nurs. 2012, 29, 272–282. [Google Scholar] [CrossRef]
- Marcus, D.A.; Blazek-O’Neill, B.; Kopar, J.L. Symptom Reduction Identified after Offering Animal-Assisted Activity at a Cancer Infusion Center. Am. J. Hosp. Palliat. Care 2014, 31, 420–421. [Google Scholar] [CrossRef]
- White, J.H.; Quinn, M.; Garland, S.; Dirkse, D.; Wiebe, P.; Hermann, M.; Carlson, L.E. Animal-Assisted Therapy and Counseling Support for Women with Breast Cancer: An Exploration of Patient’s Perceptions. Integr. Cancer Ther. 2015, 14, 460–467. [Google Scholar] [CrossRef]
- Fleishman, S.B.; Homel, P.; Chen, M.R.; Rosenwald, V.; Abolencia, V.; Gerber, J.; Nadesan, S. Beneficial Effects of Animal-Assisted Visits on Quality of Life during Multimodal Radiation-Chemotherapy Regimens. J. Community Support. Oncol. 2015, 13, 22–26. [Google Scholar] [CrossRef]
- Moreira, R.L.; Gubert, F.d.A.; de Sabino, L.M.M.; Benevides, J.L.; Tomé, M.A.B.G.; Martins, M.C.; Brito, M.d.A. Assisted therapy with dogs in pediatric oncology: Relatives’ and nurses’ perceptions. Rev. Bras. Enferm. 2016, 69, 1188–1194. [Google Scholar] [CrossRef]
- Chubak, J.; Hawkes, R.; Dudzik, C.; Foose-Foster, J.M.; Eaton, L.; Johnson, R.H.; Macpherson, C.F. Pilot Study of Therapy Dog Visits for Inpatient Youth with Cancer. J. Pediatr. Oncol. Nurs. 2017, 34, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Silva, N.B.; Osório, F.L. Impact of an Animal-Assisted Therapy Programme on Physiological and Psychosocial Variables of Paediatric Oncology Patients. PLoS ONE 2018, 13, e0194731. [Google Scholar] [CrossRef] [PubMed]
- Ginex, P.; Montefusco, M.; Zecco, G.; Trocchia Mattessich, N.; Burns, J.; Hedal-Siegel, J.; Kopelman, J.; Tan, K.S. Animal-Facilitated Therapy Program: Outcomes from Caring Canines, a Program for Patients and Staff on an Inpatient Surgical Oncology Unit. Clin. J. Oncol. Nurs. 2018, 22, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, A.I.; Neu, M. Youth and Pet Survivors: Exploring the Experiences of Pediatric Oncology and Bone Marrow Transplant Patients in a Virtual Animal-Assisted Therapy Pen Pal Program. J. Pediatr. Oncol. Nurs. 2020, 37, 368–376. [Google Scholar] [CrossRef]
- Holder, T.R.N.; Gruen, M.E.; Roberts, D.L.; Somers, T.; Bozkurt, A. A Systematic Literature Review of Animal-Assisted Interventions in Oncology (Part I): Methods and Results. Integr. Cancer Ther. 2020, 19, 1534735420943278. [Google Scholar] [CrossRef]
- Silva Carvalho, F.; Carvalho, S.; Conde, R.; Esteves, S. Cynotherapy in Cancer Pain Management: A Pilot Study. Pain Med. 2021, 22, 3051–3061. [Google Scholar] [CrossRef]
- Feng, Y.; Lin, Y.; Zhang, N.; Jiang, X.; Zhang, L. Effects of Animal-Assisted Therapy on Hospitalized Children and Teenagers: A Systematic Review and Meta-Analysis. J. Pediatr. Nurs. 2021, 60, 11–23. [Google Scholar] [CrossRef]
- Joint FAO/WHO Expert Committee on Zoonoses; World Health Organization; Food and Agriculture Organization of the United Nations. Joint WHO/FAO Expert Committee on Zoonoses [Meeting Held in Stockholm from 11 to 16 August 1958]: Second Report; World Health Organization: Geneva, Switzerland, 1959. [Google Scholar]
- Grant, S.; Olsen, C.W. Preventing Zoonotic Diseases in Immunocompromised Persons: The Role of Physicians and Veterinarians. Emerg. Infect. Dis. 1999, 5, 159–163. [Google Scholar] [CrossRef]
- Lartigue, M.-F.; Monnet, X.; Le Flèche, A.; Grimont, P.A.D.; Benet, J.-J.; Durrbach, A.; Fabre, M.; Nordmann, P. Corynebacterium ulcerans in an Immunocompromised Patient with Diphtheria and Her Dog. J. Clin. Microbiol. 2005, 43, 999–1001. [Google Scholar] [CrossRef]
- Amman, B.R.; Pavlin, B.I.; Albariño, C.G.; Comer, J.A.; Erickson, B.R.; Oliver, J.B.; Sealy, T.K.; Vincent, M.J.; Nichol, S.T.; Paddock, C.D.; et al. Pet Rodents and Fatal Lymphocytic Choriomeningitis in Transplant Patients. Emerg. Infect. Dis. 2007, 13, 719–725. [Google Scholar] [CrossRef]
- Goldberg, J.D.; Kamboj, M.; Ford, R.; Kiehn, T.E.; Gilhuley, K.; Perales, M.-A. “Kennel Cough” in a Patient Following Allogeneic Hematopoietic Stem Cell Transplant. Bone Marrow Transpl. 2009, 44, 381–382. [Google Scholar] [CrossRef]
- Redelman-Sidi, G.; Grommes, C.; Papanicolaou, G. Kitten-Transmitted Bordetella Bronchiseptica Infection in a Patient Receiving Temozolomide for Glioblastoma. J. Neurooncol. 2011, 102, 335–339. [Google Scholar] [CrossRef]
- Hernández, M.; González-Lama, Y.; Ramos, A.; Martínez-Ruiz, R.; Calvo, M.; Matallana, V.; Vera, M.I.; Suarez, C.; Blázquez, I.; Abreu, L. Visceral Leishmaniasis as an Unusual Infectious Complication in a Patient with Crohn’s Disease Treated with Infliximab. Gastroenterol. Hepatol. 2015, 38, 411–412. [Google Scholar] [CrossRef]
- Bienz, M.; Tomaszewski, M.; McDonald, E.G. Severe Pet-Transmitted Zoonosis in a Patient with a Compromised Immune System. CMAJ 2018, 190, E1332–E1336. [Google Scholar] [CrossRef]
- van der Reijden, M.; Riethoff, L.F.V.; van der Reijden, W.A.; Griffioen-Keijzer, A. Infection of Lung Cavitations in a Young Dog Owner with Hodgkin’s Lymphoma Caused by Pasteurella multocida, without a Dog Bite: Confirmed Zoonotic Transmission by Tagmentation Microbiome Analysis. BMJ Case Rep. 2018, 11, bcr-2018-226646. [Google Scholar] [CrossRef]
- James, S.; Thozhuthumparambil, K.P. Cat Scratch Disease Sepsis in an Immunocompromised Patient. BMJ Case Rep. 2021, 14, e239932. [Google Scholar] [CrossRef]
- Lothstein, K.; Fisher, B.; Li, Y.; Seif, A.; Harris, T.; Torp, K.; Kavcic, M.; Huang, Y.-S.V.; Rheingold, S.R.; Aplenc, R. Zoonotic Infections in Pediatric Patients with Acute Leukemia. Pediatr. Blood Cancer 2013, 60, E160–E162. [Google Scholar] [CrossRef]
- Gradel, K.O.; Nørgaard, M.; Dethlefsen, C.; Schønheyder, H.C.; Kristensen, B.; Ejlertsen, T.; Nielsen, H. Increased Risk of Zoonotic Salmonella and Campylobacter Gastroenteritis in Patients with Haematological Malignancies: A Population-Based Study. Ann. Hematol. 2009, 88, 761–767. [Google Scholar] [CrossRef]
- Mani, I.; Maguire, J.H. Small Animal Zoonoses and Immuncompromised Pet Owners. Top. Companion Anim. Med. 2009, 24, 164–174. [Google Scholar] [CrossRef]
- Rice, L.B. Federal Funding for the Study of Antimicrobial Resistance in Nosocomial Pathogens: No ESKAPE. J. Infect. Dis 2008, 197, 1079–1081. [Google Scholar] [CrossRef]
- Santaniello, A.; Sansone, M.; Fioretti, A.; Menna, L.F. Systematic Review and Meta-Analysis of the Occurrence of ESKAPE Bacteria Group in Dogs, and the Related Zoonotic Risk in Animal-Assisted Therapy, and in Animal-Assisted Activity in the Health Context. Int. J. Environ. Res. Public Health 2020, 17, 3278. [Google Scholar] [CrossRef] [PubMed]
- Trevejo, R.T.; Barr, M.C.; Robinson, R.A. Important Emerging Bacterial Zoonotic Infections Affecting the Immunocompromised. Vet. Res. 2005, 36, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Meazza, C.; Galaverna, F.; Petris, M.G.; Zama, D.; La Spina, M.; Muggeo, P.; Ziino, O.; Cellini, M.; Soncini, E.; Barone, A.; et al. Prophylaxis with Trimethoprim/Sulfamethoxazole Is Not Necessary in Children with Solid Tumors Treated with Low-Medium Intensity Chemotherapy. Pediatr. Infect. Dis. J. 2021, 40, 354–355. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority; European Centre for Disease Prevention and Control. The European Union One Health 2020 Zoonoses Report. EFSA J. 2021, 19, e06971. [Google Scholar] [CrossRef]
- Gurry, G.A.; Campion, V.; Premawardena, C.; Woolley, I.; Shortt, J.; Bowden, D.K.; Kaplan, Z.; Dendle, C. High Rates of Potentially Infectious Exposures between Immunocompromised Patients and Their Companion Animals: An Unmet Need for Education. Intern. Med. J. 2017, 47, 333–335. [Google Scholar] [CrossRef]
- Nowotny, N. Preventing Zoonotic Diseases in Immunocompromised Persons: The Role of Physicians and Veterinarians. Emerg. Infect. Dis. 2000, 6, 208. [Google Scholar] [CrossRef]
- Kahn, L.H. Confronting Zoonoses, Linking Human and Veterinary Medicine. Emerg. Infect. Dis. 2006, 12, 556–561. [Google Scholar] [CrossRef]
- Larson, B.R.; Looker, S.; Herrera, D.M.; Creagan, E.T.; Hayman, S.R.; Kaur, J.S.; Jatoi, A. Cancer Patients and Their Companion Animals: Results from a 309-Patient Survey on Pet-Related Concerns and Anxieties during Chemotherapy. J. Cancer Educ. 2010, 25, 396–400. [Google Scholar] [CrossRef]
- Von Matthiessen, P.W.; Sansone, R.A.; Meier, B.P.; Gaither, G.A.; Shrader, J. Zoonotic Diseases and At-Risk Patients: A Survey of Veterinarians and Physicians. AIDS 2003, 17, 1404–1406. [Google Scholar] [CrossRef]
- Peña, A.; Abarca, K.; Weitzel, T.; Gallegos, J.; Cerda, J.; García, P.; López, J. One Health in Practice: A Pilot Project for Integrated Care of Zoonotic Infections in Immunocompromised Children and Their Pets in Chile. Zoonoses Public Health 2016, 63, 403–409. [Google Scholar] [CrossRef]
- Stull, J.W.; Stevenson, K.B. Zoonotic Disease Risks for Immunocompromised and Other High-Risk Clients and Staff: Promoting Safe Pet Ownership and Contact. Vet. Clin. N. Am. Small Anim. Pract. 2015, 45, 377–392. [Google Scholar] [CrossRef]
- Robinson, R.A.; Pugh, R.N. Dogs, Zoonoses and Immunosuppression. J. R. Soc. Promot. Health 2002, 122, 95–98. [Google Scholar] [CrossRef]
- Mofenson, L.M.; Brady, M.T.; Danner, S.P.; Dominguez, K.L.; Hazra, R.; Handelsman, E.; Havens, P.; Nesheim, S.; Read, J.S.; Serchuck, L.; et al. Guidelines for the Prevention and Treatment of Opportunistic Infections among HIV-Exposed and HIV-Infected Children: Recommendations from CDC, the National Institutes of Health, the HIV Medicine Association of the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the American Academy of Pediatrics. Morb. Mortal. Wkly. Rep. Recomm. Rep. 2009, 58, 1–166. [Google Scholar]
- Avery, R.K.; Michaels, M.G.; The AST Infectious Diseases Community of Practice. Strategies for Safe Living Following Solid Organ Transplantation. Am. J. Transplant. 2009, 9, S252–S257. [Google Scholar] [CrossRef]
- Stull, J.W.; Brophy, J.; Sargeant, J.M.; Peregrine, A.S.; Lawson, M.L.; Ramphal, R.; Samson, L.; Bowes, J.; Weese, J.S. Knowledge, Attitudes, and Practices Related to Pet Contact by Immunocompromised Children with Cancer and Immunocompetent Children with Diabetes. J. Pediatr. 2014, 165, 348–355.e2. [Google Scholar] [CrossRef]
- Healthy Pets, Healthy People. CDC. Available online: https://www.cdc.gov/healthypets/index.html (accessed on 9 September 2022).
- Kotton, C.N. Zoonoses in Solid-Organ and Hematopoietic Stem Cell Transplant Recipients. Clin. Infect. Dis. 2007, 44, 857–866. [Google Scholar] [CrossRef]
- Caring for Pets During Cancer Treatment. Available online: https://www.cancer.org/treatment/treatments-and-side-effects/physical-side-effects/low-blood-counts/infections/safety.html (accessed on 9 September 2022).
- Moore, J.E.; Rendall, J.C.; Millar, B.C. A Doggy Tale: Risk of Zoonotic Infection with Bordetella bronchiseptica for Cystic Fibrosis (CF) Patients from Live Licenced Bacterial Veterinary Vaccines for Cats and Dogs. J. Clin. Pharm. Ther. 2022, 47, 139–145. [Google Scholar] [CrossRef]
- Morris, D.O. Malassezia Pachydermatis Carriage in Dog Owners. Emerg. Infect. Dis. 2005, 11, 83–88. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention; Infectious Disease Society of America; American Society of Blood and Marrow Transplantation. Guidelines for Preventing Opportunistic Infections among Hematopoietic Stem Cell Transplant Recipients. Morb. Mortal. Wkly. Rep. Recomm. Rep. 2000, 49, 1–125. [Google Scholar]
- Olshtain-Pops, K.; Yinnon, A.M. Pasteurella multocida Sepsis--Should Immunocompromised Patients Give up Their Pets? Isr. Med. Assoc. J. 2008, 10, 648–649. [Google Scholar] [PubMed]
| Indications to Patients | % (N) | % (N) | ||
|---|---|---|---|---|
| At your center, are families informed about the risks of zoonoses, and are they given indications for prevention? | Yes 95.5% (21) | No 4.5% (1) | ||
| Information provided is derived from: | Experts’ indications 9.5% (2) | Literature 9.5% (2) | Comparison with other centers 4.8% (1) | All of the above 76.2% (16) |
| How is the information provided? | Brochures 33.3% (7) | Orally 66.7% (14) | ||
| Who is in charge of giving the information? | Medical staff 68.2% (15) | Nurses 18.2% (4) | Both medical staff and nurses 13.6% (3) | Veterinarians 0 (0%) |
| When is the information provided? | At diagnosis 52.4% (11) | During certain moments (transplantation/important immunosuppression) 19% (4) | At several moments during the treatment 23.8% (5) | At the request of families 4.8% (1) |
| Is the removal, even temporarily, of pets recommended? | Yes, all animals 18.2% (4) | Only some species 23.8% (5) | Only if the animal is sick 40.9% (9) | Never 18.2% (4) |
| How long is the pet removed after the cancer/immunodeficiency diagnosis? | For the entire duration of the treatment 26.7% (4) | Over a limited period 73.3% (18) | ||
| How do you behave if a family requests to adopt a pet during the treatment? | We do not advise against 50% (11) but we suggest not adopting some species 23.8% (5) | Case-by-case assessment 13.6% (3) | We always advise against 36.4% (8) | |
| Is the family advised to have a veterinary checkup for their pet? | Yes 100% (22) | No 0% (0) | ||
| Are families advised not to let patients take care of the animals daily (e.g., changing the litter box, cleaning an aquarium, etc.)? | Yes 95.5% (21) | No 4.5% (1) | ||
| Is the family advised to avoid animals outside the domestic environment (e.g., zoos, educational farms, etc.)? | For the entire duration of the treatment 36.4% (8) | Over a limited period 45.4% (10) | No 18.2% (4) | |
| Animal-Assisted Therapy | ||||
| At your center, do you use AAI? | Yes 36.4% (8) | No 63.6% (14) | ||
| Is it recommended to start AAI outside your center? | Yes 4.5% (1) | No 95.5% (21) | ||
| Do household pets have access to your center at particular moments (e.g., terminally ill)? | Yes 31.8% (7) | No 68.2% (15) | ||
| Zoonoses | ||||
| Have there been any cases of animal-related infections at your center? | Yes 18.2% (4) | No 81.8% (18) | ||
| Was it possible to trace the source? | Yes, pet 25% (1) | Yes, animals outside the family 25% (1) | Yes, environmental origin 25% (1) | No 25% (1) |
| What consequences did such infections bring? | No complications 33.3% (1) | Hospitalization 66.7% (2) | Therapy delays 0% (0) | Death 0% (0) |
| Would you welcome a document for the management of zoonoses with indications to be given to patients in your center? | Yes 100% (22) | No 0% (0) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiumana, G.; Botta, D.; Dalla Porta, M.F.; Macchi, S.; Soncini, E.; Santaniello, A.; Paciello, O.; Amicucci, M.; Cellini, M.; Cesaro, S. Consensus Statement on Animals’ Relationship with Pediatric Oncohematological Patients, on Behalf of Infectious Diseases and Nurse Working Groups of the Italian Association of Pediatric Hematology-Oncology. J. Clin. Med. 2023, 12, 2481. https://doi.org/10.3390/jcm12072481
Fiumana G, Botta D, Dalla Porta MF, Macchi S, Soncini E, Santaniello A, Paciello O, Amicucci M, Cellini M, Cesaro S. Consensus Statement on Animals’ Relationship with Pediatric Oncohematological Patients, on Behalf of Infectious Diseases and Nurse Working Groups of the Italian Association of Pediatric Hematology-Oncology. Journal of Clinical Medicine. 2023; 12(7):2481. https://doi.org/10.3390/jcm12072481
Chicago/Turabian StyleFiumana, Giulia, Debora Botta, Maria Francesca Dalla Porta, Simone Macchi, Elena Soncini, Antonio Santaniello, Orlando Paciello, Matteo Amicucci, Monica Cellini, and Simone Cesaro. 2023. "Consensus Statement on Animals’ Relationship with Pediatric Oncohematological Patients, on Behalf of Infectious Diseases and Nurse Working Groups of the Italian Association of Pediatric Hematology-Oncology" Journal of Clinical Medicine 12, no. 7: 2481. https://doi.org/10.3390/jcm12072481
APA StyleFiumana, G., Botta, D., Dalla Porta, M. F., Macchi, S., Soncini, E., Santaniello, A., Paciello, O., Amicucci, M., Cellini, M., & Cesaro, S. (2023). Consensus Statement on Animals’ Relationship with Pediatric Oncohematological Patients, on Behalf of Infectious Diseases and Nurse Working Groups of the Italian Association of Pediatric Hematology-Oncology. Journal of Clinical Medicine, 12(7), 2481. https://doi.org/10.3390/jcm12072481









