Causal Link between Inflammatory Bowel Disease and Fistula: Evidence from Mendelian Randomization Study
Abstract
1. Introduction
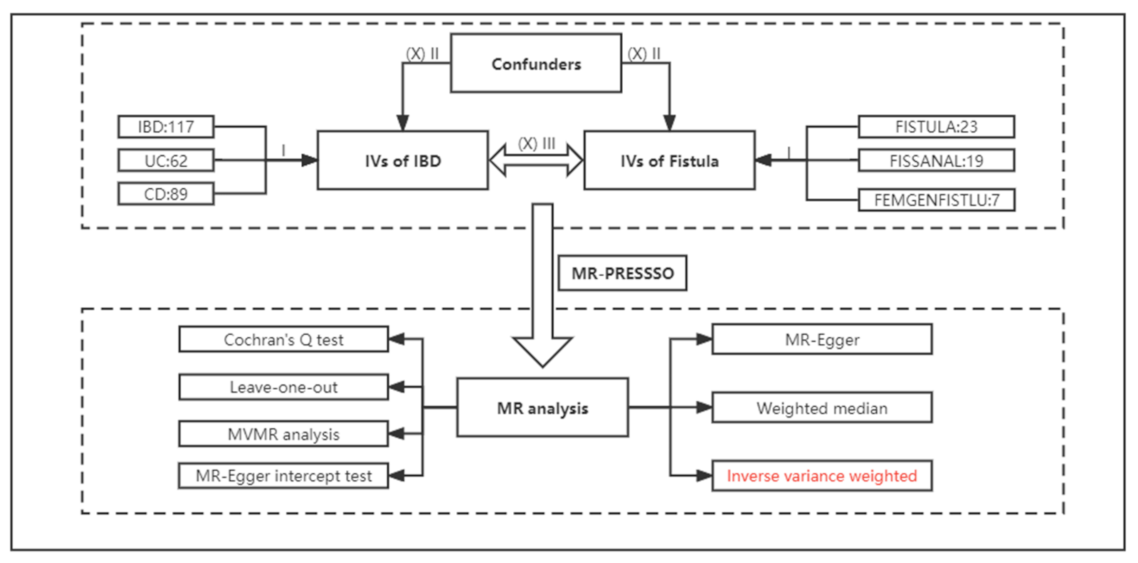
2. Materials and Methods
2.1. Data Sources
2.2. Selection of Instrumental Variables
2.3. MR Analyses
2.4. Statistics Analysis and Visualization
3. Results
3.1. Selection of Instrumental Variables
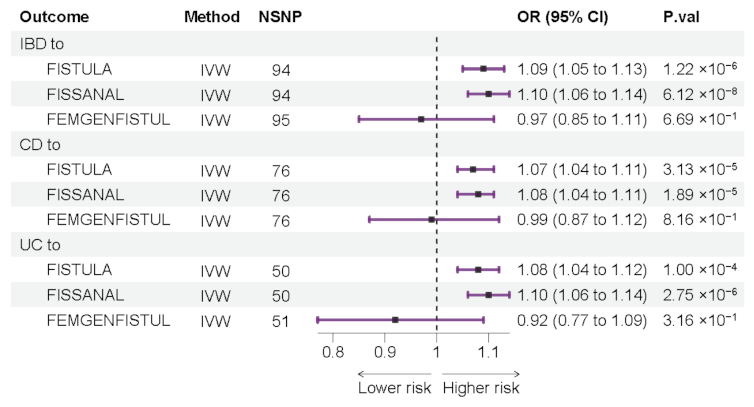
3.2. Forward Mendelian Randomization Analyze
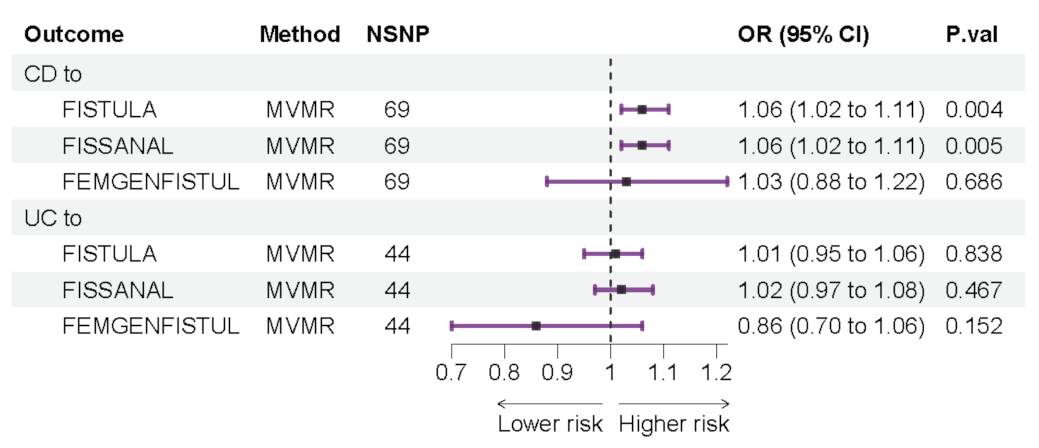
3.3. Forward Multivariable Mendelian Randomization Analyze
3.4. Reverse Mendelian Randomization Analyze
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef] [PubMed]
- Jairath, V.; Feagan, B.G. Global burden of inflammatory bowel disease. Lancet Gastroenterol. Hepatol. 2020, 5, 2–3. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Dalal, R.L.; Shaffer, S.; Schwartz, D.A.; Rubin, D.T. Inpatient Management of Inflammatory Bowel Disease-Related Complications. Clin. Gastroenterol. Hepatol. 2020, 18, 1346–1355. [Google Scholar] [CrossRef] [PubMed]
- Rieder, F.; Zimmermann, E.M.; Remzi, F.H.; Sandborn, W.J. Crohn’s disease complicated by strictures: A systematic review. Gut 2013, 62, 1072–1084. [Google Scholar] [CrossRef]
- Geldof, J.; Iqbal, N.; LeBlanc, J.F.; Anandabaskaran, S.; Sawyer, R.; Buskens, C.; Bemelman, W.; Gecse, K.; Lundby, L.; Lightner, A.L.; et al. Classifying perianal fistulising Crohn’s disease: An expert consensus to guide decision-making in daily practice and clinical trials. Lancet Gastroenterol. Hepatol. 2022, 7, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Kochar, B.; Cai, W.; Cagan, A.; Ananthakrishnan, A.N. Pretreatment Frailty Is Independently Associated With Increased Risk of Infections After Immunosuppression in Patients With Inflammatory Bowel Diseases. Gastroenterology 2020, 158, 2104–2111.e2. [Google Scholar] [CrossRef]
- Shah, S.C.; Itzkowitz, S.H. Colorectal Cancer in Inflammatory Bowel Disease: Mechanisms and Management. Gastroenterology 2022, 162, 715–730.e3. [Google Scholar] [CrossRef]
- Thia, K.T.; Sandborn, W.J.; Harmsen, W.S.; Zinsmeister, A.R.; Loftus, E.V., Jr. Risk factors associated with progression to intestinal complications of Crohn’s disease in a population-based cohort. Gastroenterology 2010, 139, 1147–1155. [Google Scholar] [CrossRef]
- Householder, S.; Picoraro, J.A. Diagnosis and Classification of Fistula from Inflammatory Bowel Disease and Inflammatory Bowel Disease-Related Surgery. Gastrointest. Endosc. Clin. North Am. 2022, 32, 631–650. [Google Scholar] [CrossRef]
- Tjandra, D.; Garg, M.; Behrenbruch, C.; McCormick, J.; Simkin, P.; Prentice, R.; Trinh, A.; Al-Ani, A.; Vaughan, R.; Macrae, F.; et al. Review article: Investigation and management of internal fistulae in Crohn’s disease. Aliment. Pharmacol. Ther. 2021, 53, 1064–1079. [Google Scholar] [CrossRef]
- Rackovsky, O.; Hirten, R.; Ungaro, R.; Colombel, J.F. Clinical updates on perianal fistulas in Crohn’s disease. Expert Rev. Gastroenterol. Hepatol. 2018, 12, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Sarveazad, A.; Bahardoust, M.; Shamseddin, J.; Yousefifard, M. Prevalence of anal fistulas: A systematic review and meta-analysis. Gastroenterol. Hepatol. Bed Bench 2022, 15, 1–8. [Google Scholar] [PubMed]
- De la Poza, G.; Lopez-Sanroman, A.; Taxonera, C.; Marín-Jimenez, I.; Gisbert, J.P.; Bermejo, F.; Opio, V.; Muriel, A. Genital fistulas in female Crohn’s disease patients.: Clinical characteristics and response to therapy. J. Crohn’s Colitis 2012, 6, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.S.; Kim, D.S.; Lee, D.H.; Lee, J.B.; Lee, E.J.; Lee, S.D.; Song, K.H.; Jung, H.J. Clinical Characteristics and Incidence of Perianal Diseases in Patients With Ulcerative Colitis. Ann. Coloproctology 2018, 34, 138–143. [Google Scholar] [CrossRef]
- Heimann, T.M.; Swaminathan, S.; Slater, G.I.; Kurtz, R.J. Perianal Fistula After Ileoanal Pouch in Patients With Ulcerative Colitis: A Review of 475 Patients Operated on at a Major IBD Center. Dis. Colon Rectum 2022, 65, 76–82. [Google Scholar] [CrossRef]
- Emdin, C.A.; Khera, A.V.; Kathiresan, S. Mendelian Randomization. JAMA 2017, 318, 1925–1926. [Google Scholar] [CrossRef]
- Burgess, S.; Scott, R.A.; Timpson, N.J.; Smith, G.D.; Thompson, S.G.; EPIC-InterAct Consortium. Using published data in Mendelian randomization: A blueprint for efficient identification of causal risk factors. Eur. J. Epidemiol. 2015, 30, 543–552. [Google Scholar] [CrossRef]
- Luo, J.; Xu, Z.; Noordam, R.; van Heemst, D.; Li-Gao, R. Depression and Inflammatory Bowel Disease: A Bidirectional Two-sample Mendelian Randomization Study. J. Crohn’s Colitis 2022, 16, 633–642. [Google Scholar] [CrossRef]
- Li, Y.; Guo, J.; Cao, Z.; Wu, J. Causal Association Between Inflammatory Bowel Disease and Psoriasis: A Two-Sample Bidirectional Mendelian Randomization Study. Front. Immunol. 2022, 13, 916645. [Google Scholar] [CrossRef]
- Meisinger, C.; Freuer, D. Causal Association Between Atopic Dermatitis and Inflammatory Bowel Disease: A 2-Sample Bidirectional Mendelian Randomization Study. Inflamm. Bowel Dis. 2022, 28, 1543–1548. [Google Scholar] [CrossRef]
- Meisinger, C.; Freuer, D. Rheumatoid arthritis and inflammatory bowel disease: A bidirectional two-sample Mendelian randomization study. Semin. Arthritis Rheum. 2022, 55, 151992. [Google Scholar] [CrossRef] [PubMed]
- De Lange, K.M.; Moutsianas, L.; Lee, J.C.; Lamb, C.A.; Luo, Y.; Kennedy, N.A.; Jostins, L.; Rice, D.L.; Gutierrez-Achury, J.; Ji, S.-G.; et al. Genome-wide association study implicates immune activation of multiple integrin genes in inflammatory bowel disease. Nat. Genet. 2017, 49, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Lamb, C.A.; Kennedy, N.A.; Raine, T.; Hendy, P.A.; Smith, P.J.; Limdi, J.K.; Hayee, B.; Lomer, M.C.E.; Parkes, G.C.; Selinger, C.; et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 2019, 68 (Suppl. 3), s1–s106. [Google Scholar] [CrossRef] [PubMed]
- Kurki, M.I.; Karjalainen, J.; Palta, P.; Sipilä, T.P.; Kristiansson, K.; Donner, K.; Reeve, M.P.; Laivuori, H.; Aavikko, M.; Kaunisto, M.A.; et al. FinnGen: Unique genetic insights from combining isolated population and national health register data. medRxiv 2022, 3, 22271360. [Google Scholar] [CrossRef]
- Hartwig, F.P.; Davies, N.M.; Hemani, G.; Davey Smith, G. Two-sample Mendelian randomization: Avoiding the downsides of a powerful, widely applicable but potentially fallible technique. Int. J. Epidemiol. 2016, 45, 1717–1726. [Google Scholar] [CrossRef]
- Bowden, J.; Smith, G.D.; Haycock, P.C.; Burgess, S. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet. Epidemiol. 2016, 40, 304–314. [Google Scholar] [CrossRef]
- Bowden, J.; Davey Smith, G.; Burgess, S. Mendelian randomization with invalid instruments: Effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 2015, 44, 512–525. [Google Scholar] [CrossRef]
- Sanderson, E. Multivariable Mendelian Randomization and Mediation. Cold Spring Harb. Perspect. Med. 2021, 11, a038984. [Google Scholar] [CrossRef]
- Agrawal, M.; Spencer, E.A.; Colombel, J.F.; Ungaro, R.C. Approach to the Management of Recently Diagnosed Inflammatory Bowel Disease Patients: A User’s Guide for Adult and Pediatric Gastroenterologists. Gastroenterology 2021, 161, 47–65. [Google Scholar] [CrossRef]
- Scharl, M.; Rogler, G. Pathophysiology of fistula formation in Crohn’s disease. World J. Gastrointest. Pathophysiol. 2014, 5, 205–212. [Google Scholar] [CrossRef]
- Lee, M.J.; Parker, C.E.; Taylor, S.R.; Guizzetti, L.; Feagan, B.G.; Lobo, A.J.; Jairath, V. Efficacy of Medical Therapies for Fistulizing Crohn’s Disease: Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2018, 16, 1879–1892. [Google Scholar] [CrossRef]
- Vogel, J.D.; Johnson, E.K.; Morris, A.M.; Paquette, I.M.; Saclarides, T.J.; Feingold, D.L.; Steele, S.R. Clinical Practice Guideline for the Management of Anorectal Abscess, Fistula-in-Ano, and Rectovaginal Fistula. Dis. Colon Rectum 2016, 59, 1117–1133. [Google Scholar] [CrossRef] [PubMed]
- Guan, Q. A Comprehensive Review and Update on the Pathogenesis of Inflammatory Bowel Disease. J. Immunol. Res. 2019, 2019, 7247238. [Google Scholar] [CrossRef] [PubMed]
- Gecse, K.; Khanna, R.; Stoker, J.; Jenkins, J.T.; Gabe, S.; Hahnloser, D.; D’Haens, G. Fistulizing Crohn’s disease: Diagnosis and management. United Eur. Gastroenterol. J. 2013, 1, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Tozer, P.J.; Lung, P.; Lobo, A.J.; Sebastian, S.; Brown, S.R.; Hart, A.L.; Fearnhead, N.; On behalf of ENiGMA Collaboration. Review article: Pathogenesis of Crohn’s perianal fistula-understanding factors impacting on success and failure of treatment strategies. Aliment. Pharmacol. Ther. 2018, 48, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Schofield, J.B.; Haboubi, N. Histopathological Mimics of Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2020, 26, 994–1009. [Google Scholar] [CrossRef]
- Van Onkelen, R.; Mitalas, L.; Gosselink, M.; van Belkum, A.; Laman, J.; Schouten, W. Assessment of microbiota and peptidoglycan in perianal fistulas. Diagn. Microbiol. Infect. Dis. 2013, 75, 50–54. [Google Scholar] [CrossRef]
- Thia, K.T.; Mahadevan, U.; Feagan, B.G.; Wong, C.; Cockeram, A.; Bitton, A.; Bernstein, C.N.; Sandborn, W.J. Ciprofloxacin or metronidazole for the treatment of perianal fistulas in patients with Crohn’s disease: A randomized, double-blind, placebo-controlled pilot study. Inflamm. Bowel Dis. 2009, 15, 17–24. [Google Scholar] [CrossRef]
- Siegmund, B.; Feakins, R.M.; Barmias, G.; Ludvig, J.C.; Teixeira, F.V.; Rogler, G.; Scharl, M. Results of the Fifth Scientific Workshop of the ECCO (II): Pathophysiology of Perianal Fistulizing Disease. J. Crohn’s Colitis 2016, 10, 377–386. [Google Scholar] [CrossRef]
- Boros, E.; Nagy, I. The Role of MicroRNAs upon Epithelial-to-Mesenchymal Transition in Inflammatory Bowel Disease. Cells 2019, 8, 1461. [Google Scholar] [CrossRef]
- Lovisa, S.; Genovese, G.; Danese, S. Role of Epithelial-to-Mesenchymal Transition in Inflammatory Bowel Disease. J. Crohn’s Colitis 2019, 13, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Leeb, S.N.; Vogl, D.; Gunckel, M.; Kiessling, S.; Falk, W.; Göke, M.; Schölmerich, J.; Gelbmann, C.M.; Rogler, G. Reduced migration of fibroblasts in inflammatory bowel disease: Role of inflammatory mediators and focal adhesion kinase. Gastroenterology 2003, 125, 1341–1354. [Google Scholar] [CrossRef] [PubMed]
- Das, B.; Snyder, M. Rectovaginal Fistulae. Clin. Colon Rectal Surg. 2016, 29, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Ngongo, C.J.; Raassen, T.; Mahendeka, M.; Lombard, L.; van Roosmalen, J.; Temmerman, M. Rare causes of genital fistula in nine African countries: A retrospective review. BMC Womens Health 2022, 22, 497. [Google Scholar] [CrossRef] [PubMed]
- Ngongo, C.J.; Raassen, T.; Mahendeka, M.; Lombard, L.; van Roosmalen, J.; Temmerman, M. A retrospective review of genital fistula occurrence in nine African countries. BMC Pregnancy Childbirth 2022, 22, 744. [Google Scholar] [CrossRef] [PubMed]
- Kotze, P.G.; Shen, B.; Lightner, A.; Yamamoto, T.; Spinelli, A.; Ghosh, S.; Panaccione, R. Modern management of perianal fistulas in Crohn’s disease: Future directions. Gut 2018, 67, 1181–1194. [Google Scholar] [CrossRef]

| Variable | Number of Cases | Number of Controls | Data Resource | Population | PMID | Year |
|---|---|---|---|---|---|---|
| IBD | 25,042 | 34915 | ebi-a-GCST004131 | Mix | 28067908 | 2017 |
| UC | 12,366 | 33,609 | ebi-a-GCST004132 | Mix | 28067908 | 2017 |
| CD | 12,194 | 28,072 | ebi-a-GCST004133 | Mix | 28067908 | 2017 |
| FISTULA | 6926 | 30,228 | finn-b-K11_FISTULA | European | - | 2021 |
| FISSANAL | 6610 | 253,186 | finn-b-K11_FISSANAL | European | - | 2021 |
| FEMGENFISTUL | 327 | 94,394 | finn-b-N14_FEMGENFISTUL | European | - | 2021 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, Z.; Zhu, S.; Liu, C.; Meng, Y.; Li, J.; Zhang, J.; Dong, W. Causal Link between Inflammatory Bowel Disease and Fistula: Evidence from Mendelian Randomization Study. J. Clin. Med. 2023, 12, 2482. https://doi.org/10.3390/jcm12072482
Tan Z, Zhu S, Liu C, Meng Y, Li J, Zhang J, Dong W. Causal Link between Inflammatory Bowel Disease and Fistula: Evidence from Mendelian Randomization Study. Journal of Clinical Medicine. 2023; 12(7):2482. https://doi.org/10.3390/jcm12072482
Chicago/Turabian StyleTan, Zongbiao, Shijie Zhu, Chuan Liu, Yang Meng, Jiao Li, Jixiang Zhang, and Weiguo Dong. 2023. "Causal Link between Inflammatory Bowel Disease and Fistula: Evidence from Mendelian Randomization Study" Journal of Clinical Medicine 12, no. 7: 2482. https://doi.org/10.3390/jcm12072482
APA StyleTan, Z., Zhu, S., Liu, C., Meng, Y., Li, J., Zhang, J., & Dong, W. (2023). Causal Link between Inflammatory Bowel Disease and Fistula: Evidence from Mendelian Randomization Study. Journal of Clinical Medicine, 12(7), 2482. https://doi.org/10.3390/jcm12072482







