Cardiopulmonary Exercise Testing in Children and Young Adolescents after a Multisystem Inflammatory Syndrome: Physical Deconditioning or Residual Pathology?
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Participants
2.3. First-Level Evaluation
2.4. Cardio-Pulmonary Exercise Test
2.5. Statistical Analysis
3. Results
- -
- A total of 7 out of 20 subjects (35%) showed a slight (less than 80% but more than 70% of the predicted) reduction in VO2p values compared to the expected [17], while 3 cases (15%) showed a moderate VO2p reduction with VO2p values below 70% of the expected (Figure 1). Among them, two children showed a major cardiological involvement with the need for ventilatory support and inotropic therapy;
- -
- The mean value of the oxygen pulse was 9.0 ± 3.0 mL/beat; similarly to VO2p, its results were lower than 80% of predicted [17] in 10 cases and in 3 subjects lower than 70% of predicted;
- -
- A total of 15 out of 20 cases (75%) showed VO2 at AT values lower than the 60% of predicted [17];
- -
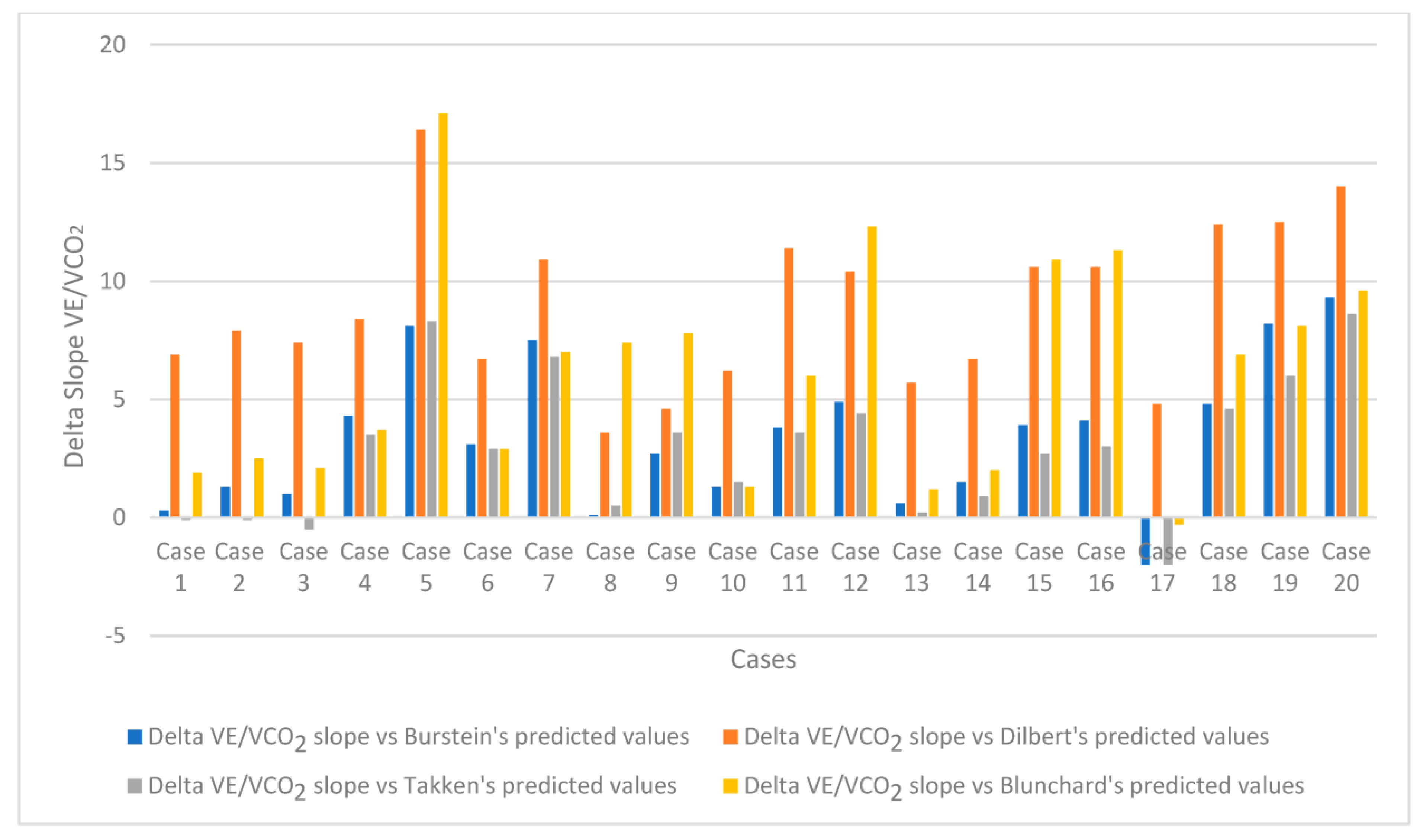
- The mean value of the VE/VCO2 slope was 33.4, with 19 of the 20 children recruited in the study (95%) showing higher VE/VCO2 slope values than the Burstein predicted values [17]. Similar results were observed using different prediction formulas that can be adopted in healthy paediatric populations [18,19,20] Figure 2.
- -
- CRP values at admission and peak VO2/kg values (p = 0.034);
- -
- Uric acid values at admission and peak VO2 expressed as a percentage of predicted (p = 0.011);
- -
- Uric acid values at admission and peak oxygen pulse expressed as a percentage of predicted (p = 0.021);
- -
- NT-proBNP values at admission and peak VO2 expressed as a percentage of predicted (p = 0.046).
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kaushik, S.; Aydin, S.I.; Derespina, K.R.; Bansal, P.B.; Kowalsky, S.; Trachtman, R.; Gillen, J.K.; Perez, M.M.; Soshnick, S.H.; Conway, E.E., Jr.; et al. Multisystem inflammatory syndrome in children (MIS-C) associated with SARS-CoV-2 infection: A multiinstitutional study from New York City. J. Pediatr. 2020, 224, 24–29. [Google Scholar] [CrossRef]
- Radia, T.; Williams, N.; Agrawal, P.; Harman, K.; Weale, J.; Cook, J.; Gupta, A. Multi-system inflammatory syndrome in children & adolescents (MIS-C): A systematic review of clinical features and presentation. Paediatr. Respir. Rev. 2021, 38, 51–57. [Google Scholar] [PubMed]
- Soma, V.L.; Shust, G.F.; Ratner, A.J. Multisystem inflammatory syndrome in children. Curr. Opin. Pediatr. 2021, 33, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Algarni, A.S.; Alamri, N.M.; Khayat, N.Z.; Alabdali, R.A.; Alsubhi, R.S.; Alghamdi, S.H. Clinical practice guidelines in multisystem inflammatory syndrome (MIS-C) related to COVID-19: A critical review and recommendations. World J. Pediatr. 2022, 18, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Capone, C.A.; Misra, N.; Ganigara, M.; Epstein, S.; Rajan, S.; Acharya, S.S.; Hayes, D.A.; Kearney, M.B.; Romano, A.; Friedman, R.A.; et al. Six Month Follow-up of Patients With Multi-System Inflammatory Syndrome in Children. Pediatrics 2021, 148, e2021050973. [Google Scholar] [CrossRef]
- Sperotto, F.; Friedman, K.G.; Son, M.B.F.; VanderPluym, C.J.; Newburger, J.W.; Dionne, A. Cardiac manifestations in SARS-CoV-2-associated multisystem inflammatory syndrome in children: A comprehensive review and proposed clinical approach. Eur. J. Pediatr. 2021, 180, 307–322. [Google Scholar] [CrossRef]
- Messiah, S.E.; Xie, L.; Mathew, M.S.; Shaikh, S.; Veeraswamy, A.; Rabi, A.; Francis, J.; Lozano, A.; Ronquillo, C.; Sanchez, V.; et al. Comparison of Long-Term Complications of COVID-19 Illness among a Diverse Sample of Children by MIS-C Status. Int. J. Environ. Res. Public Health 2022, 19, 13382. [Google Scholar] [CrossRef]
- Schlapbach, L.J.; Andre, M.C.; Grazioli, S.; Schöbi, N.; Ritz, N.; Aebi, C.; Agyeman, P.; Albisetti, M.; Bailey, D.G.N.; Berger, C.; et al. Best Practice Recommendations for the Diagnosis and Management of Children With Pediatric Inflammatory Multisystem Syndrome Temporally Associated With SARS-CoV-2 (PIMS-TS; Multisystem Inflammatory Syndrome in Children, MIS-C) in Switzerland. Front. Pediatr. 2021, 9, 667507. [Google Scholar] [CrossRef]
- American Thoracic Society; American College of Chest Physicians. ATS/ACCP Statement on cardiopulmonary exercise testing. Am. J. Respir. Crit. Care Med. 2003, 167, 211–277. [Google Scholar] [CrossRef]
- Magrì, D.; Mastromarino, V.; Gallo, G.; Zachara, E.; Re, F.; Agostoni, P.; Giordano, D.; Rubattu, S.; Forte, M.; Cotugno, M.; et al. Risk Stratification in Hypertrophic Cardiomyopathy. Insights from Genetic Analysis and Cardiopulmonary Exercise Testing. J. Clin. Med. 2020, 9, 1636. [Google Scholar] [CrossRef]
- Eligibility for Competitive Sports Activity in Non-Professional COVID-19 Positive Healed Athletes and in Athletes with Symptoms Suggestive of COVID-19 in the absence Of SARS-CoV-2 Diagnosis. Vol. DGPRE 0015502 P-02/03/2022 (Up-dating Circular n.3566 of 18 January 2022). Available online: https://www.salute.gov.it/imgs/C_17_eventiEpidemici_2402_comunicato_itemComunicato0_files_itemFiles0_fileAzione.pdf (accessed on 19 January 2023).
- World Health Organization (WHO). Multisystem Inflammatory Syndrome in Children and Adolescents with COVID-19; WHO: Geneva, Switzerland, 2020; pp. 1–3. [Google Scholar]
- Will, P.M.; Walter, J.D. Exercise testing: Improving performance with a ramped Bruce protocol. Am. Heart J. 1999, 138 Pt 1, 1033–1037. [Google Scholar] [CrossRef] [PubMed]
- Beaver, W.L.; Wasserman, K.; Whipp, B.J. A new method for detecting anaerobic threshold by gas exchange. J. Appl. Physiol 1985, 60, 2020–2027. [Google Scholar] [CrossRef] [PubMed]
- Wasserman, K.; Hansen, J.E.; Sue, D.Y.; Casaburi, R.; Whipp, B.J. Measurement during integrative cardiopulmonary exercise testing. In Principle of Exercise Testing and Interpretation, 4th ed.; Wasserman, K., Hansen, J.E., Sue, D.Y., Stringer, W.W., Whipp, B.J., Eds.; Lippincott, Williams and Wilkins: Philadelphia, PA, USA, 2005. [Google Scholar]
- Kim, K.J.; Rivas, E.; Prejean, B.; Frisco, D.; Young, M.; Downs, M. Novel Computerized Method for Automated Determination of Ventilatory Threshold and Respiratory Compensation Point. Front. Physiol. 2021, 12, 782167. [Google Scholar] [CrossRef]
- Burstein, D.S.; McBride, M.G.; Min, J.; Paridon, A.A.; Perelman, S.; Huffman, E.M.; O’Malley, S.; Del Grosso, J.; Groepenhoff, H.; Paridon, S.M.; et al. Normative Values for Cardiopulmonary Exercise Stress Testing Using Ramp Cycle Ergometry in Children and Adolescents. J. Pediatr. 2021, 229, 61–69. [Google Scholar] [CrossRef]
- Dilbert, D.; Malcić, I.; Čaleta, T.; Zovko, A. Reference values for cardiopulmonaryexercise testing in children and adolescents in nortwestCroatia. Paediatria Croat. 2015, 59, 195–201. [Google Scholar] [CrossRef]
- Ten Harkel, A.D.J.; Takken, T. Normal values for cardiopulmonary exercise testing in children. Eur. J. Cardiovasc. Prev. Rehabil. 2011, 18, 676–677. [Google Scholar] [CrossRef]
- Blanchard Blanchard, J.; Blais, S.; Chetaille, P.; Bisson, M.; Counil, F.P.; Huard-Girard, T.; Berbari, J.; Boulay, P.; Dallaire, F. New Reference Values for Cardiopulmonary Exercise Testing in Children. Med. Sci. Sport. Exerc. 2018, 50, 1125–1133. [Google Scholar] [CrossRef] [PubMed]
- Truong, D.T.; Trachtenberg, F.L.; Pearson, G.D.; Dionne, A.; Elias, M.D.; Friedman, K.; Hayes, K.H.; Mahony, L.; McCrindle, B.W.; Oster, M.E.; et al. The NHLBI Study on Long-terM OUtcomes after the Multisystem Inflammatory Syndrome In Children (MUSIC): Design and Objectives. Am. Heart J. 2022, 243, 43–53. [Google Scholar] [CrossRef]
- Cantarutti, N.; Battista, V.; Stagnaro, N.; Labate, M.E.; Cicenia, M.; Campisi, M.; Vitali, V.; Secinaro, A.; Campana, A.; Trocchio, G.; et al. Long-Term Cardiovascular Outcome in Children with MIS-C Linked to SARS-CoV-2 Infection-An Italian Multicenter Experience. Biology 2022, 11, 1474. [Google Scholar] [CrossRef]
- Astley, C.; Badue Pereira, M.F.; Lima, M.S.; Buchpiguel, C.A.; Carneiro, C.G.; Sapienza, M.T.; Leal, G.N.; do Prado, D.M.L.; Peçanha, T.; Sieczkowska, S.M.; et al. In-depth cardiovascular and pulmonary assessments in children with multisystem inflammatory syndrome after SARS-CoV-2 infection: A case series study. Physiol. Rep. 2022, 10, e15201. [Google Scholar] [CrossRef]
- López-Bueno, R.; Calatayud, J.; Andersen, L.L.; Casaña, J.; Ezzatvar, Y.; Casajús, J.A.; López-Sánchez, G.F.; Smith, L. Cardiorespiratory fitness in adolescents before and after the COVID-19 confinement: A prospective cohort study. Eur. J. Pediatr. 2021, 180, 2287–2293. [Google Scholar] [CrossRef]
- Gentili, F.; Cafiero, G.; Perrone, M.A.; Bianco, M.; Salvati, A.; Giordano, U.; Silva Kikina, S.; Guccione, P.; De Zorzi, A.; Galletti, L.; et al. The Effects of Physical Inactivity and Exercise at Home in Young Patients with Congenital Heart Disease during the COVID-19 Pandemic. Int. J. Environ. Res. Public Health 2021, 18, 10065. [Google Scholar] [CrossRef] [PubMed]
- Gallo, G.; Mastromarino, V.; Limongelli, G.; Calcagni, G.; Maruotti, A.; Ragni, L.; Valente, F.; Musumeci, M.B.; Adorisio, R.; Rubino, M.; et al. Insights from Cardiopulmonary Exercise Testing in Pediatric Patients with Hypertrophic Cardiomyopathy. Biomolecules 2021, 11, 376. [Google Scholar] [CrossRef]
- Magrì, D. Peak oxygen uptake in heart failure: Look behind the number! Eur. J. Prev. Cardiol. 2018, 25, 1934–1936. [Google Scholar] [CrossRef] [PubMed]
- Thijssen, D.H.J.; Bruno, R.M.; van Mil, A.C.C.M.; Holder, S.M.; Faita, F.; Greyling, A.; Zock, P.L.; Taddei, S.; Deanfield, J.E.; Luscher, T.; et al. Expert consensus and evidence-based recommendations for the assessment of flow-mediated dilation in humans. Eur. Heart J. 2019, 40, 2534–2547. [Google Scholar] [CrossRef]
- Chen, B.; Lu, C.; Gu, H.Q.; Li, Y.; Zhang, G.; Lio, J.; Luo, X.; Zhang, L.; Hu, Y.; Lan, X.; et al. Serum Uric Acid Concentrations and Risk of Adverse Outcomes in Patients With COVID-19. Front. Endocrinol. 2021, 12, 633767. [Google Scholar] [CrossRef]
- Olexa, P.; Olexová, M.; Gonsorcík, J.; Tkác, I.; Kisel’ová, J.; Olejníková, M. Uric acid--a marker for systemic inflammatory response in patients with congestive heart failure? Wien. Klin. Wochenschr. 2002, 114, 211–215. [Google Scholar]
- Phillips, D.B.; Collins, S.É.; Stickland, M.K. Measurement and Interpretation of Exercise Ventilatory Efficiency. Front. Physiol. 2020, 11, 659. [Google Scholar] [CrossRef]
- Neder, J.A.; Berton, D.C.; Arbex, F.F.; Alencar, M.C.; Rocha, A.; Sperandio, P.A.; Palange, P.; O’Donnell, D.E. Physiological and clinical relevance of exercise ventilatory efficiency in COPD. Eur. Respir. J. 2017, 49, 1602036. [Google Scholar] [CrossRef]
- Guazzi, M.; Adams, V.; Conraads, V.; Halle, M.; Mezzani, A.; Vanhees, L.; Arena, R.; Fletcher, G.F.; Forman, D.E.; Kitzman, D.W.; et al. Clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Eur. Heart J. 2012, 33, 2917–2927. [Google Scholar] [CrossRef] [PubMed]
- Dorelli, G.; Braggio, M.; Gabbiani, D.; Busti, F.; Caminati, M.; Senna, G.; Girelli, D.; Laveneziana, P.; Ferrari, M.; Sartori, G.; et al. Importance of Cardiopulmonary Exercise Testing amongst Subjects Recovering from COVID-19. Diagnostics 2021, 11, 507. [Google Scholar] [CrossRef] [PubMed]
- Nagano, Y.; Baba, R.; Kuraishi, K.; Yasuda, T.; Ikoma, M.; Nishibata, K.; Yokota, M.; Nagashima, M. Ventilatory control during exercise in normal children. Pediatr. Res. 1998, 43, 704–707. [Google Scholar] [CrossRef]
- Giardini, A.; Odendaal, D.; Khambadkone, S.; Derrick, G. Physiologic decrease of ventilatory response to exercise in the second decade of life in healthy children. Am. Heart J. 2011, 161, 1214–1219. [Google Scholar] [CrossRef]
- Gavotto, A.; Huguet, H.; Picot, M.C.; Guillaumont, S.; Matecki, S.; Amedro, P. The Ve/VCO2 slope: A useful tool to evaluate the physiological status of children with congenital heart disease. J. Appl. Physiol. 2020, 129, 1102–1110. [Google Scholar] [CrossRef]
- Rhodes, J.; Ubeda Tikkanen, A.; Jenkins, K.J. Exercise testing and training in children with congenital heart disease. Circulation 2010, 122, 1957–1967. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, C.; Kelly, A.S.; Kaiser, D.R.; Steinberger, J.; Dengel, D.R. Aerobic-exercise training improves ventilatory efficiency in overweight children. Pediatr. Exerc. Sci. 2007, 19, 82–92. [Google Scholar] [CrossRef]
- Hoffor, A.S.; Harrison, A.C.; Kirk, P.A. Anaerobic threshold alterations caused by interval training in 11-year-olds. J. Sports Med. Phys. Fit. 1990, 30, 53–56. [Google Scholar]
- Nourry, C.; Deruelle, F.; Guinhouva, C.; Baquet, G.; Fabre, C.; Bart, F.; Berthoin, S.; Mucci, P. High-intensity intermittent running training improves pulmonary function and alters exercise breathing pattern in children. Eur. J. Appl. Physiol. 2005, 94, 415–423. [Google Scholar] [CrossRef]
- López-Viñas, L.; Vega-Villar, J.; Rocío-Martín, E.; García-García, P.; De La Rosa Santiago, E.; Galván-Román, J.M.; Wix-Ramos, R. Diaphragm impairment in patients admitted for severe COVID-19. Eur. J. Transl. Myol. 2022, 32, 10460. [Google Scholar] [CrossRef]
- Knoke, L.; Schlegtendal, A.; Maier, C.; Eitner, L.; Lücke, T.; Brinkmann, F. Pulmonary Function and Long-Term Respiratory Symptoms in Children and Adolescents After COVID-19. Front. Pediatr. 2022, 10, 851008. [Google Scholar] [CrossRef]
- Spiesshoefer, J.; Friedrich, J.; Regmi, B.; Geppert, J.; Jörn, B.; Kersten, A.; Giannoni, A.; Boentert, M.; Marx, G.; Marx, N.; et al. Diaphragm dysfunction as a potential determinant of dyspnea on exertion in patients 1 year after COVID-19-related ARDS. Respir. Res. 2022, 23, 187. [Google Scholar] [CrossRef] [PubMed]
- Frésard, I.; Genecand, L.; Altarelli, M.; Gex, G.; Vremaroiu, P.; Vremaroiu-Coman, A.; Lawi, D.; Bridevaux, P.O. Dysfunctional breathing diagnosed by cardiopulmonary exercise testing in ‘long COVID’ patients with persistent dyspnoea. BMJ Open Respir. Res. 2022, 9, e001126. [Google Scholar] [CrossRef] [PubMed]
- Peyer, K.; Pivarnik, J.M.; Coe, D.P. The relationship among HRpeak, RERpeak, and VO2peak during treadmill testing in girls. Res. Q. Exerc. Sport 2011, 82, 685–692. [Google Scholar] [CrossRef] [PubMed]

| Demographic and Anthropometric Characteristics | |
|---|---|
| Age (years) | 11.76 ± 3.29 |
| Gender | |
| Male | 13 (65%) |
| Female | 7 (35%) |
| Weight (kg) | 50.91 ± 17.67 |
| Height (cm) | 149.20 ± 14.67 |
| BMI (kg/m2) | 22.22 ± 4.76 |
| At Admission | At Enrolment | |
|---|---|---|
| Symptoms | 20 (100%) | 0 (0%) |
| School physical activity | 20 (100%) | 0 (0%) |
| Extra-curricular non-competitive sport activity | 7 (35%) | 0 (0%) |
| High Inflammatory markers | 20 (100%) | 0 (0%) |
| Heart involvement | 20 (100%) | 0 (0%) ^ |
| R-VLEF | 10 (50.0%) | 0 (0%) |
| Signs of myo-pericarditis ^^ | 8 (40.0%) | 0 (0%) |
| Kawasaki-like alterations ^^^ | 2 (10.0%) | 0 (0%) |
| Lung involvement * | 11 (55%) | 0 (0%) |
| Bowel involvement ** | 13 (65%) | 0 (0%) |
| Need of immunoglobulin therapy | 20 (100%) | 0 (0%) |
| Need of steroids | 20 (100%) | 0 (0%) |
| Need of inotropic support | 4 (23.5%) | 0 (0%) |
| Need of respiratory support | 3 (17.6%) | 0 (0%) |
| Need of CRRT purification treatment | 1 (5.6%) | 0 (0%) |
| ECG -and Holter ECG anomalies | ||
| Complex or frequent arrhythmias | 0 (100%) | 0 (0%) |
| VRAs | 18 (90%) | 3 (15%) |
| AVB II or III | 0 (0%) | 0 (%) |
| hs-TnT (pg/mL) | 148.47 (±363.54) (increased in 16 patients, 80%) | <14 ng/mL ° |
| NT-proBNP (pg/mL) | 4434.78 (±6233.53) (increased in 17 patients, 85%) | <317 ng/mL °° |
| Hemoglobin (g/dL) | 11.5 ± 1.7 | 13.7 ± 1 |
| Leukocytes (103/µL) | 9.9 ± 3.4 | 6.6 ± 1.9 |
| Lymphocytes (103/µL) | 1.2 ± 0.6 | 2.5 ± 0.8 |
| Lymphocytes (%) | 12.7 ± 8.8 | 38.9 ± 8.1 |
| Platelets (103/µL) | 258.1 ± 173.7 | 261.9 ± 80.1 |
| Serum albumin (g/dL) | 3.2 ± 0.6 | 4.7 ± 0.3 |
| Ferritin (ng/mL) | 862.1 ± 717.5 | 52.7 ± 26.4 |
| Triglycerides (mg/dL) | 173.8 ± 72.1 | 87.9 ± 47.4 |
| Uric acid (mg/dL) | 3.8 ± 1.4 | 4.5 ± 1.4 |
| Serum sodium (mEq/L) | 133.3 ± 4.5 | 139.9 ± 0.8 |
| C-reactive protein (mg/dL) | 13.4 ± 8 | 0.1 ± 0.1 |
| D-dimers (µg/mL FEU) | 2.8 ± 4.2 | 0.3 ± 0.0 |
| Fibrinogen (mg/dL) | 616.8 ± 193.6 | 306.6 ± 62.6 |
| Basal HR (bpm) | 83.75 ± 12.9 |
| Peak HR (bpm) | 192.35 ± 7.59 |
| Peak HR (% of expected) | 92.39 ± 4.05 |
| Peak RER | 1.12 ± 0.04 |
| Peak systolic blood pressure | 140 ± 18 |
| Peak diastolic blood pressure | 69 ± 8 |
| Basal systolic blood pressure | 104 ± 13 |
| Basal diastolic blood pressure | 62 ± 9 |
| Peak VO2 (mL/min) | 1676.20 ± 521.03 (lower than expected in 10 out of 20) |
| Peak VO2/kg (mL/min/kg) | 34.16 ± 6.99 (lower than expected in 10 out of 20) |
| Peak VO2 (% of expected) | 84.95 ± 16.53 (lower than expected in 10 out of 20) |
| VO2 at AT (ml/min) | 1157 ± 383 |
| VO2 at AT/Kg (ml/min/kg) | 23 ± 4 |
| VO2 at AT (% of predicted VO2max) | 55 ± 10 (lower than 60% of predicted VO2max in 15 out of 20) |
| Oxygen Pulse (mL/beat) | 9.0 ± 3.0 |
| Oxygen Pulse (% of expected) | 85 ± 16 (lower than 80% of predicted in 10 out of 20 and lower than 70% in 3 out 20) |
| Slope VE/VCO2 (VCP) | 33.40 ± 3.54 |
| Slope delta Burstein 1 | 3.45 ± 3.08 (higher than expected in 19 out of 20) |
| Slope delta Dilbert 2 | 8.84 ± 3.37 (higher than expected in 16 out of 20) |
| Delta Takken 3 | 2.92 ± 2.95 (higher than expected in 16 out of 20) |
| Delta Blunchard 4 | 6.43 ± 4.97 (higher than expected in 19 out of 20) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gentili, F.; Calcagni, G.; Cantarutti, N.; Manno, E.C.; Cafiero, G.; Tranchita, E.; Salvati, A.; Palma, P.; Giordano, U.; Drago, F.; et al. Cardiopulmonary Exercise Testing in Children and Young Adolescents after a Multisystem Inflammatory Syndrome: Physical Deconditioning or Residual Pathology? J. Clin. Med. 2023, 12, 2375. https://doi.org/10.3390/jcm12062375
Gentili F, Calcagni G, Cantarutti N, Manno EC, Cafiero G, Tranchita E, Salvati A, Palma P, Giordano U, Drago F, et al. Cardiopulmonary Exercise Testing in Children and Young Adolescents after a Multisystem Inflammatory Syndrome: Physical Deconditioning or Residual Pathology? Journal of Clinical Medicine. 2023; 12(6):2375. https://doi.org/10.3390/jcm12062375
Chicago/Turabian StyleGentili, Federica, Giulio Calcagni, Nicoletta Cantarutti, Emma Concetta Manno, Giulia Cafiero, Eliana Tranchita, Annamaria Salvati, Paolo Palma, Ugo Giordano, Fabrizio Drago, and et al. 2023. "Cardiopulmonary Exercise Testing in Children and Young Adolescents after a Multisystem Inflammatory Syndrome: Physical Deconditioning or Residual Pathology?" Journal of Clinical Medicine 12, no. 6: 2375. https://doi.org/10.3390/jcm12062375
APA StyleGentili, F., Calcagni, G., Cantarutti, N., Manno, E. C., Cafiero, G., Tranchita, E., Salvati, A., Palma, P., Giordano, U., Drago, F., & Turchetta, A. (2023). Cardiopulmonary Exercise Testing in Children and Young Adolescents after a Multisystem Inflammatory Syndrome: Physical Deconditioning or Residual Pathology? Journal of Clinical Medicine, 12(6), 2375. https://doi.org/10.3390/jcm12062375





