Dyspnea in Post-Acute COVID-19: A Multi-Parametric Cardiopulmonary Evaluation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Protocol
2.2. Transthoracic Echocardiography
2.3. Pulmonary Functional Test
2.4. Six-Minute Walking Test
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Clinical Evaluation at Follow-Up
3.3. Cardiopulmonary Evaluation at Follow-Up
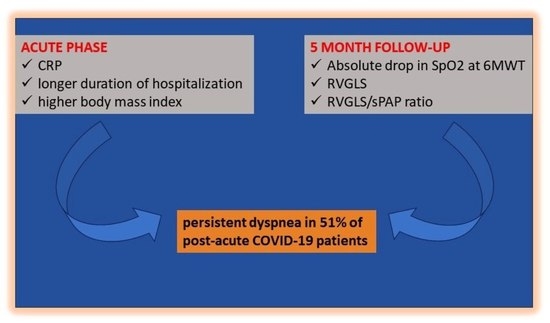
3.4. Acute Predictors of Persistent Dyspnea at Follow-Up
3.5. Association between Persistent Dyspnea and Cardiopulmonary Measures at Follow-Up
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef] [PubMed]
- Al-Aly, Z.; Xie, Y.; Bowe, B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature 2021, 594, 259–264. [Google Scholar] [CrossRef]
- Huang, C.; Huang, L.; Wang, Y.; Li, X.; Ren, L.; Gu, X.; Kang, L.; Guo, L.; Liu, M.; Zhou, X.; et al. 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet 2021, 397, 220–232. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Yao, Q.; Gu, X.; Wang, Q.; Ren, L.; Wang, Y.; Hu, P.; Guo, L.; Liu, M.; Xu, J.; et al. 1-year outcomes in hospital survivors with COVID-19: A longitudinal cohort study. Lancet 2021, 398, 747–758. [Google Scholar] [CrossRef]
- Goërtz, Y.M.J.; Van Herck, M.; Delbressine, J.; Vaes, A.W.; Meys, R.; Machado, F.V.C.; Houben-Wilke, S.; Burtin, C.; Posthuma, R.; Franssen, F.M.E.; et al. Persistent symptoms 3 months after a SARS-CoV2 infection: The post-COVID-19 syndrome? ERJ Open Res. 2020, 6, 00542–2020. [Google Scholar] [CrossRef]
- Carfì, A.; Bernabei, R.; Landi, F.; Gemelli against COVID-19 Post-Acute Care Study Group. Persistent symptoms in patients after acute COVID-19. JAMA 2020, 324, 603–605. [Google Scholar] [CrossRef]
- Lala, A.; Johnson, K.W.; Januzzi, J.L.; Russak, A.J.; Paranjpe, I.; Richter, F.; Zhao, S.; Somani, S.; Van Vleck, T.; Vaid, A.; et al. Prevalence and Impact of Myocardial Injury in Patients Hospitalized with COVID-19 Infection. J. Am. Coll. Cardiol. 2020, 76, 533–546. [Google Scholar] [CrossRef] [PubMed]
- Lerum, T.V.; Aaløkken, T.M.; Brønstad, E.; Aarli, B.; Ikdahl, E.; Lund, K.M.A.; Durheim, M.T.; Rodriguez, J.R.; Meltzer, C.; Tonby, K.; et al. Dyspnoea, lung function and CT findings 3 months after hospital admission for COVID-19. Eur. Respir. J. 2021, 57, 2003448. [Google Scholar] [CrossRef]
- Shah, A.S.; Ryu, M.H.; Hague, C.J.; Murphy, D.T.; Johnston, J.C.; Ryerson, C.J.; Carlsten, C.; Wong, A.W. Changes in pulmonary function and patient-reported outcomes during COVID-19 recovery: A longitudinal, prospective cohort study. ERJ Open Res. 2021, 7, 00243–2021. [Google Scholar] [CrossRef]
- Lam, G.Y.; Befus, A.D.; Damant, R.W.; Ferrara, G.; Fuhr, D.P.; Stickland, M.K.; Varughese, R.A.; Wong, E.Y.; Smith, M.P. Exertional intolerance and dyspnea with preserved lung function: An emerging long COVID phenotype? Respir. Res. 2021, 22, 222. [Google Scholar] [CrossRef]
- Beaudry, R.I.; Brotto, A.R.; Varughese, R.A.; de Waal, S.; Fuhr, D.P.; Damant, R.W.; Ferrara, G.; Lam, G.Y.; Smith, M.P.; Stickland, M.K. Persistent dyspnea after COVID-19 is not related to cardiopulmonary impairment; A cross-sectional study of persistently dyspneic COVID-19, non-dyspneic COVID-19 and controls. Front Physiol. 2022, 13, 917886. [Google Scholar] [CrossRef] [PubMed]
- Singh, I.; Joseph, P.; Heerdt, P.M.; Cullinan, M.; Lutchmansingh, D.D.; Gulati, M.; Possick, J.D.; Systrom, D.M.; Waxman, A.B. Persistent Exertional Intolerance after COVID-19: Insights from Invasive Cardiopulmonary Exercise Testing. Chest 2022, 161, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Mancini, D.M.; Brunjes, D.L.; Lala, A.; Trivieri, M.G.; Contreras, J.P.; Natelson, B.H. Use of Cardiopulmonary Stress Testing for Patients with Unexplained Dyspnea Post-Coronavirus Disease. JACC Heart Fail. 2021, 9, 927–937. [Google Scholar] [CrossRef]
- Matta, J.; Wiernik, E.; Robineau, O.; Carrat, F.; Touvier, M.; Severi, G.; de Lamballerie, X.; Blanché, H.; Deleuze, J.F.; Gouraud, C.; et al. Association of self-reported COVID-19 infection and SARS-CoV-2 serology test results with persistent physical symptoms among french adults during the COVID-19 pandemic. JAMA Intern. Med. 2022, 182, 19–25. [Google Scholar] [CrossRef]
- Bestall, J.C.; Paul, E.A.; Garrod, R.; Garnham, R.; Jones, P.W.; Wedzicha, J.A. Usefulness of the Medical Research Council (MRC) dyspnea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax 1999, 54, 5816. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39. [Google Scholar] [CrossRef]
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.F.; Brida, M.; Carlsen, J.; Coats, A.J.S.; Escribano-Subias, P.; Ferrari, P.; et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension Developed by the task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS). Eur. Heart J. 2022, 43, 3618–3731. [Google Scholar] [CrossRef]
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; van der Grinten, C.P.M.; Gustafsson, P.; et al. Standardisation of spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef]
- Macintyre, N.; Crapo, R.O.; Viegi, G.; Johnson, D.C.; van der Grinten, C.P.M.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Enright, P.; et al. Standardisation of single-breath determination of carbon monoxide uptake in the lung. Eur. Respir. J. 2005, 26, 720–735. [Google Scholar] [CrossRef]
- Quanjer, P.H.; Stanojevic, S.; Cole, T.J.; Baur, X.; Hall, G.L.; Culver, B.H.; Enright, P.L.; Hankinson, J.L.; Ip, M.S.M.; Zheng, J.; et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: The global lung function 2012 equations. Eur. Respir. J. 2012, 40, 1324–1343. [Google Scholar] [CrossRef]
- Cotes, J.E.; Chinn, D.J.; Quanjer, P.H.; Roca, J.; Yernault, J.C. Standardization of the measurement of transfer factor (diffusing capacity). Eur. Respir. J. 1993, 6 (Suppl. 16), 41–52. [Google Scholar] [CrossRef]
- Evans, J.A.; Whitelaw, W.A. The assessment of maximal respiratory mouth pressures in adults. Respir. Care 2009, 54, 1348–1359. [Google Scholar] [PubMed]
- Singh, S.J.; Puhan, M.A.; Andrianopoulos, V.; Hernandes, N.A.; Mitchell, K.E.; Hill, C.J.; Lee, A.L.; Camillo, C.A.; Troosters, T.; Spruit, M.A.; et al. An official systematic review of the European Respiratory Society/American Thoracic Society: Measurement properties of field walking tests in chronic respiratory disease. Eur. Respir. J. 2014, 44, 1447–1478. [Google Scholar] [CrossRef] [PubMed]
- Camarria, B.; Eastwood, P.R.; Cecins, N.M.; Thompson, P.J.; Jenkins, S. Six minute walk distance in healthy subjects aged 55–75 years. Respir. Med. 2006, 100, 658–665. [Google Scholar] [CrossRef]
- Stoica, P.; Selen, Y. Model-order selection: A review of information criterion rules. IEEE Signal Process. Mag. 2004, 21, 36–47. [Google Scholar] [CrossRef]
- Cecchetto, A.; Torreggiani, G.; Guarnieri, G.; Vianello, A.; Baroni, G.; Palermo, C.; Bertagna De Marchi, L.; Lorenzoni, G.; Bartolotta, P.; Bertaglia, E.; et al. Subclinical Myocardial Injury in Patients Recovered from COVID-19 Pneumonia: Predictors and Longitudinal Assessment. J. Cardiovasc. Dev. Dis. 2023, 10, 179. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Daines, L.; Han, Q.; Hurst, J.R.; Pfeffer, P.; Shankar-Hari, M.; Elneima, O.; Walker, S.; Brown, J.S.; Siddiqui, S.; et al. Prevalence, risk factors and treatments for post-COVID-19 breathlessness: A systematic review and meta-analysis. Eur. Respir. Rev. 2022, 31, 220071. [Google Scholar] [CrossRef]
- Arnold, D.T.; Hamilton, F.W.; Milne, A.; Morley, A.J.; Viner, J.; Attwood, M.; Noel, A.; Gunning, S.; Hatrick, J.; Hamilton, S.; et al. Patient outcomes after hospitalisation with COVID-19 and implications for follow-up: Results from a prospective UK cohort. Thorax 2021, 76, 399–401. [Google Scholar] [CrossRef]
- Michelen, M.; Manoharan, L.; Elkheir, N.; Cheng, V.; Dagens, A.; Hastie, C.; O’Hara, M.; Suett, J.; Dahmash, D.; Bugaeva, P.; et al. Characterising long COVID: A living systematic review. BMJ Glob. 2021, 6, e005427. [Google Scholar] [CrossRef]
- Sudre, H.D.; Murray, B.; Varsavsky, T.; Graham, M.S.; Penfold, R.S.; Bowyer, R.C.; Capdevila Pujol, J.; Klaser, K.; Antonelli, M.; Canas, L.S.; et al. Attributes and predictors of long COVID. Nat. Med. 2021, 27, 626–631. [Google Scholar] [CrossRef]
- Vimercati, L.; De Maria, L.; Quarato, M.; Caputi, A.; Gesualdo, L.; Migliore, G.; Cavone, D.; Sponselli, S.; Pipoli, A.; Inchingolo, F.; et al. Association between Long COVID and Overweight/Obesity. J. Clin. Med. 2021, 10, 4143. [Google Scholar] [CrossRef]
- Daitch, V.; Yelin, D.; Awwad, M.; Guaraldi, G.; Milic, J.; Mussini, C.; Falcone, M.; Tiseo, G.; Carrozzi, L.; Pistelli, F.; et al. Characteristics of long-COVID among older adults: A cross-sectional study. Int. J. Infect. Dis. 2022, 125, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Grewal, J.S.; Carlsten, C.; Johnston, J.C.; Shah, A.S.; Wong, A.W.; Ryerson, C.J. Post-COVID dyspnea: Prevalence, predictors, and outcomes in a longitudinal, prospective cohort. BMC Pulm. Med. 2023, 23, 84. [Google Scholar] [CrossRef] [PubMed]
- Robey, R.C.; Kemp, K.; Hayton, P.; Mudawi, D.; Wang, R.; Greaves, M.; Yioe, V.; Rivera-Ortega, P.; Avram, C.; Chaudhuri, N. Pulmonary Sequelae at 4 Months After COVID-19 Infection: A Single-Centre Experience of a COVID Follow-Up Service. Adv. Ther. 2021, 38, 4505–4519. [Google Scholar] [CrossRef]
- Shi, Z.; de Vries, H.J.; Vlaar, A.P.J.; van der Hoeven, J.; Boon, R.A.; Heunks, L.M.A.; Ottenheijm, C.A.C. Diaphragm pathology in critically Ill patients with COVID-19 and postmortem findings from 3 medical centers. JAMA Intern. Med. 2021, 181, 122–124. [Google Scholar] [CrossRef] [PubMed]
- Farr, E.; Wolfe, A.R.; Deshmukh, S.; Rydberg, L.; Soriano, R.; Walter, J.M.; Boon, A.J.; Wolfe, L.F.; Franz, C.K. Diaphragm dysfunction in severe COVID-19 as determined by neuromuscular ultrasound. Ann. Clin. Transl. Neurol. 2021, 8, 1745–1749. [Google Scholar] [CrossRef]
- Abodonya, A.M.; Abdelbasset, W.K.; Awad, E.A.; Elalfy, I.E.; Salem, H.A.; Elsayed, S.H. Inspiratory muscle training for recovered COVID-19 patients after weaning from mechanical ventilation: A pilot control clinical study. Medicine 2021, 100, e25339. [Google Scholar] [CrossRef]
- Dosbabaa, F.; Hartmana, M.; Batalika, L.; Senkyra, V.; Radkovcovaa, I.; Richterd, S.; Brate, K.; Cahalinf, L.P.; Formigag, M.F. A temporal examination of inspiratory muscle strength and endurance in hospitalized COVID-19 patients. Heart Lung 2023, 60, 95–101. [Google Scholar] [CrossRef]
- Hennigs, J.K.; Huwe, M.; Hennigs, A.; Oqueka, T.; Simon, M.; Harbaum, L.; Körbelin, J.; Schmiedel, S.; zur Wiesch, J.S.; Addo, M.M.; et al. Respiratory muscle dysfunction in long COVID patients. Infection 2022, 50, 1391–1397. [Google Scholar] [CrossRef]
- Rinaldo, R.F.; Mondoni, M.; Parazzini, E.M.; Pitari, F.; Brambilla, E.; Luraschi, S.; Balbi, M.; Sferrazza Papa, G.M.; Sotgiu, G.; Guazzi, M.; et al. Deconditioning as main mechanism of impaired exercise response in COVID-19 survivors. Eur. Respir. J. 2021, 58, 2100870. [Google Scholar] [CrossRef]
- Skjørten, I.; Ankerstjerne, O.A.W.; Trebinjac, D.; Brønstad, E.; Rasch-Halvorsen, Ø.; Einvik, G.; Lerum, T.V.; Stavem, K.; Edvardsen, A.; Björk Ingul, C. Cardiopulmonary exercise capacity and limitations 3 months after COVID-19 hospitalisation. Eur. Respir. J. 2021, 58, 2100996. [Google Scholar] [CrossRef] [PubMed]
- Ozer, P.K.; Govdeli, E.A.; Baykiz, D.; Karaayvaz, E.B.; Medetalibeyoglu, A.; Catma, Y.; Elitok, A.; Cagatay, A.; Umman, B.; Oncul, A. Impairment of right ventricular longitudinal strain associated with severity of pneumonia in patients recovered from COVID-19. Int. J. Cardiovasc. Imaging 2021, 37, 2387–2397. [Google Scholar] [CrossRef] [PubMed]
- Tryfou, E.S.; Kostakou, P.M.; Chasikidis, C.G.; Kostopoulos, V.S.; Serafetinidis, I.I.; Ferdianaki, E.K.; Mihas, C.; Olympios, C.D.; Kouris, N.T. Biventricular myocardial function in COVID-19 recovered patients assessed by speckle tracking echocardiography: A prospective cohort echocardiography study. Int. J. Cardiovasc. Imaging 2022, 38, 995–1003. [Google Scholar] [CrossRef]
- Stockenhuber, A.; Vrettos, A.; Androschuck, V.; George, M.; Robertson, C.; Bowers, N.; Clifford, P.; Firoozan, F. A pilot study on right ventricular longitudinal strain as a predictor of outcome in COVID-19 patients with evidence of cardiac involvement. Echocardiography 2021, 38, 222–229. [Google Scholar] [CrossRef]
- Pestelli, G.; Fiorencis, A.; Trevisan, F.; Luisi, G.A.; Smarrazzo, M.; Mele, D. New measures of right ventricle-pulmonary artery coupling in heart failure: An all-cause mortality echocardiographic study. Int. J. Cardiol. 2021, 329, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Guazzi, M.; Dixon, D.; Labate, V.; Beussink-Nelson, L.; Bandera, F.; Cuttica, M.J.; Shah, S.J. RV Contractile Function and its Coupling to Pulmonary Circulation in Heart Failure with Preserved Ejection Fraction: Stratification of Clinical Phenotypes and Outcomes. JACC Cardiovasc. Imaging 2017, 10, 1211–1221. [Google Scholar] [CrossRef]
- Iacoviello, M.; Monitillo, F.; Citarelli, G.; Leone, M.; Grande, D.; Antoncecchi, V.; Rizzo, C.; Terlizzese, P.; Romito, R.; Caldarola, P.; et al. Right ventriculo-arterial coupling assessed by two-dimensional strain: A new parameter of right ventricular function independently associated with prognosis in chronic heart failure patients. Int. J. Cardiol. 2017, 241, 318–321. [Google Scholar] [CrossRef]
- Polito, M.V.; Silverio, A.; Di Maio, M.; Bellino, M.; Scudiero, F.; Russo, V.; Rasile, B.; Alfano, C.; Citro, R.; Parodi, G.; et al. Prognostic Implications of Right Ventricular Function and Pulmonary Pressures Assessed by Echocardiography in Hospitalized Patients with COVID-19. J. Pers. Med. 2021, 11, 1245. [Google Scholar] [CrossRef]
| Variables | Total Patients (n = 225) | Dyspnea Absent at FU (n = 110) | Dyspnea Present at FU (n = 115) | p Value |
|---|---|---|---|---|
| Age (y) (median IQR, (range)) | 65 (55, 73) | 65 (54, 73) | 65 (55, 72) | NS |
| Gender (n(%)) F | 88 (39%) | 36 (33%) | 52 (45%) | NS |
| M | 137 (61%) | 74 (67%) | 63 (55%) | |
| Smoke (n (%)) no | 125 (56%) | 64 (59%) | 61 (53%) | NS |
| user | 11 (5%) | 8 (7.3%) | 3 (2.6%) | |
| former smoker | 88 (39%) | 37 (34%) | 51 (44%) | |
| Body mass index (kg/m2) (median IQR (range)) | 28.1 (24, 31.4) | 28.1 (25.1, 31.2) | 28.4 (24.5, 31.8) | NS |
| Previous cardiovascular disease (n (%)) | NS | |||
| Absent | 213 (95%) | 106 ((96%) | 107 (93%) | |
| Present | 12 (5.3%) | 4 (3.6%) | 8 (7.0%) | |
| Type of cardiovascular disease (n (%)) | - | - | ||
| Ischemic | 8 (3.5%) | |||
| Valvular | 3 (1.3%) | |||
| Hypertrophic cardiomyopathy | 1 (0.4%) | |||
| Duration of hospitalization (days) (median IQR (range)) | 18 (12, 28) | 17 (12, 25) | 20 (13, 35) | NS |
| Respiratory ventilation (Type; (n (%)) | NS | |||
| Absent | 36 (16%) | 12 (11%) | 24 (21%) | |
| NC-SM | 25 (11%) | 12 (11%) | 13 (11%) | |
| RM | 6 (3%) | 3 (2.8) | 3 (2.6%) | |
| HFNC | 69 (31%) | 42 (40%) | 27 (24%) | |
| NIV | 28 (13%) | 13 (12%) | 15 (13%) | |
| MV | 56 (26%) | 24 (23%) | 32 (28%) | |
| Troponin I (ng/L) (median IQR (range)) n.v. < 34 ng/L | 9 (5, 26) | 8 (4, 17) | 12 (6, 65) | 0.035 |
| CRP (mg/l) (median IQR (range)) n.v. < 5 mg/L | 120 (81, 180) | 110 (78, 142) | 135 (86, 218) | 0.024 |
| D-dimer (ng/mL) (median IQR (range)) n.v. < 500 ng/mL | 662 (295, 1927) | 564 (290, 1166) | 767 (324, 2620) | NS |
| BNP (pg/mL) (median IQR (range)) n.v. < 125 pg/mL | 70 (26, 120) | 40 (16, 102) | 80 (48, 210) | 0.001 |
| Cardiovascular risk factors (n (%)) | NS | |||
| Absent | 74 (33%) | 36 (33%) | 38 (33%) | |
| Present | 151 (67%) | 74 (67%) | 77 (67%) | |
| Cardiovascular risk factors type (n (%)) | - | - | ||
| Hypertension | 101 (45%) | |||
| Dyslipidemia | 33 (14%) | |||
| Diabetes Mellitus | 34 (15%) | |||
| Obesity | 58 (26%) | |||
| CKD | 7 (3%) |
| Measures | Overall Patients (n = 225) | No Dyspnea at FU (n = 110) | Dyspnea at FU (n = 115) | p Value |
|---|---|---|---|---|
| LV EDVi biplane (mL/m2) (median IQR (range)) | 51 (43, 58) | 52 (46, 59) | 50 (42, 57) | NS |
| LV EDVi 3D (mL/m2) (median IQR (range)) | 54 (47, 62) | 56 (48, 62) | 53 (46, 62) | NS |
| LV ESVi biplane (mL/m2) (median IQR (range)) | 20 (16, 23) | 20 (16, 24) | 20 (16, 22) | NS |
| LV ESVi 3D (mL/m2) (median IQR (range)) | 21 (18, 25) | 21 (18, 25) | 20 (18, 25) | NS |
| LV EF biplane (%) (median IQR (range)) | 61 (57, 65) | 61 (57, 65) | 61 (57, 64) | NS |
| LV EF 3D (%) (median IQR (range)) | 61 (58, 64) | 61 (58, 63) | 60 (57, 64) | NS |
| LV GLS (%) (median IQR (range)) | −18.6 (−20.4, −17.0) | −18.6 (−20.8, −17.0) | −18.4 (−20.1, −26.7) | NS |
| LV E/A ratio (median IQR (range)) | 0.85 (0.71, 1.08) | 0.85 (0.71, 1.08) | 0.85 (0.70, 1.08) | NS |
| LV E/e’ ratio (median IQR (range)) | 7.5 (6.0, 9.1) | 7.7 (6.0, 8.9) | 7.1 (5.9, 9.7) | NS |
| Left atrial volume index (mL/m2) median IQR (range)) | 30 (25, 35) | 30 (27, 36) | 29 (24, 35) | NS |
| RV EDAi (cm2/m2) (median IQR (range)) | 11 (9, 12) | 11 (10, 12) | 11 (9, 12) | NS |
| RV ESAi (cm2/m2) (median IQR (range)) | 6 (5, 7) | 6 (5, 7) | 6 (5, 7) | NS |
| FAC (%) (median IQR (range)) | 44 (40, 47) | 44 (40, 47) | 44 (40, 48) | NS |
| RV EDVi 3D (mL/m2) (median IQR (range)) | 50 (41, 61) | 53 (42, 61) | 44 (39, 58) | NS |
| RV ESVi 3D (mL/m2) (median IQR (range)) | 23 (19, 30) | 25 (20, 32) | 23 (19, 29) | NS |
| RV EF 3D (%) (median IQR (range)) | 52 (47, 56) | 52 (48, 56) | 51 (47, 55) | NS |
| RV FWS (%) (median IQR (range)) | −24.4(−27.0, −22.0) | −25.0 (−27.3, −22.0) | −24.0 (−26.0, −21.4) | NS |
| RV GLS (%) (median IQR (range)) | −20.3 (−22.5, −18.4) | −20.4 (−22.6, −18.7) | −20.0 (−22.0, −18.2) | NS |
| RV GLS/sPAP (%/mmHg) (median IQR (range)) | 0.78 (0.97, 0.64) | 0.78 (0.93, 0.66) | 0.78 (1.04, 0.63) | NS |
| TAPSE (mm) (median IQR (range)) | 22 (20, 24) | 22 (20, 24) | 22 (19, 24) | NS |
| TAPSE/sPAP (mm/mmHg) | 0.86 (0.71, 1.04) | 0.82 (0.71, 1.01) | 0.88 (0.71, 1.06) | NS |
| Probability of pulmonary hypertension (n (%)) | NS | |||
| -Low | 193 (93%) | 96 (93%) | 97 (92%) | |
| -Intermediate | 11 (5.3%) | 6 (5.8%) | 5 (4.8%) | |
| -Intermediate-high | 3 (1.4%) | 1 (1%) | 2 (1.9%) | |
| -High | 1 (0.5%) | 0 (0%) | 1 (1%) | |
| sPAP (mmHg) (median IQR (range)) | 26 (21,29) | 26 (22, 29) | 25 (21, 29) | NS |
| PVR (WU) (median IQR (range)) | 1.73 (1.49, 1.99) | 1.77 (1.5, 2.00) | 1.71 (1.42, 1.98) | NS |
| AT (msec) (median IQR (range)) | 129 (116, 146) | 130 (120, 145) | 128 (114, 146) | NS |
| Measures | Total Patients (n = 225) | Dyspnea Absent at FU (n = 110) | Dyspnea Present at FU (n = 115) | p Value |
|---|---|---|---|---|
| VC (l) (median IQR (range)) | 3.56 (2.86, 4.18) | 3.63 (3.05, 4.27) | 3.34 (2.64, 4.05) | NS |
| VC (%) (median IQR (range)) | 102 (91, 114) | 104 (93, 115) | 101 (86, 113) | NS |
| TLC (l) (median IQR (range)) | 5.64 (4.64, 6.61) | 5.85 (4.92, 6.66) | 5.37 (4.41, 6.51) | 0.044 |
| TLC (%) (median IQR (range)) | 95 (86, 103) | 96 (88, 103) | 94 (83, 103) | NS |
| DLCO (mL/min/mmHg) (median IQR (range)) | 19 (14, 23) | 20 (15, 24) | 18 (14, 22) | 0.036 |
| DLCO (%) (median IQR (range)) | 76 (61, 87) | 79 (66, 90) | 73 (58, 84) | 0.015 |
| KCO (l) (median IQR (range)) | 3.63 (2.94, 4.12) | 3.63 (2.99, 4.02) | 3.63 (2.93, 4.16) | NS |
| MIP (cm H2O) (median IQR (range)) | 74 (56, 103) | 83 (61, 103) | 70 (53, 103) | NS |
| MEP (cm H2O) (median IQR (range)) | 90 (68, 113) | 93 (73, 119) | 85 (63, 104) | 0.034 |
| Tiffeneau index (%) (median IQR (range)) | 0.84 (0.79, 0.88) | 0.84 (0.80, 0.88) | 0.84 (0.79, 0.88) | NS |
| Distance at 6MWT (m) (median IQR (range)) | 420 (360, 480) | 450 (385, 495) | 420 (360, 480) | NS |
| Final SpO2 at 6MWT (%) (median IQR (range)) | 96 (95, 98) | 97 (96, 98) | 96 (94, 98) | NS |
| Absolute drop in SpO2 at 6MWT (%) (median IQR (range)) | 2 (1, 3) | 2 (1, 3) | 2 (1, 4) | NS |
| Univariable Analysis | Multivariable Analysis | |||||
|---|---|---|---|---|---|---|
| Variables | OR | 95% CI | p Value | OR | 95% CI | p Value |
| Age | 1.00 | 0.98, 1.02 | NS | |||
| Gender (male) | 0.58 | 0.33, 1.00 | NS | |||
| Smoke | NS | |||||
| -User | 0.39 | 0.08, 1.43 | ||||
| -Former smoker | 1.45 | 0.84, 2.52 | ||||
| BMI (kg/m2) | 1.02 | 0.97, 1.07 | NS | 1.15 | 1.06, 1.28 | 0.001 |
| Previous CVD | 1.98 | 0.61, 7.61 | NS | |||
| Duration of hospitalization | 1.02 | 1.00, 1.04 | 0.029 | 1.05 | 1.01, 1.10 | 0.005 |
| Respiratory | NS | |||||
| Ventilation | ||||||
| -NC-SM | 0.54 | 0.19, 1.54 | ||||
| -RM | 0.50 | 0.08, 3.05 | ||||
| -HFNC | 0.32 | 0.13, 0.74 | ||||
| -NIV | 0.58 | 0.21, 1.59 | ||||
| -MV | 0.67 | 0.27, 1.61 | ||||
| Troponin I | 1.00 | 1.00, 1.00 | NS | |||
| CRP | 1.00 | 1.00, 1.01 | 0.013 | 1.01 | 1.00, 1.02 | 0.025 |
| D-dimer | 1.00 | 1.00, 1.00 | NS | |||
| BNP | 1.00 | 1.00, 1.00 | NS | |||
| Cardiovascular risk factors | 0.99 | 0.56, 1.72 | NS | |||
| Univariable Analysis | Multivariable Analysis | |||||
|---|---|---|---|---|---|---|
| Measures | OR | 95% CI | p Value | OR | 95% CI | p Value |
| Laboratory tests | ||||||
| CRP | 1.02 | 0.97, 1.07 | NS | |||
| D-dimer | 1.00 | 1.00, 1.00 | NS | |||
| Troponin I | 1.02 | 0.99, 1.07 | NS | |||
| BNP | 1.00 | 1.00, 1.00 | NS | |||
| 6MWT | ||||||
| Distance | 1.00 | 0.99, 1.00 | 0.038 | |||
| Final SpO2 | 0.88 | 0.78, 0.99 | 0.040 | |||
| Absolute drop in SpO2 | 1.12 | 0.97, 1.30 | NS | 1.37 | 1.13, 1.69 | 0.001 |
| Pulmonary functional tests | ||||||
| VC (l) | 1.02 | 0.97, 1.13 | NS | |||
| VC (%) | 0.99 | 0.97, 1.00 | NS | |||
| TLC (l) | 1.01 | 0.96, 1.11 | NS | |||
| TLC (%) | 0.98 | 0.96, 1.00 | NS | |||
| DLCO | 0.98 | 0.94, 1.01 | NS | |||
| DLCO (%) | 0.98 | 0.97, 1.00 | 0.007 | |||
| KCO (l) | 1.06 | 0.95, 1.32 | NS | |||
| MIP | 1.00 | 0.99, 1.00 | NS | |||
| MEP | 0.99 | 0.98, 1.00 | 0.038 | |||
| Tiffeneau index | 0.23 | 0.01, 8.41 | NS | |||
| Echocardiography | ||||||
| LV EDVi biplane | 0.98 | 0.96, 1.00 | NS | |||
| LV EDVi 3D | 0.98 | 0.96, 1.01 | NS | |||
| LV ESVi biplane | 0.97 | 0.93, 1.02 | NS | |||
| LV ESVi 3D | 1.00 | 0.96, 1.04 | NS | |||
| LV EF biplane | 0.99 | 0.94, 1.04 | NS | |||
| LV EF 3D | 0.98 | 0.92, 1.04 | NS | |||
| LV GLS | 1.06 | 0.97, 1.17 | NS | |||
| LV E/A ratio | 1.76 | 0.86, 4.00 | NS | |||
| LV E/e’ ratio | 1.05 | 0.94, 1.18 | NS | |||
| LAVi | 0.98 | 0.95, 1.00 | NS | |||
| RV EDAi | 0.91 | 0.79, 1.05 | NS | |||
| RV ESAi | 0.88 | 0.72, 1.05 | NS | |||
| FAC | 1.00 | 0.96, 1.05 | NS | |||
| RV EDVi 3D | 0.98 | 0.96, 1.00 | NS | |||
| RV ESVi 3D | 0.98 | 0.94, 1.02 | NS | |||
| RV EF 3D (%) | 0.98 | 0.93, 1.02 | NS | |||
| RV FWS | 1.03 | 0.98, 1.09 | NS | |||
| RV GLS | 1.02 | 0.98, 1.08 | NS | 1.12 | 1.02, 1.25 | 0.016 |
| RV GLS/sPAP | 0.92 | 0.38, 2.18 | NS | 0.14 | 0.02, 0.86 | 0.034 |
| TAPSE | 0.96 | 0.90, 1.02 | NS | |||
| TAPSE/sPAP | 1.23 | 0.45, 3.45 | NS | |||
| Probability of pulmonary hypertension | NS | |||||
| -Low | 0.82 | 0.23, 2.83 | ||||
| -Intermediate | 1.98 | 0.19, 43.0 | ||||
| -High | NA | NA | ||||
| sPAP | 0.99 | 0.95, 1.03 | NS | |||
| PVR | 0.83 | 0.46, 1.18 | NS | |||
| AT | 0.83 | 0.46, 1.18 | NS | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cecchetto, A.; Guarnieri, G.; Torreggiani, G.; Vianello, A.; Baroni, G.; Palermo, C.; Bertagna De Marchi, L.; Lorenzoni, G.; Bartolotta, P.; Bertaglia, E.; et al. Dyspnea in Post-Acute COVID-19: A Multi-Parametric Cardiopulmonary Evaluation. J. Clin. Med. 2023, 12, 4658. https://doi.org/10.3390/jcm12144658
Cecchetto A, Guarnieri G, Torreggiani G, Vianello A, Baroni G, Palermo C, Bertagna De Marchi L, Lorenzoni G, Bartolotta P, Bertaglia E, et al. Dyspnea in Post-Acute COVID-19: A Multi-Parametric Cardiopulmonary Evaluation. Journal of Clinical Medicine. 2023; 12(14):4658. https://doi.org/10.3390/jcm12144658
Chicago/Turabian StyleCecchetto, Antonella, Gabriella Guarnieri, Gianpaolo Torreggiani, Andrea Vianello, Giulia Baroni, Chiara Palermo, Leonardo Bertagna De Marchi, Giulia Lorenzoni, Patrizia Bartolotta, Emanuele Bertaglia, and et al. 2023. "Dyspnea in Post-Acute COVID-19: A Multi-Parametric Cardiopulmonary Evaluation" Journal of Clinical Medicine 12, no. 14: 4658. https://doi.org/10.3390/jcm12144658
APA StyleCecchetto, A., Guarnieri, G., Torreggiani, G., Vianello, A., Baroni, G., Palermo, C., Bertagna De Marchi, L., Lorenzoni, G., Bartolotta, P., Bertaglia, E., Donato, F., Aruta, P., Iliceto, S., & Mele, D. (2023). Dyspnea in Post-Acute COVID-19: A Multi-Parametric Cardiopulmonary Evaluation. Journal of Clinical Medicine, 12(14), 4658. https://doi.org/10.3390/jcm12144658