Cardiopulmonary Long-Term Sequelae in Patients after Severe COVID-19 Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Population
2.3. Outcome Baseline Examinations
2.4. Clinical Assessment
2.5. Six-Minute Walk Test
2.6. Laboratory Parameters
2.7. Transthoracic Echocardiography
2.8. Cardiac Magnetic Resonance Imaging
2.9. Chest Computed Tomography
2.10. Pulmonary Function Test
2.11. Statistical Analysis
3. Results
3.1. Study Participants
3.2. Clinical Parameters
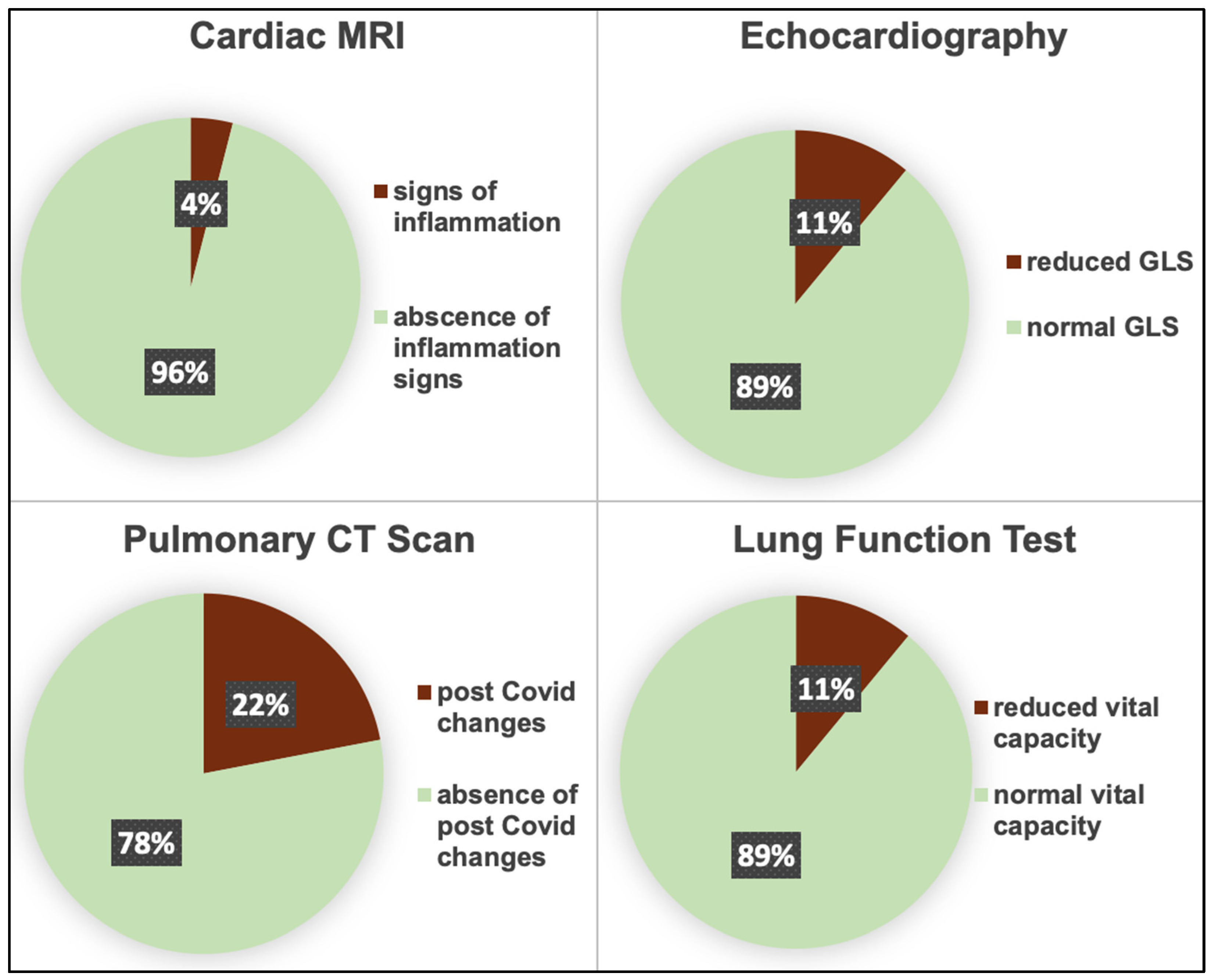
3.3. Imaging Parameters
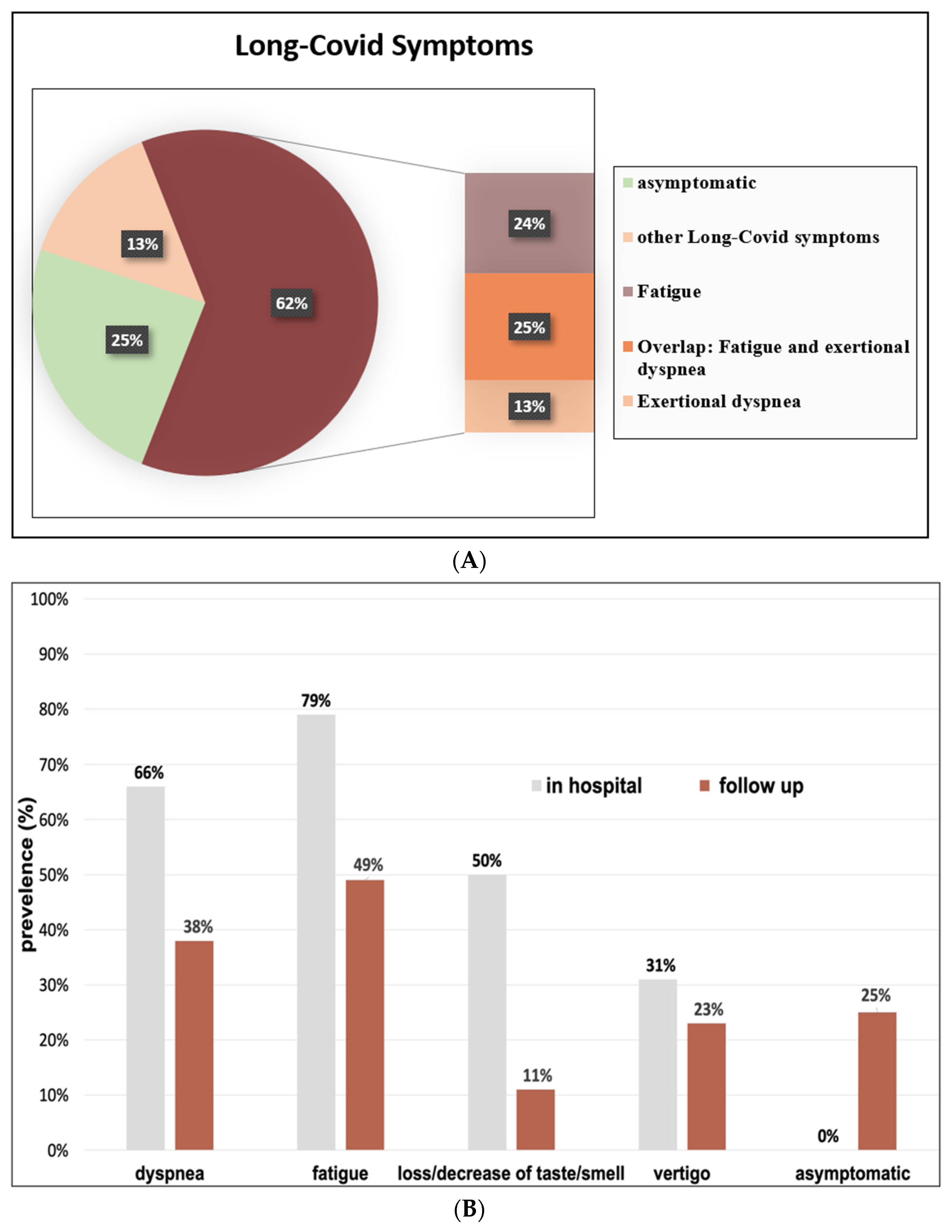
3.4. COVID-19-Related Symptoms
3.5. Long-COVID Risk Factors
3.6. Long-COVID Symptoms of Fatigue and Exertional Dyspnea in Relation to Organ Manifestations
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Di Fazio, N.; Delogu, G.; Bertozzi, G.; Fineschiw, V.; Frati, P. SARS-CoV-2 Vaccination Adverse Events Trend in Italy: A Retrospective Interpretation of the Last Year (December 2020–September 2021). Vaccines 2022, 10, 216. [Google Scholar] [CrossRef]
- Dixit, N.M.; Churchill, A.; Nsair, A.; Hsu, J.J. Post-Acute COVID-19 Syndrome and the cardiovascular system: What is known? Am. Heart J. Plus Cardiol. Res. Pract. 2021, 5, 100025. [Google Scholar] [CrossRef]
- De Sire, A.; Moggio, L.; Marotta, N.; Agostini, F.; Tasselli, A.; Ferrante, V.D.; Curci, C.; Calafiore, D.; Ferraro, F.; Bernetti, A.; et al. Impact of Rehabilitation on Fatigue in Post-COVID-19 Patients: A Systematic Review and Meta-Analysis. Appl. Sci. 2022, 12, 8593. [Google Scholar] [CrossRef]
- Wolf, S.; Erdos, E.J. Epidemiologie von Long-COVID: Ein Vorläufiger Bericht. Deutsche Kurzfassung zum Gleichnamigen KCE-Bericht; AIHTA-Projektberic 135a; AIHTA: Vienna, Austria, 2021. [Google Scholar]
- Huang, L.; Yao, Q.; Gu, X.; Wang, Q.; Ren, L.; Wang, Y.; Hu, P.; Guo, L.; Liu, M.; Xu, J.; et al. 1-year outcomes in hospital survivors with COVID-19: A longitudinal cohort study. Lancet 2021, 398, 747–758. [Google Scholar] [CrossRef] [PubMed]
- Kersten, J.; Wolf, A.; Hoyo, L.; Hüll, E.; Tadic, M.; Andreß, S.; D’Almeida, S.; Scharnbeck, D.; Roder, E.; Beschoner, P.; et al. Symptom burden correlates to impairment of diffusion capacity and exercise intolerance in long COVID patients. Sci. Rep. 2022, 12, 8801. [Google Scholar] [CrossRef] [PubMed]
- Maiese, A.; Frati, P.; Del Duca, F.; Santoro, P.; Manetti, A.C.; La Russa, R.; Di Paolo, M.; Turillazzi, E.; Fineschi, V. Myocardial Pathology in COVID-19-Associated Cardiac Injury: A Systematic Review. Diagnostics 2021, 11, 1647. [Google Scholar] [CrossRef]
- Raman, B.; Bluemke, D.A.; Lüscher, T.F.; Neubauer, S. Long COVID: Post-acute sequelae of COVID-19 with a cardiovascular focus. Eur. Heart J. 2022, 43, 1157–1172. [Google Scholar] [CrossRef]
- Ehrenfeld, M.; Tincani, A.; Andreoli, L.; Cattalini, M.; Greenbaum, A.; Kanduc, D.; Alijotas-Reig, J.; Zinserling, V.; Semenova, N.; Amital, H.; et al. COVID-19 and autoimmunity. Autoimmun. Rev. 2020, 19, 102597. [Google Scholar] [CrossRef]
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef]
- Taboada, M.; Cariñena, A.; Moreno, E.; Rodríguez, N.; Domínguez, M.J.; Casal, A.; Riveiro, V.; Diaz-Vieito, M.; Valdés, L.; Álvarez, J.; et al. Post-COVID-19 functional status six-months after hospitalization. J. Infect. 2021, 82, e31–e33. [Google Scholar] [CrossRef]
- Raman, B.; Cassar, M.P.; Tunnicliffe, E.M.; Filippini, N.; Griffanti, L.; Alfaro-Almagro, F.; Okell, T.; Sheerin, F.; Xie, C.; Mahmod, M.; et al. Medium-term effects of SARS-CoV-2 infection on multiple vital organs, exercise capacity, cognition, quality of life and mental health, post-hospital discharge. eClinicalMedicine 2021, 31, 100683. [Google Scholar] [CrossRef] [PubMed]
- Sivan, M.; Taylor, S. NICE guideline on long covid. BMJ 2020, 371, m4938. [Google Scholar] [CrossRef] [PubMed]
- Koczulla, A.; Ankermann, T.; Behrends, U. S1-Leitlinie Long/Post-COVID. Pneumologie 2022, 76, 855–907. [Google Scholar] [CrossRef] [PubMed]
- Brooks, D.; Solway, S.; Gibbons, W.J. ATS Statement: Guidelines for the six-minute walk test. Am. J. Respir. Crit. Care Med. 2002, 166, 111–117. [Google Scholar] [CrossRef]
- Voigt, J.-U.; Pedrizzetti, G.; Lysyansky, P.; Marwick, T.H.; Houle, H.; Baumann, R.; Pedri, S.; Ito, Y.; Abe, Y.; Metz, S.; et al. Definitions for a Common Standard for 2D Speckle Tracking Echocardiography: Consensus Document of the EACVI/ASE/Industry Task Force to Standardize Deformation Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Galderisi, M.; Cosyns, B.; Edvardsen, T.; Cardim, N.; Delgado, V.; Di Salvo, G.; Donal, E.; Sade, L.E.; Ernande, L.; Garbi, M.; et al. Standardization of adult transthoracic echocardiography reporting in agreement with recent chamber quantification, diastolic function, and heart valve disease recommendations: An expert consensus document of the European association of cardiovascular imaging. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 1301–1310. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef]
- Ramadan, M.S.; Bertolino, L.; Zampino, R.; Durante-Mangoni, E.; Iossa, D.; Ursi, M.P.; D‘Amico, F.; Karruli, A.; Andini, R.; Bernardo, M.; et al. Cardiac sequelae after coronavirus disease 2019 recovery: A systematic review. Clin. Microbiol. Infect. 2021, 27, 1250–1261. [Google Scholar] [CrossRef]
- Roca-Fernández, A.; Wamil, M.; Telford, A.; Carapella, V.; Borlotti, A.; Monteiro, D.; Thomaides-Brears, H.; Dennis, A.; Banerjee, R.; Robson, M.D.; et al. Cardiac impairment in Long COVID 1-year post-SARS-CoV-2 infection. Eur. Heart J. 2022, 43 (Suppl. S2), ehac544-219. [Google Scholar] [CrossRef]
- Puntmann, V.O.; Carerj, M.L.; Wieters, I.; Fahim, M.; Arendt, C.; Hoffmann, J.; Shchendrygina, A.; Escher, F.; Vasa-Nicotera, M.; Zeiher, A.M.; et al. Outcomes of Cardiovascular Magnetic Resonance Imaging in Patients Recently Recovered From Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 1265–1273. [Google Scholar] [CrossRef]
- Kotecha, T.; Knight, D.S.; Razvi, Y.; Kumar, K.; Vimalesvaran, K.; Thornton, G.; Patel, R.; Chacko, L.; Brown, J.T.; Coyle, C.; et al. Patterns of myocardial injury in recovered troponin-positive COVID-19 patients assessed by cardiovascular magnetic resonance. Eur. Heart J. 2021, 42, 1866–1878. [Google Scholar] [CrossRef] [PubMed]
- Tran, D.H.; Gupta, A.; Verceles, A.C.; Chow, R.D. COVID-19 Presenting as Recurrent Pericardial Effusion. Cureus 2021, 13, e18652. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Fan, Y.; Alwalid, O.; Li, N.; Jia, X.; Yuan, M.; Li, Y.; Cao, Y.; Gu, J.; Wu, H.; et al. Six-month Follow-up Chest CT Findings after Severe COVID-19 Pneumonia. Radiology 2021, 299, E177–E186. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Zhang, L.; Ni-Jia-Ti, M.-Y.; Zhang, J.; Hu, F.; Chen, L.; Dong, Y.; Yang, K.; Zhang, B.; Zhang, S. Anormal pulmonary function and residual CT abnormalities in rehabilitating COVID-19 patients after discharge. J. Infect. 2020, 81, e150–e152. [Google Scholar] [CrossRef] [PubMed]
- Mo, X.; Jian, W.; Su, Z.; Chen, M.; Peng, H.; Peng, P.; Lei, C.; Chen, R.; Zhong, N.; Li, S. Abnormal pulmonary function in COVID-19 patients at time of hospital discharge. Eur. Respir. J. 2020, 55, 2001217. [Google Scholar] [CrossRef]
- Peghin, M.; Palese, A.; Venturini, M.; De Martino, M.; Gerussi, V.; Graziano, E.; Bontempo, G.; Marrella, F.; Tommasini, A.; Fabris, M.; et al. Post-COVID-19 symptoms 6 months after acute infection among hospitalized and non-hospitalized patients. Clin. Microbiol. Infect. 2021, 27, 1507–1513. [Google Scholar] [CrossRef]
- Carfì, A.; Bernabei, R.; Landi, F. Persistent Symptoms in Patients After Acute COVID-19. JAMA 2020, 324, 603–605. [Google Scholar] [CrossRef]
- Aiyegbusi, O.L.; Hughes, S.E.; Turner, G.; Rivera, S.C.; McMullan, C.; Chandan, J.S.; Haroon, S.; Price, G.; Davies, E.H.; Nirantharakumar, K.; et al. Symptoms, complications and management of long COVID: A review. J. R. Soc. Med. 2021, 114, 428–442. [Google Scholar] [CrossRef]
- Groff, D.; Sun, A.; Ssentongo, A.E.; Ba, D.M.; Parsons, N.; Poudel, G.R.; Lekoubou, A.; Oh, J.S.; Ericson, J.E.; Ssentongo, P.; et al. Short-term and Long-term Rates of Postacute Sequelae of SARS-CoV-2 Infection. JAMA Netw. Open 2021, 4, e2128568. [Google Scholar] [CrossRef]
- Antonelli, M.; Penfold, R.S.; Merino, J.; Sudre, C.H.; Molteni, E.; Berry, S.; Canas, L.S.; Graham, M.S.; Klaser, K.; Modat, M.; et al. Risk factors and disease profile of post-vaccination SARS-CoV-2 infection in UK users of the COVID Symptom Study app: A prospective, community-based, nested, case-control study. Lancet Infect. Dis. 2022, 22, 43–55. [Google Scholar] [CrossRef]
- Sandler, C.X.; Wyller, V.B.B.; Moss-Morris, R.; Buchwald, D.; Crawley, E.; Hautvast, J.; Katz, B.Z.; Knoop, H.; Little, P.; Taylor, R.; et al. Long COVID and Post-infective Fatigue Syndrome: A Review. Open Forum Infect. Dis. 2021, 8, ofab440. [Google Scholar] [CrossRef] [PubMed]
- Ostojic, S. Diagnostic and Pharmacological Potency of Creatine in Post-Viral Fatigue Syndrome. Nutrients 2021, 13, 503. [Google Scholar] [CrossRef] [PubMed]
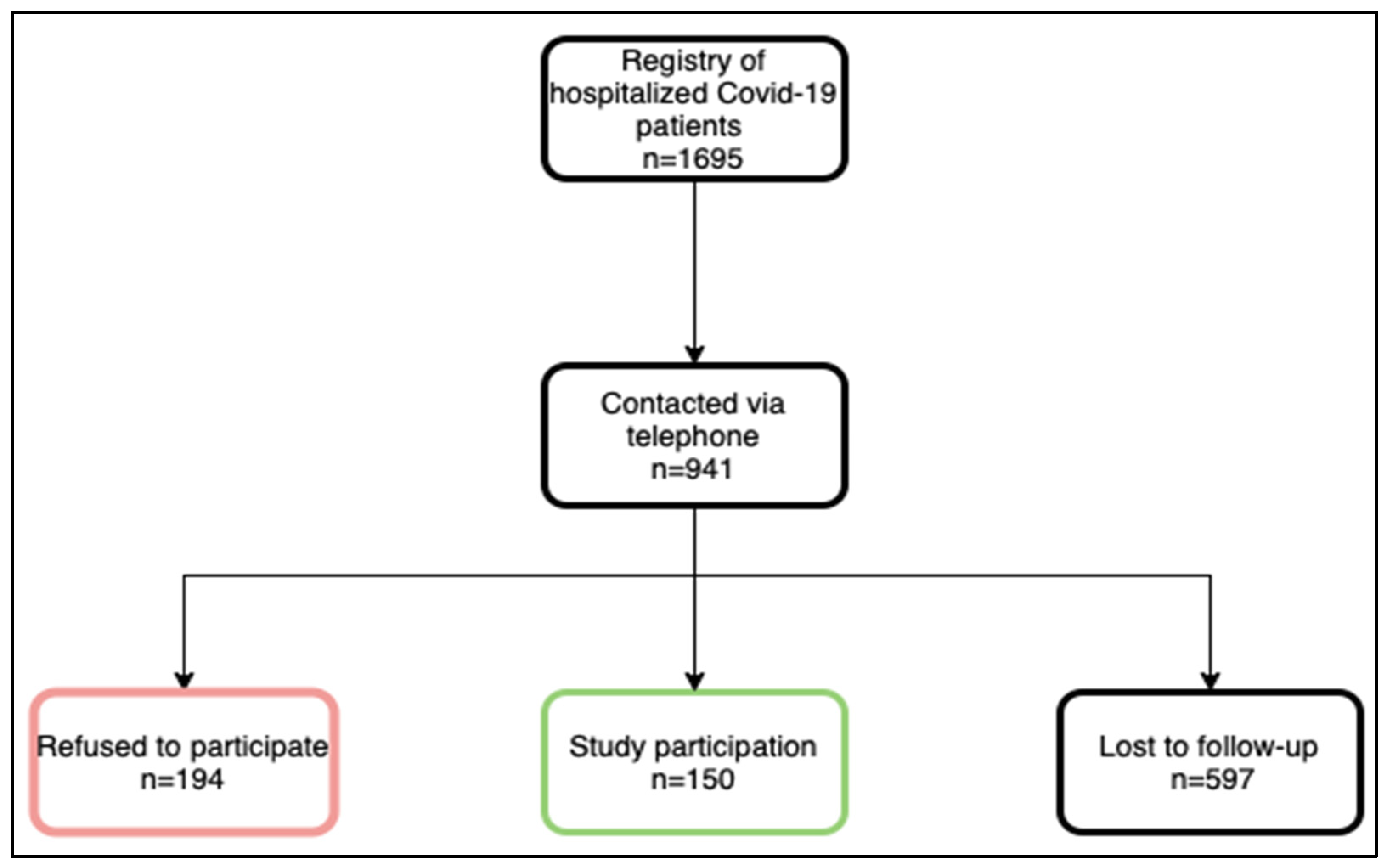
| Patient Characteristics | Total Study Population (n = 150) | Long-COVID (n = 113) | Asymptomatic at Follow-Up (n = 37) | p-Value |
|---|---|---|---|---|
| Age, years | 53.5 ± 14.49 | 56.48 ± 12.56 | 53.67 ± 12.80 | 0.37 |
| Female, % | 60.0 (40.0) | 48.0 (42.5) | 12.0 (32.4) | 0.28 |
| BMI, kg/m2 | 29.01 ± 5.44 | 29.75 ± 4.99 | 30.16 ± 4.80 | 0.73 |
| SBP, mmHg | 135.55 ± 19.21 | 137.52 ± 20.91 | 137.29 ± 19.44 | 0.47 |
| DBP, mmHg | 84.37 ± 10.94 | 83.97 ±12.27 | 85.79 ± 10.39 | 0.99 |
| Heart rate, bpm | 70.91 ± 9.81 | 70.60 ± 9.62 | 69.58 ± 9.82 | 0.57 |
| SpO2, % | 97.79 ± 1.20 | 97.62 ± 1.29 | 98.25 ± 1.15 | 0.03 |
| 6-MWT, m | 556.7 ± 99.30 | 549.6 ± 97.30 | 577.7 ± 104.10 | 0.23 |
| Arterial hypertension, % | 68 (45.3) | 53 (46.9) | 15 (40.5) | 0.50 |
| Coronary artery disease, % | 11 (7.3) | 10 (8.8) | 1 (2.7) | 0.213 |
| Pre-existing heart failure, % | 4 (2.7) | 4 (3.5) | 0 (0) | 0.25 |
| Overweight, % | 112 (75.7) | 85 (75.9) | 27 (75.0) | 0.91 |
| Diabetes mellitus, % | 29 (19.3) | 20 (17.7) | 9 (24.3) | 0.38 |
| COPD or Asthma, % | 16 (10.7) | 14 (12.4) | 2 (5.4) | 0.23 |
| Hemoglobin, g/dL | 15.15 ± 11.30 | 16.57 ± 17.70 | 14.86 ± 1.22 | 0.51 |
| eGFR, mL/min/1.73 m2 | 86.56 ± 21.42 | 88.08 ± 20.76 | 93.11 ± 17.08 | 0.25 |
| CRP, mg/dl | 0.25 ± 0.39 | 0.3 ± 0.52 | 0.27 ± 0.29 | 0.83 |
| NT-proBNP, pg/mL # | 1.79 ± 0.52 | 1.84 ± 0.51 | 1.62 ± 0.53 | 0.024 |
| Troponin T, ng/L # | 0.78 ± 0.28 | 0.81 ±0.27 | 0.70 ± 0.27 | 0.041 |
| CK, U/L | 139.1 ± 94.00 | 160 ± 121.9 | 132.2 ± 82.10 | 0.21 |
| Normal ward, % | 138 (92.0) | 101 (89.4) | 37 (100.0) | <0.00001 |
| Intensive care ward, % | 12 (8.0) | 12 (10.6) | 0 (0.0) | 0.039 |
| Length of in-hospital stay | 9 (5.0–12.0) | 9.0 (5.5–13.5) | 7.0 (4.5–9.0) | 0.001 |
| Ventilation | ||||
| No oxygen, % | 60 (40.0) | 41 (36.3) | 19 (51.4) | 0.12 |
| Oxygen, % | 61 (40.7) | 48 (42.5) | 13 (35.1) | 0.45 |
| Nasal high flow, % | 18 (12.0) | 13 (11.5) | 5 (13.5) | 0.77 |
| NIV, % | 2 (1.3) | 2 (1.8) | 0 (0.0) | 1.0 |
| Intubation, % | 9 (6.0) | 9 (8.0) | 0 (0.0) | 0.11 |
| Imaging Parameters | Total Study Population (n = 150) | Long-COVID (n = 113) | Asymptomatic at Follow-Up (n = 37) | p-Value |
|---|---|---|---|---|
| Cardiac organ manifestation (n, %) | 31 (20.7) | 26 (23.4) | 5 (13.8) | 0.25 |
| Echocardiography | ||||
| EF, % | 60.69 ± 6.71 | 60.51 ± 6.81 | 61.21 ± 6.34 | 0.60 |
| GLS, % | −18.77 ± 2.93 | −18.54 ± 2.78 | 19.47 ± 3.30 | 0.21 |
| Diastolic dysfunction, (n, %) | 6 (4.0) | 5 (4.7) | 1 (3.1) | 0.64 |
| Cardiac MRI | ||||
| EF, % | 59.12 ± 8.84 | 59.11 ± 9.76 | 59.35 ± 4.78 | 0.88 |
| LGE, (n, %) | 10 (6.7) | 3 (12.0) | 7 (8.4) | 0.69 |
| Myocarditis/pericarditis, (n, %) | 5 (4.6) | 4 (3.7) | 1 (0.9) | 0.93 |
| Pericardial effusion, (n, %) | 19 (18.0) | 5 (20.0) | 14 (16.8) | 0.71 |
| T1 time, ms | 1025.20 ± 108.17 | 1021.59 ± 121.43 | 1037.04 ± 41.49 | 0.38 |
| ECV, % | 25.63 ± 3.59 | 25.59 ± 3.86 | 25.73 ± 2.56 | 0.82 |
| Pulmonary organ manifestation (n, %) | 34 (23.6) | 27 (24.7) | 7 (20.0) | 0.65 |
| Chest CT | ||||
| Post COVID changes, (n, %) | 28 (21.5) | 23 (23.5) | 5 (15.6) | 0.35 |
| Pulmonary function test | ||||
| Reduced VC (n, %) | 13 (11) | 3 (11.2) | 10 (10.3) | 0.91 |
| Risk Factors for Long-COVID | p-Value # | Odds Ratio | 95% CI |
|---|---|---|---|
| Age | 0.07 | 1.03 | 0.998–1.05 |
| Gender | 0.28 | 0.65 | 0.30–1.42 |
| Admission to intensive care unit | 0.001 | - c | - c |
| Overweight | 0.91 | 1.05 | 0.44–2.50 |
| Previous illness | 0.56 | 1.27 | 0.57–2.83 |
| NT-proBNP a | 0.026 | 2.54 | 1.19–5.78 |
| Troponin T a | 0.054 | 2.04 | 0.999–4.17 |
| Ventilation | |||
| No oxygen | - | 1 b | - |
| Oxygen | 0.20 | 1.71 | 0.75–3.88 |
| Nasal high flow | 0.75 | 1.21 | 0.38–3.89 |
| NIV | 1.00 | - c | - c |
| Intubation | 1.00 | - c | - c |
| Length of in-hospital stay | 0.004 | 1.14 | 1.05–1.26 |
| Pericardial effusion in MRI | 0.72 | 0.81 | 0.26–2.53 |
| Reduced LVF in echocardiography | 0.57 | 1.89 | 0.21–16.8 |
| Normal GLS in echocardiography | - | 1.0 b | - |
| Borderline GLS in echocardiography | 0.25 | 2.0 | 0.61–6.59 |
| Reduced GLS in echocardiography | 0.37 | 1.87 | 0.48–7.2 |
| CT postcovid Changes | 0.35 | 1.66 | 0.57–4.79 |
| Reduced pulmonary function | 0.89 | 1.10 | 0.28–4.29 |
| Risk Factors for Exertional Dyspnea | p-Value # | Odds Ratio | 95% CI |
| Age | 0.11 | 1.02 | 0.996–1.04 |
| Gender | 0.15 | 0.61 | 0.31–1.12 |
| Admission to intensive care unit | 0.012 | 5.63 | 1.45–21.8 |
| Overweight | 0.001 | 5.37 | 1.95–14.8 |
| Previous illness | 0.22 | 1.61 | 0.75–3.43 |
| NT-proBNP a | 0.16 | 1.00 | 1.00–1.00 |
| Troponin T a | 0.78 | 1.08 | 0.60–1.93 |
| Ventilation | 0.02 | 5.1 | 1.13–22.6 |
| No oxygen | - | 1.0 b | - |
| Oxygen | 0.36 | 1.43 | 0.66–3.07 |
| Nasal high flow | 0.014 | 3.98 | 1.32–12.0 |
| NIV | 0.52 | 2.52 | 0.15–42.8 |
| Intubation | 0.034 | 5.06 | 1.13–22.6 |
| Length of in-hospital stay | 0.002 | 1.096 | 1.04–1.16 |
| Pericardial effusion in MRI | 0.87 | 1.1 | 0.39–3.1 |
| Reduced LVF in echocardiography | 0.04 | 9.76 | 1.11–86.7 |
| Normal GLS in echocardiography | - | 1 b | - |
| Borderline GLS in echocardiography | 0.33 | 0.57 | 0.19–1.74 |
| Reduced GLS in echocardiography | 0.005 | 5.24 | 1.64–16.7 |
| Diastolic dysfunction | 0.03 | 2.41 | 1.09–5.35 |
| CT postcovid Changes | 0.73 | 1.16 | 0.50–2.71 |
| Reduced pulmonary function | 0.028 | 3.62 | 1.15–11.4 |
| Risk factors for Fatigue | p-Value # | Odds Ratio | 95% CI |
| Age | 0.57 | 1.01 | 0.98–1.03 |
| Gender | 0.26 | 0.69 | 0.36–1.32 |
| Admission to intensive care unit | 0.22 | 2.18 | 0.63–7.58 |
| Overweight | 0.77 | 1.12 | 0.53–2.37 |
| Previous illness | 0.43 | 1.34 | 0.66–2.72 |
| NT-proBNP a | 0.55 | 1.09 | 0.83–1.43 |
| Troponin T a | 0.64 | 1.14 | 0.65–2.02 |
| Ventilation | |||
| No oxygen | - | 1.0 b | - |
| Oxygen | 0.17 | 1.65 | 0.80–3.39 |
| Nasal high flow | 0.30 | 1.75 | 0.61–5.06 |
| NIV | 1.0 | - c | - c |
| Intubation | 0.17 | 2.8 | 0.64–12.3 |
| Length of in-hospital stay | 0.021 | 1.06 | 1.01–1.11 |
| Reduced LVF in echocardiography | 0.94 | 0.94 | 0.18–4.83 |
| Normal GLS in echocardiography | - | 1 b | - |
| Borderline GLS in echocardiography | 0.052 | 2.5 | 0.96–6.50 |
| Reduced GLS in echocardiography | 0.061 | 2.75 | 0.91–8.29 |
| Diastolic dysfunction | 0.74 | 1.13 | 0.55–2.30 |
| CT postcovid Changes | 0.395 | 1.44 | 0.62–3.35 |
| Reduced pulmonary function | 0.91 | 0.94 | 0.32–2.796 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niebauer, J.H.; Binder-Rodriguez, C.; Iscel, A.; Schedl, S.; Capelle, C.; Kahr, M.; Cadjo, S.; Schamilow, S.; Badr-Eslam, R.; Lichtenauer, M.; et al. Cardiopulmonary Long-Term Sequelae in Patients after Severe COVID-19 Disease. J. Clin. Med. 2023, 12, 1536. https://doi.org/10.3390/jcm12041536
Niebauer JH, Binder-Rodriguez C, Iscel A, Schedl S, Capelle C, Kahr M, Cadjo S, Schamilow S, Badr-Eslam R, Lichtenauer M, et al. Cardiopulmonary Long-Term Sequelae in Patients after Severe COVID-19 Disease. Journal of Clinical Medicine. 2023; 12(4):1536. https://doi.org/10.3390/jcm12041536
Chicago/Turabian StyleNiebauer, Julia Hanne, Christina Binder-Rodriguez, Ahmet Iscel, Sarah Schedl, Christophe Capelle, Michael Kahr, Simona Cadjo, Simon Schamilow, Roza Badr-Eslam, Michael Lichtenauer, and et al. 2023. "Cardiopulmonary Long-Term Sequelae in Patients after Severe COVID-19 Disease" Journal of Clinical Medicine 12, no. 4: 1536. https://doi.org/10.3390/jcm12041536
APA StyleNiebauer, J. H., Binder-Rodriguez, C., Iscel, A., Schedl, S., Capelle, C., Kahr, M., Cadjo, S., Schamilow, S., Badr-Eslam, R., Lichtenauer, M., Toma, A., Zoufaly, A., Valenta, R., Hoffmann, S., Charwat-Resl, S., Krestan, C., Hitzl, W., Wenisch, C., & Bonderman, D. (2023). Cardiopulmonary Long-Term Sequelae in Patients after Severe COVID-19 Disease. Journal of Clinical Medicine, 12(4), 1536. https://doi.org/10.3390/jcm12041536







