Incidence and Prognostic Role of Pleural Effusion in Patients with Pulmonary Embolism: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection
2.3. Data Extraction and Quality Assessment
2.4. Statistical Analysis
3. Results
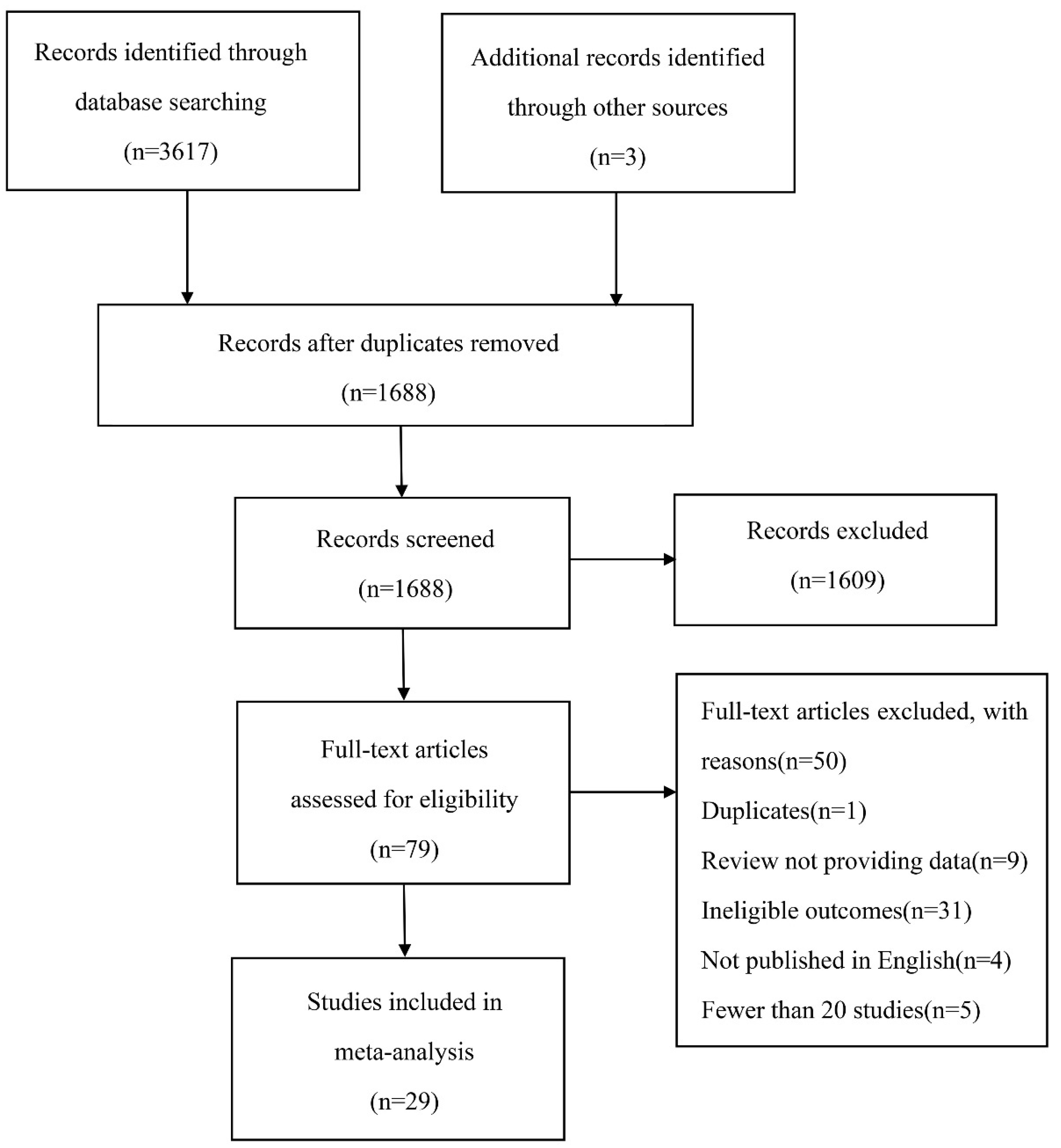
3.1. Study Selection and Characteristics
3.2. Risk of Bias
3.3. Pleural Effusion in PE
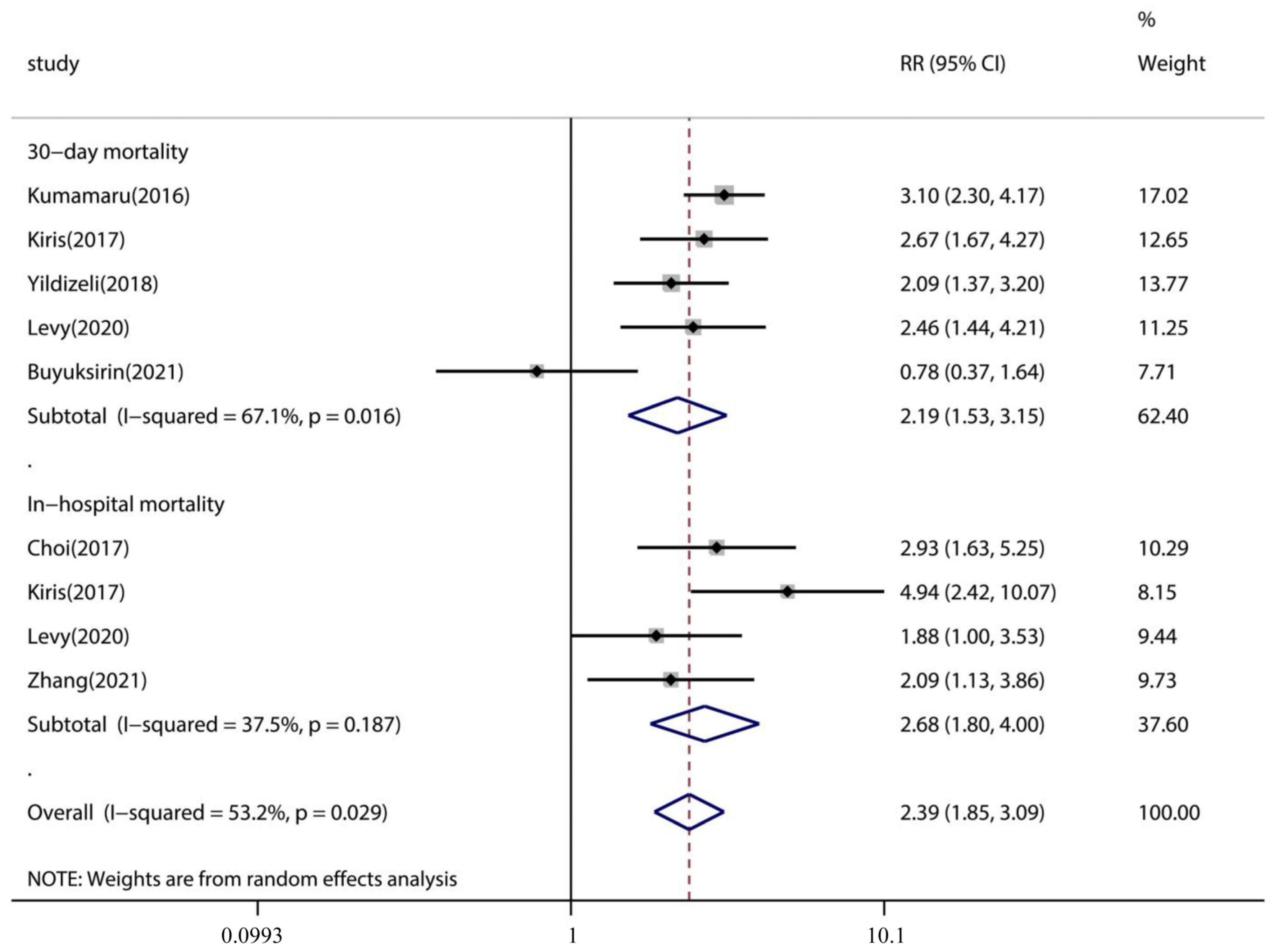
3.4. Association of Pleural Effusion with PE Patient Outcome
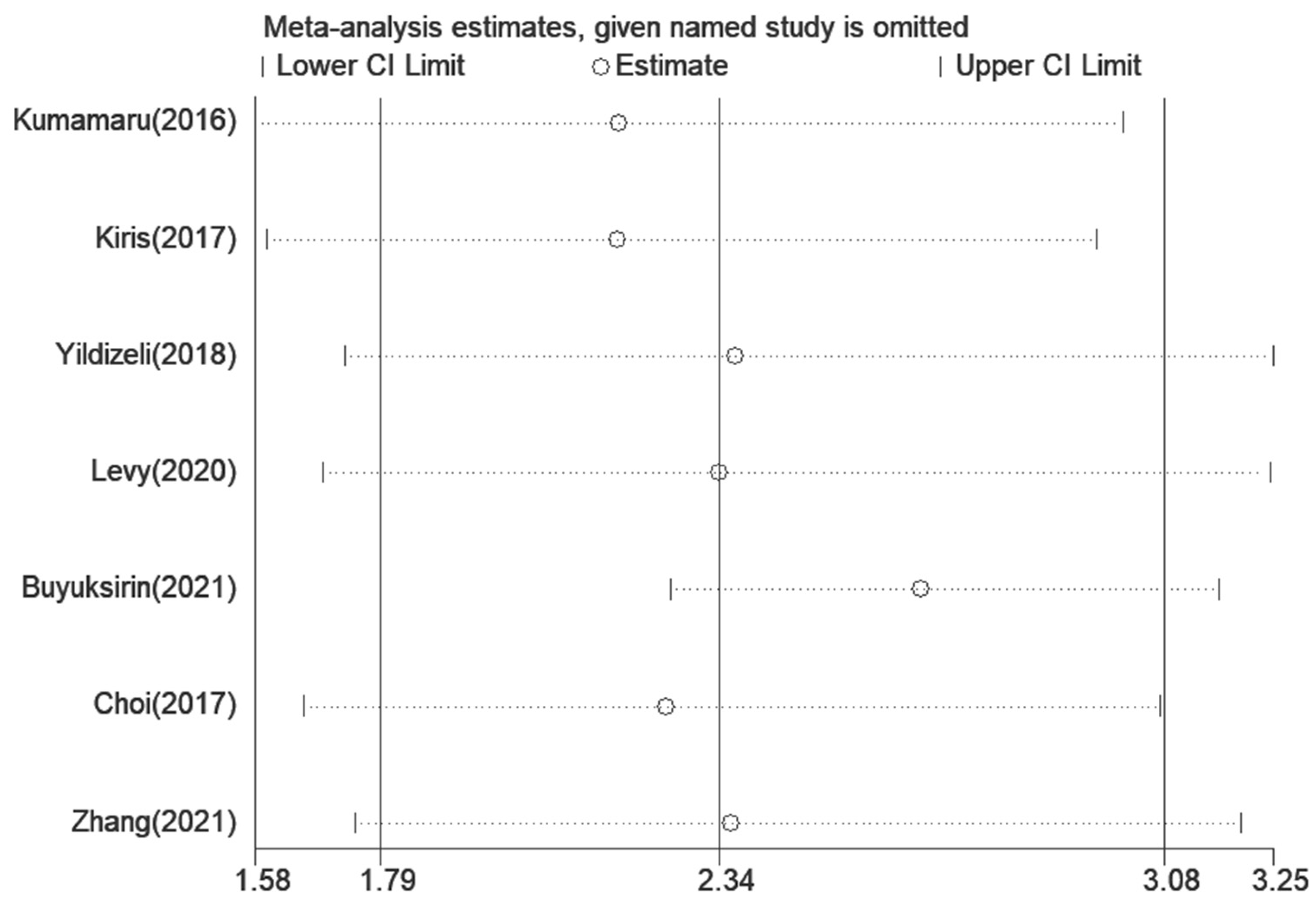
3.5. Assessment of Sensitivity Analysis and Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hepburn-Brown, M.; Darvall, J.; Hammerschlag, G. Acute pulmonary embolism: A concise review of diagnosis and management. Intern. Med. J. 2019, 49, 15–27. [Google Scholar] [CrossRef]
- Alikhan, R.; Peters, F.; Wilmott, R.; Cohen, A.T. Fatal pulmonary embolism in hospitalised patients: A necropsy review. J. Clin. Pathol. 2004, 57, 1254–1257. [Google Scholar] [CrossRef]
- Bikdeli, B.; Wang, Y.; Jimenez, D.; Parikh, S.A.; Monreal, M.; Goldhaber, S.Z.; Krumholz, H.M. Pulmonary Embolism Hospitalization, Readmission, and Mortality Rates in US Older Adults, 1999-2015. JAMA 2019, 322, 574–576. [Google Scholar] [CrossRef] [PubMed]
- Konstantinides, S.V.; Meyer, G.; Becattini, C.; Bueno, H.; Geersing, G.J.; Harjola, V.P.; Huisman, M.V.; Humbert, M.; Jennings, C.S.; Jiménez, D.; et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur. Heart J. 2020, 41, 543–603. [Google Scholar] [CrossRef]
- Furfaro, D.; Stephens, R.S.; Streiff, M.B.; Brower, R. Catheter-directed Thrombolysis for Intermediate-Risk Pulmonary Embolism. Ann. Am. Thorac. Soc. 2018, 15, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Itagaki, S.; Chiang, Y.P.; Egorova, N.N.; Adams, D.H.; Chikwe, J. Survival and recurrence after acute pulmonary embolism treated with pulmonary embolectomy or thrombolysis in New York State, 1999 to 2013. J. Thorac. Cardiovasc. Surg. 2018, 155, 1084–1090.e12. [Google Scholar] [CrossRef]
- Porcel, J.M.; Light, R.W. Pleural effusions due to pulmonary embolism. Curr. Opin. Pulm. Med. 2008, 14, 337–342. [Google Scholar] [CrossRef]
- Light, R.W. Clinical practice. Pleural effusion. N. Engl. J. Med. 2002, 346, 1971–1977. [Google Scholar] [CrossRef] [PubMed]
- Jany, B.; Welte, T. Pleural Effusion in Adults—Etiology, Diagnosis, and Treatment. Dtsch. Arztebl. Int. 2019, 116, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Light, R.W. Pleural effusion in pulmonary embolism. Semin. Respir. Crit. Care Med. 2010, 31, 716–722. [Google Scholar] [CrossRef]
- Findik, S. Pleural effusion in pulmonary embolism. Curr. Opin. Pulm. Med. 2012, 18, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhang, Z.; Zhai, Z.; Zhang, Y.; Miao, R.; Yang, Y.; Xie, W.; Wan, J.; Wang, C. Pleural effusions as a predictive parameter for poor prognosis for patients with acute pulmonary thromboembolism. J. Thromb. Thrombolysis 2016, 42, 432–440. [Google Scholar] [CrossRef]
- Calder, K.K.; Herbert, M.; Henderson, S.O. The mortality of untreated pulmonary embolism in emergency department patients. Ann. Emerg. Med. 2005, 45, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Wells, G.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. 2021. Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 30 September 2022).
- Ades, A.E.; Lu, G.; Higgins, J.P. The interpretation of random-effects meta-analysis in decision models. Med. Decis. Mak. Int. J. Soc. Med. Decis. Mak. 2005, 25, 646–654. [Google Scholar] [CrossRef]
- Chrysikos, S.; Papaioannou, O.; Karampitsakos, T.; Tavernaraki, K.; Thanou, I.; Filippousis, P.; Anyfanti, M.; Hillas, G.; Tzouvelekis, A.; Thanos, L.; et al. Diagnostic Accuracy of Multiple D-Dimer Cutoff Thresholds and Other Clinically Applicable Biomarkers for the Detection and Radiographic Evaluation of Pulmonary Embolism. Adv. Respir. Med. 2022, 90, 300–309. [Google Scholar] [CrossRef]
- Panjwani, A.; Zaid, T.; Alawi, S.; Al Shehabi, D.; Abdulkarim, E.S. Pleural effusion in acute pulmonary embolism in Bahrain: Radiological and pleural fluid characteristics. Lung India 2019, 36, 112–117. [Google Scholar] [CrossRef]
- Bynum, L.J.; Wilson, J.E., 3rd. Radiographic features of pleural effusions in pulmonary embolism. Am. Rev. Respir. Dis. 1978, 117, 829–834. [Google Scholar] [CrossRef]
- Stein, P.D.; Saltzman, H.A.; Weg, J.G. Clinical characteristics of patients with acute pulmonary embolism. Am. J. Cardiol. 1991, 68, 1723–1724. [Google Scholar] [CrossRef]
- Coche, E.E.; Müller, N.L.; Kim, K.I.; Wiggs, B.R.; Mayo, J.R. Acute pulmonary embolism: Ancillary findings at spiral CT. Radiology 1998, 207, 753–758. [Google Scholar] [CrossRef]
- Johnson, P.T.; Wechsler, R.J.; Salazar, A.M.; Fisher, A.M.; Nazarian, L.N.; Steiner, R.M. Spiral CT of acute pulmonary thromboembolism: Evaluation of pleuroparenchymal abnormalities. J. Comput. Assist. Tomogr. 1999, 23, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.A.; Davis, S.D.; Gamsu, G.; Intriere, L. Parenchymal and pleural findings in patients with and patients without acute pulmonary embolism detected at spiral CT. Radiology 1999, 211, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Elliott, C.G.; Goldhaber, S.Z.; Visani, L.; DeRosa, M. Chest radiographs in acute pulmonary embolism. Results from the International Cooperative Pulmonary Embolism Registry. Chest 2000, 118, 33–38. [Google Scholar] [CrossRef]
- Reissig, A.; Heyne, J.P.; Kroegel, C. Sonography of lung and pleura in pulmonary embolism: Sonomorphologic characterization and comparison with spiral CT scanning. Chest 2001, 120, 1977–1983. [Google Scholar] [CrossRef]
- Enden, T.; Kløw, N.E. CT pulmonary angiography and suspected acute pulmonary embolism. Acta Radiol. 2003, 44, 310–315. [Google Scholar] [CrossRef]
- Reibig, A.; Heyne, J.-P.; Kroegel, C. Ancillary lung parenchymal findings at spiral CT scanning in pulmonary embolism. Relationship to chest sonography. Eur. J. Radiol. 2004, 49, 250–257. [Google Scholar] [CrossRef]
- Lobo, J.L.; Zorrilla, V.; Aizpuru, F.; Uresandi, F.; Garcia-Bragado, F.; Conget, F.; Monreal, M. Clinical syndromes and clinical outcome in patients with pulmonary embolism: Findings from the RIETE registry. Chest 2006, 130, 1817–1822. [Google Scholar] [CrossRef]
- Porcel, J.M.; Madronero, A.B.; Pardina, M.; Vives, M.; Esquerda, A.; Light, R.W. Analysis of pleural effusions in acute pulmonary embolism: Radiological and pleural fluid data from 230 patients. Respirology 2007, 12, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Zubairi, A.B.; Husain, S.J.; Irfan, M.; Fatima, K.; Zubairi, M.A.; Islam, M. Chest radiographs in acute pulmonary embolism. JAMC: J. Ayub Med. Coll. Abbottabad. 2007, 19, 29–31. [Google Scholar]
- Karabulut, N.; Kiroğlu, Y. Relationship of parenchymal and pleural abnormalities with acute pulmonary embolism: CT findings in patients with and without embolism. Diagn. Interv. Radiol. 2008, 14, 189–196. [Google Scholar]
- Yap, E.; Anderson, G.; Donald, J.; Wong, C.A.; Lee, Y.C.; Sivakumaran, P. Pleural effusion in patients with pulmonary embolism. Respirology 2008, 13, 832–836. [Google Scholar] [CrossRef]
- Lee, E.Y.; Zurakowski, D.; Diperna, S.; d’Almeida Bastos, M.; Strauss, K.J.; Boiselle, P.M. Parenchymal and pleural abnormalities in children with and without pulmonary embolism at MDCT pulmonary angiography. Pediatr. Radiol. 2010, 40, 173–181. [Google Scholar] [CrossRef]
- Pfeil, A.; Schmidt, P.; Hermann, R.; Bottcher, J.; Wolf, G.; Hansch, A. Parenchymal and pleural findings in pulmonary embolism visualized by multi-channel detector computed tomography. Acta Radiol. 2010, 51, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Sandevski, A.; Jovkovska Kaeva, B.; Gligorovski, L.; Simonovska, L.; Sandevska, E. Frequency and characteristics of pleural effusions in pulmonary embolism. Contrib. Sec. Biol. Med. Sci. MASA 2012, 33, 93–104. [Google Scholar]
- Comert, S.S.; Caglayan, B.; Akturk, U.; Fidan, A.; Kiral, N.; Parmaksiz, E.; Salepci, B.; Kurtulus, B.A. The role of thoracic ultrasonography in the diagnosis of pulmonary embolism. Ann. Thorac. Med. 2013, 8, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Rad, M.P.; Tehrani, D.F.; Reihani, H.; Sabzevari, S.H.F.; Rajabi, M. Incidental Findings in Patients Evaluated for Pulmonary Embolism Using Computed Tomography Angiography. J. Cardio-Thorac. Med. 2014, 2, 162–166. [Google Scholar]
- Liu, M.; Cui, A.; Zhai, Z.G.; Guo, X.J.; Li, M.; Teng, L.L.; Xu, L.L.; Wang, X.J.; Wang, Z.; Shi, H.Z. Incidence of pleural effusion in patients with pulmonary embolism. Chin. Med. J. 2015, 128, 1032–1036. [Google Scholar] [CrossRef] [PubMed]
- Kumamaru, K.K.; Saboo, S.S.; Aghayev, A.; Cai, P.; Quesada, C.G.; George, E.; Hussain, Z.; Cai, T.; Rybicki, F.J. CT pulmonary angiography-based scoring system to predict the prognosis of acute pulmonary embolism. J. Cardiovasc. Comput. Tomogr. 2016, 10, 473–479. [Google Scholar] [CrossRef]
- Kiris, T.; Yazici, S.; Koc, A.; Koprulu, C.; Ilke Akyildiz, Z.; Karaca, M.; Nazli, C.; Dogan, A. Prognostic impact of pleural effusion in acute pulmonary embolism. Acta Radiol. 2017, 58, 816–824. [Google Scholar] [CrossRef]
- Choi, S.H.; Cha, S.I.; Shin, K.M.; Lim, J.K.; Yoo, S.S.; Lee, S.Y.; Lee, J.; Kim, C.H.; Park, J.Y.; Lee, D.H. Clinical Relevance of Pleural Effusion in Patients with Pulmonary Embolism. Respiration 2017, 93, 271–278. [Google Scholar] [CrossRef]
- Yildizeli, S.O.; Kasapoglu, U.S.; Arikan, H.; Cimsit, C.; Cimsit, N.C.; Suzer Aslan, M.; Kocakaya, D.; Eryuksel, E.; Ceyhan, B.; Karakurt, S. Pleural effusion as an indicator of short term mortality in acute pulmonary embolism. Tuberk Toraks 2018, 66, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Levy, O.; Fux, D.; Bartsikhovsky, T.; Vosko, S.; Tishler, M.; Copel, L. Clinical relevance of bilateral pleural effusion in patients with acute pulmonary embolism. Intern. Med. J. 2020, 50, 938–944. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhou, H.; Aili, A.; Wang, M.; Shen, Y.; Yi, Q. Prevalence and clinical significance of pleural effusion in patients with acute pulmonary embolism: A retrospective study. J. Thorac. Dis. 2021, 13, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Buyuksirin, M.; Anar, C.; Polat, G.; Karadeniz, G. Can the Level of CRP in Acute Pulmonary Embolism Determine Early Mortality? Turk. Thorac. J. 2021, 22, 4–10. [Google Scholar] [CrossRef]
- Roy, P.M.; Meyer, G.; Vielle, B.; Le Gall, C.; Verschuren, F.; Carpentier, F.; Leveau, P.; Furber, A. Appropriateness of diagnostic management and outcomes of suspected pulmonary embolism. Ann. Intern. Med. 2006, 144, 157–164. [Google Scholar] [CrossRef]
- Van Maanen, R.; Trinks-Roerdink, E.M.; Rutten, F.H.; Geersing, G.J. A systematic review and meta-analysis of diagnostic delay in pulmonary embolism. Eur. J. Gen. Pract. 2022, 28, 165–172. [Google Scholar] [CrossRef]
- Agarwal, R.; Singh, N.; Gupta, D. Pleural Effusions Associated with Pulmonary Thromboembolism: A Systematic Review. Indian J. Chest Dis. Allied Sci. 2009, 51, 159–164. [Google Scholar]
- Zuin, M.; Rigatelli, G.; Turchetta, S.; Pasquetto, G.; Roncon, L.; Bilato, C. Mortality Risk in Patients with Pulmonary Embolism with Pleural Effusion. Am. J. Cardiol. 2022, 179, 122–123. [Google Scholar] [CrossRef] [PubMed]
- Heyer, C.M.; Lemburg, S.P.; Knoop, H.; Holland-Letz, T.; Nicolas, V.; Roggenland, D. Multidetector-CT angiography in pulmonary embolism-can image parameters predict clinical outcome? Eur. Radiol. 2011, 21, 1928–1937. [Google Scholar] [CrossRef] [PubMed]
- Wiener-Kronish, J.P.; Broaddus, V.C.; Albertine, K.H.; Gropper, M.A.; Matthay, M.A.; Staub, N.C. Relationship of pleural effusions to increased permeability pulmonary edema in anesthetized sheep. J. Clin. Investig. 1988, 82, 1422–1429. [Google Scholar] [CrossRef] [PubMed]
- Brixey, A.G.; Light, R.W. Pleural effusions occurring with right heart failure. Curr. Opin. Pulm. Med. 2011, 17, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Alerhand, S.; Sundaram, T.; Gottlieb, M. What are the echocardiographic findings of acute right ventricular strain that suggest pulmonary embolism? Anaesth. Crit. Care Pain Med. 2021, 40, 100852. [Google Scholar] [CrossRef] [PubMed]


| Study/Year | Study Design | Country | No. of PE Patients/Pleural Effusion in PE | Short-Term Mortality | Location of Pleural Effusion (Unilateral/Bilateral) | Size of Pleural Effusion (Small/Moderate /Large) | Assessment of Pleural Effusion |
|---|---|---|---|---|---|---|---|
| Bynum (1978) | Prospective | USA | 155/62 | NA | 61/1 | NA/NA/NA | CXR |
| Stein (1991) | Prospective | USA | 383/180 | NA | NA/NA | NA/NA/NA | CXR |
| Coche (1998) | Retrospective | British | 26/13 | NA | 7/6 | NA/NA/NA | CT |
| Shah (1999) | Retrospective | USA | 28/16 | NA | 4/12 | NA/NA/NA | CT |
| Johnson (1999) | Retrospective | USA | 31/14 | NA | 4/10 | NA/NA/NA | CT |
| Elliott (2000) | Prospective | Europe and North America | 2319/523 | NA | NA/NA | NA/NA/NA | CXR |
| Reissig (2001) | Prospective | Germany | 44/26 | NA | NA/NA | NA/NA/NA | TS |
| Enden (2003) | Retrospective | Norway | 55/27 | NA | NA/NA | NA/NA/NA | CT |
| Reibig (2004) | Retrospective | Germany | 39/23 | NA | NA/NA | NA/NA/NA | TS |
| Lobo (2006) | Prospective | Spain, France, Italy, Israel, Argentina | 3391/628 | NA | NA/NA | NA/NA/NA | CXR |
| Porcel (2007) | Retrospective | Spain | 230/73 | NA | 62/11 | 66/3/4 | CXR |
| Zubairi (2007) | Retrospective | Pakistan | 50/12 | NA | NA/NA | NA/NA/NA | CXR |
| Yap (2008) | Retrospective | UK | 60/29 | NA | 23/6 | NA/NA/NA | CT |
| Karabulut (2008) | Retrospective | Turkey | 49/27 | NA | NA/NA | NA/NA/NA | CT |
| Lee (2010) | Retrospective | USA | 22/5 | NA | NA/NA | 4/1/0 | CT |
| Pfeil (2010) | Retrospective | Germany | 45/31 | NA | NA/NA | NA/NA/NA | CT |
| Sandevski (2012) | Retrospective | Macedonia | 100/31 | NA | 25/6 | 22/8/1 | CXR |
| Comert (2013) | Prospective | Turkey | 30/18 | NA | NA/NA | NA/NA/NA | TS |
| Rad (2014) | Retrospective | Iran | 56/30 | NA | NA/NA | NA/NA/NA | CT |
| Liu (2015) | Retrospective | China | 1220/243 | NA | 162/81 | NA/NA/NA | CT |
| Panjwani (2019) | Retrospective | Bahrain | 200/70 | NA | 26/44 | 58/9/3 | CT |
| Chrysikos (2022) | Retrospective | Greece | 190/78 | NA | 55/23 | NA/NA/NA | CT |
| Kumamaru (2016) | Retrospective | United States | 1698/841 | 30-day mortality (210) | NA/NA | NA/NA/NA | CT |
| Choi (2017) | Retrospective | Korea | 778/127 | In-hospital mortality (44) | NA/NA | 112/13/2 | CT |
| Kiris (2017) | Retrospective | Turkey | 463/120 | 30-day mortality (58), In-hospital mortality (30) | 56/64 | 99/20/1 | CT |
| Yildizeli (2018) | Retrospective | Turkey | 570/205 | 30-day mortality (74) | NA/NA | NA/NA/NA | CT |
| Levy (2020) | Retrospective | Israel | 343/177 | 30-day mortality (58), In-hospital mortality (39) | 83/94 | NA/NA/NA | CT |
| Buyuksirin (2021) | Retrospective | Turkey | 220/98 | 30-day mortality (26) | NA/NA | NA/NA/NA | CT |
| Zhang (2021) | Retrospective | China | 635/362 | In-hospital mortality (49) | NA/NA | NA/NA/NA | CT |
| Subgroups | No. Studies | Pooled Estimate (%) | 95% CI | p Value | I2 |
|---|---|---|---|---|---|
| Geographic location | |||||
| North America | 6 | 45.1 | 39.5–50.6 | p < 0.001 | 66.5% |
| Europe | 9 | 47.5 | 39.0–56.1 | p < 0.001 | 82.1% |
| Asia | 7 | 36.6 | 22.9–50.3 | p < 0.001 | 98.6% |
| Europe and Asia | 5 | 41.9 | 32.2–51.7 | p < 0.001 | 90.9% |
| Methods of pleural effusion assessment | |||||
| TS | 3 | 59.3 | 50.2–68.4 | p < 0.001 | 0.0% |
| CXR | 7 | 30.5 | 24.0–37.1 | p < 0.001 | 96.2% |
| CT | 19 | 43.0 | 35.3–50.7 | p < 0.001 | 97.7% |
| Study design | |||||
| Prospective | 6 | 38.0 | 29.7–46.4 | p < 0.001 | 97.3% |
| Retrospective | 23 | 41.7 | 34.9–48.6 | p < 0.001 | 97.2% |
| Subgroups | No. Studies | Pooled Estimate (%) | 95% CI (%) | p Value | I2 |
|---|---|---|---|---|---|
| 30-day mortality | |||||
| PE with pleural effusion | 5 | 18.9 | 14.9–22.9 | p < 0.001 | 65.9% |
| PE | 5 | 8.6 | 6.4–10.8 | p < 0.001 | 57.5% |
| In-hospital mortality | |||||
| PE with pleural effusion | 4 | 12.0 | 9.7–14.2 | p < 0.001 | 26.7% |
| PE | 4 | 4.3 | 3.2–5.3 | p < 0.001 | 30.4% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, P.; An, J.; Wang, S.; Hu, X.; Zeng, T.; Wan, C.; Shen, Y.; Wang, T. Incidence and Prognostic Role of Pleural Effusion in Patients with Pulmonary Embolism: A Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 12, 2315. https://doi.org/10.3390/jcm12062315
Li P, An J, Wang S, Hu X, Zeng T, Wan C, Shen Y, Wang T. Incidence and Prognostic Role of Pleural Effusion in Patients with Pulmonary Embolism: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2023; 12(6):2315. https://doi.org/10.3390/jcm12062315
Chicago/Turabian StyleLi, Ping, Jing An, Shuyan Wang, Xueru Hu, Tingting Zeng, Chun Wan, Yongchun Shen, and Tao Wang. 2023. "Incidence and Prognostic Role of Pleural Effusion in Patients with Pulmonary Embolism: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 12, no. 6: 2315. https://doi.org/10.3390/jcm12062315
APA StyleLi, P., An, J., Wang, S., Hu, X., Zeng, T., Wan, C., Shen, Y., & Wang, T. (2023). Incidence and Prognostic Role of Pleural Effusion in Patients with Pulmonary Embolism: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 12(6), 2315. https://doi.org/10.3390/jcm12062315






