Cryopreservation of Fetal Porcine Kidneys for Xenogeneic Regenerative Medicine
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Animals
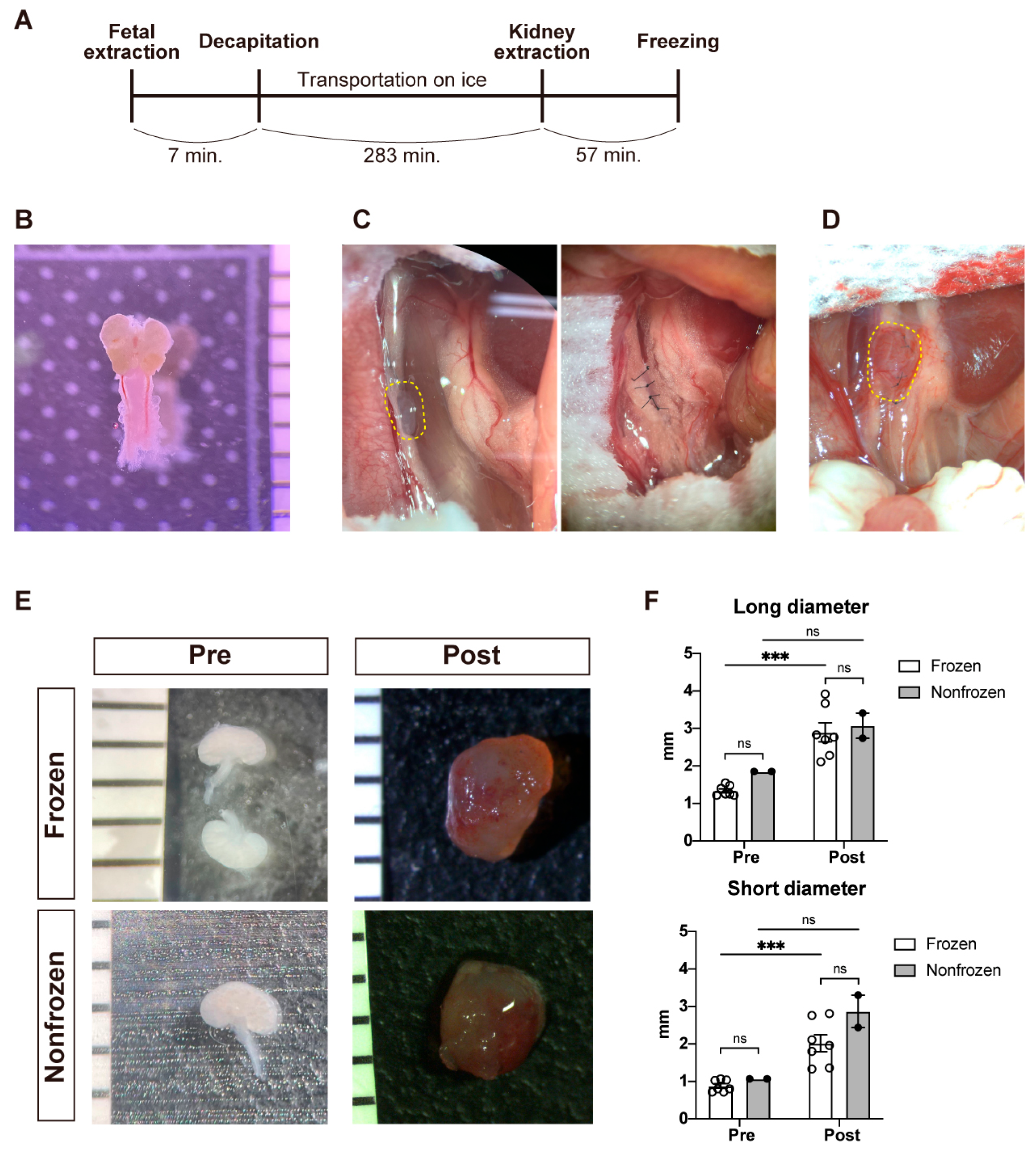
2.2. Collection of Fetal Porcine Kidneys
2.3. Cryopreservation of Fetal Porcine Kidneys
2.4. Thawing of Fetal Porcine Kidneys
2.5. Transplantation of Fetal Kidneys under the Retroperitoneum of NOG Mice
2.6. Hematoxylin-and-Eosin Staining and Immunostaining of Paraffin Sections
2.7. Statistical Analysis
3. Results
3.1. Gross Characteristics of the Fetal Porcine Kidneys before and after Transplantation
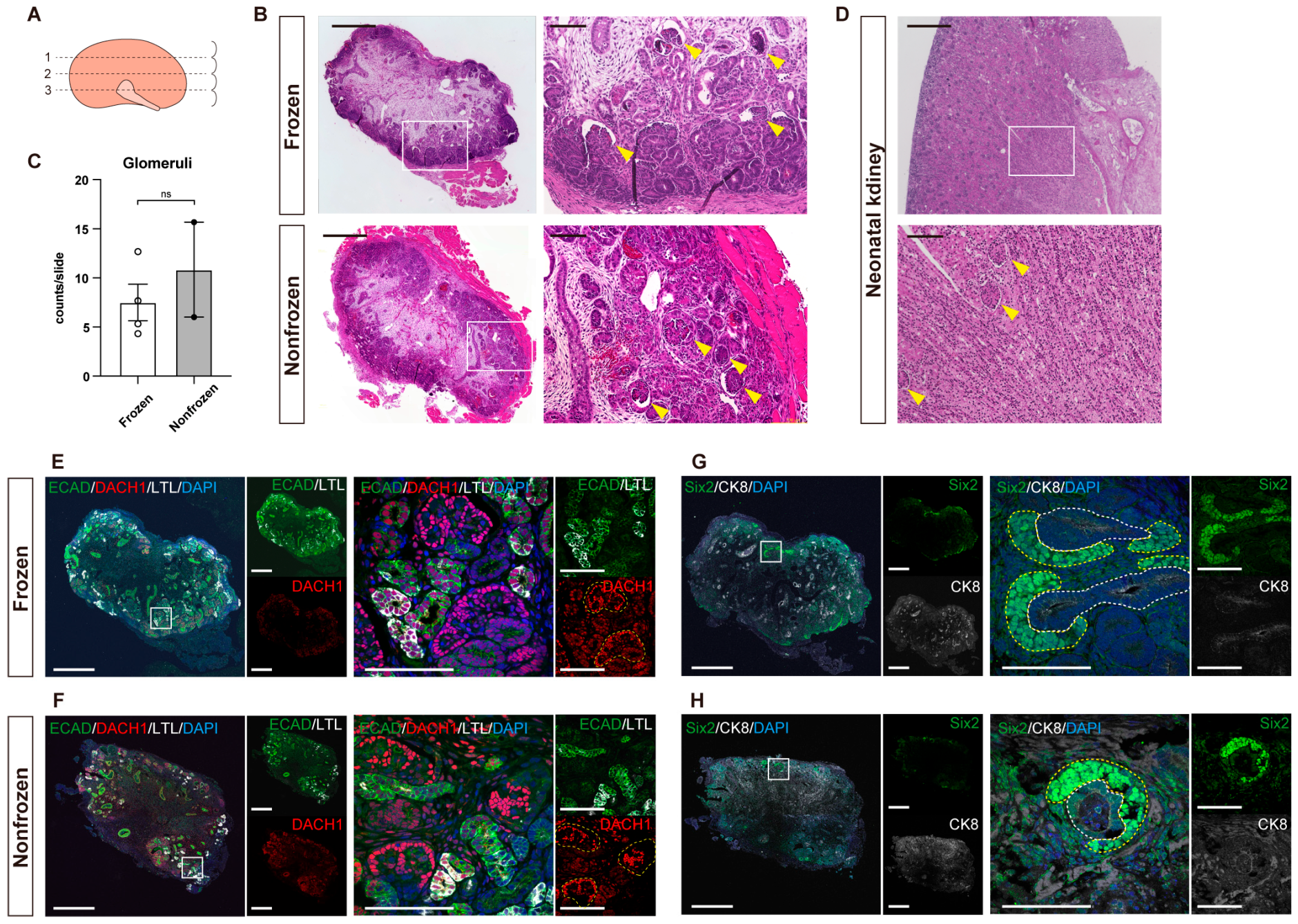
3.2. Quantitative Evaluation of Kidneys with Hematoxylin-and-Eosin Staining
3.3. Detailed Structural Evaluation by Immunostaining
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anderson, D.J.; Locke, J.E. Progress towards solving the donor organ shortage. Nat. Rev. Nephrol. 2022, 6, 83–84. [Google Scholar] [CrossRef] [PubMed]
- Porrett, P.M.; Orandi, B.J.; Kumar, V.; Houp, J.; Anderson, D.; Cozette Killian, A.; Hauptfeld-Dolejsek, V.; Martin, D.E.; Macedon, S.; Budd, N.; et al. First clinical-grade porcine kidney xenotransplant using a human decedent model. Am. J. Transplant. 2022, 22, 1037–1053. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, R.A.; Stern, J.M.; Lonze, B.E.; Tatapudi, V.S.; Mangiola, M.; Wu, M.; Weldon, E.; Lawson, N.; Deterville, C.; Dieter, R.A.; et al. Results of two cases of pig-to-human kidney xenotransplantation. N. Engl. J. Med. 2022, 386, 1889–1898. [Google Scholar] [CrossRef] [PubMed]
- Takamura, T.; Matsui, K.; Matsumoto, N.; Saito, Y.; Fujimoto, T.; Tajiri, S.; Yamanaka, S.; Matsumoto, K.; Kobayashi, A.; Yamamoto, I.; et al. In vivo development of fetal pig kidneys in mature monkeys under clinically approved immunosuppressant drugs. Engineering 2022, 10, 65–73. [Google Scholar] [CrossRef]
- Yamanaka, S.; Tajiri, S.; Fujimoto, T.; Matsumoto, K.; Fukunaga, S.; Kim, B.S.; Okano, H.J.; Yokoo, T. Generation of interspecies limited chimeric nephrons using a conditional nephron progenitor cell replacement system. Nat. Commun. 2017, 8, 1719. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, T.; Yamanaka, S.; Tajiri, S.; Takamura, T.; Saito, Y.; Matsumoto, N.; Matsumoto, K.; Tachibana, T.; Okano, H.J.; Yokoo, T. Generation of human renal vesicles in mouse organ niche using nephron progenitor cell replacement system. Cell Rep. 2020, 32, 108130. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Yamanaka, S.; Matsumoto, N.; Takamura, T.; Fujimoto, T.; Matsui, K.; Tajiri, S.; Matsumoto, K.; Kobayashi, E.; Yokoo, T. Generation of functional chimeric kidney containing exogenous progenitor-derived stroma and nephron via a conditional empty niche. Cell Rep. 2022, 39, 110933. [Google Scholar] [CrossRef]
- Saito, Y.; Matsumoto, N.; Yamanaka, S.; Yokoo, T.; Kobayashi, E. Beneficial impact of interspecies chimeric renal organoids against a xenogeneic immune response. Front. Immunol. 2022, 13, 848433. [Google Scholar] [CrossRef]
- Yokote, S.; Matsunari, H.; Iwai, S.; Yamanaka, S.; Uchikura, A.; Fujimoto, E.; Matsumoto, K.; Nagashima, H.; Kobayashi, E.; Yokoo, T. Urine excretion strategy for stem cell-generated embryonic kidneys. Proc. Natl. Acad. Sci. USA 2015, 112, 12980–12985. [Google Scholar] [CrossRef] [PubMed]
- Takamura, T.; Nagashima, H.; Matsunari, H.; Yamanaka, S.; Saito, Y.; Kinoshita, Y.; Fujimoto, T.; Matsumoto, K.; Nakano, K.; Okano, H.J.; et al. Development of a Cryopreservation Technique for Xenogeneic Kidney Grafts: Evaluation Using a Mouse Model. J. Clin. Med. 2022, 11, 7237. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.A. A method for cold storage and transport of viable embryonic kidney rudiments. Kidney Int. 2006, 70, 2031–2034. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Yokoo, T.; Matsunari, H.; Iwai, S.; Yokote, S.; Teratani, T.; Gheisari, Y.; Tsuji, O.; Okano, H.; Utsunomiya, Y.; et al. Xenotransplanted embryonic kidney provides a niche for endogenous mesenchymal stem cell differentiation into erythropoietin-producing tissue. Stem Cells 2012, 30, 1228–1235. [Google Scholar] [CrossRef] [PubMed]
- Groth, C.G.; Korsgren, O.; Tibell, A.; Tollemar, J.; Möller, E.; Bolinder, J.; Ostman, J.; Reinholt, F.P.; Hellerström, C.; Andersson, A. Transplantation of porcine fetal pancreas to diabetic patients. Lancet 1994, 344, 1402–1404. [Google Scholar] [CrossRef] [PubMed]
- Scudellari, M. Core Concept: Cryopreservation aims to engineer novel ways to freeze, store, and thaw organs. Proc. Natl. Acad. Sci. USA 2017, 114, 13060–13062. [Google Scholar] [CrossRef] [PubMed]
- Borini, A.; Bianchi, V. Cryopreservation of mature and immature oocytes. Clin. Obstet. Gynecol. 2010, 53, 763–774. [Google Scholar] [CrossRef] [PubMed]
- Rall, W.F.; Fahy, G.M. Ice-free cryopreservation of mouse embryos at -196 degrees C by vitrification. Nature 1985, 313, 573–575. [Google Scholar] [CrossRef] [PubMed]
- Kagawa, N.; Silber, S.; Kuwayama, M. Successful vitrification of bovine and human ovarian tissue. Reprod. Biomed. Online 2009, 18, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Naganuma, H.; Miike, K.; Ohmori, T.; Tanigawa, S.; Ichikawa, T.; Yamane, M.; Eto, M.; Niwa, H.; Kobayashi, A.; Nishinakamura, R. Molecular detection of maturation stages in the developing kidney. Dev. Biol. 2021, 470, 62–73. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsui, K.; Kinoshita, Y.; Inage, Y.; Matsumoto, N.; Morimoto, K.; Saito, Y.; Takamura, T.; Matsunari, H.; Yamanaka, S.; Nagashima, H.; et al. Cryopreservation of Fetal Porcine Kidneys for Xenogeneic Regenerative Medicine. J. Clin. Med. 2023, 12, 2293. https://doi.org/10.3390/jcm12062293
Matsui K, Kinoshita Y, Inage Y, Matsumoto N, Morimoto K, Saito Y, Takamura T, Matsunari H, Yamanaka S, Nagashima H, et al. Cryopreservation of Fetal Porcine Kidneys for Xenogeneic Regenerative Medicine. Journal of Clinical Medicine. 2023; 12(6):2293. https://doi.org/10.3390/jcm12062293
Chicago/Turabian StyleMatsui, Kenji, Yoshitaka Kinoshita, Yuka Inage, Naoto Matsumoto, Keita Morimoto, Yatsumu Saito, Tsuyoshi Takamura, Hitomi Matsunari, Shuichiro Yamanaka, Hiroshi Nagashima, and et al. 2023. "Cryopreservation of Fetal Porcine Kidneys for Xenogeneic Regenerative Medicine" Journal of Clinical Medicine 12, no. 6: 2293. https://doi.org/10.3390/jcm12062293
APA StyleMatsui, K., Kinoshita, Y., Inage, Y., Matsumoto, N., Morimoto, K., Saito, Y., Takamura, T., Matsunari, H., Yamanaka, S., Nagashima, H., Kobayashi, E., & Yokoo, T. (2023). Cryopreservation of Fetal Porcine Kidneys for Xenogeneic Regenerative Medicine. Journal of Clinical Medicine, 12(6), 2293. https://doi.org/10.3390/jcm12062293





