Peritoneal Mesothelioma in a High Volume Peritoneal Surface Malignancies Unit
Abstract
1. Introduction
2. Materials and Methods
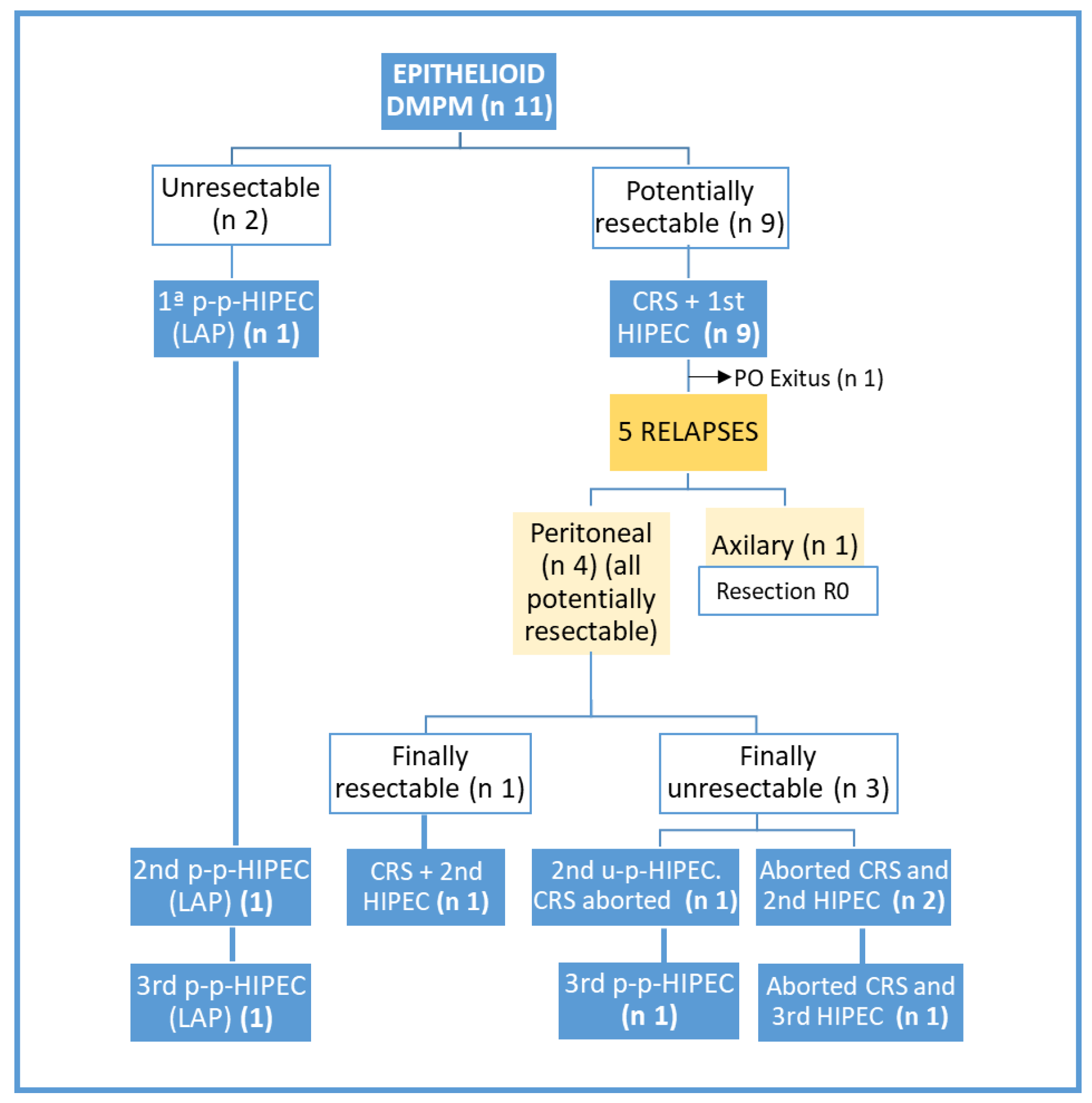
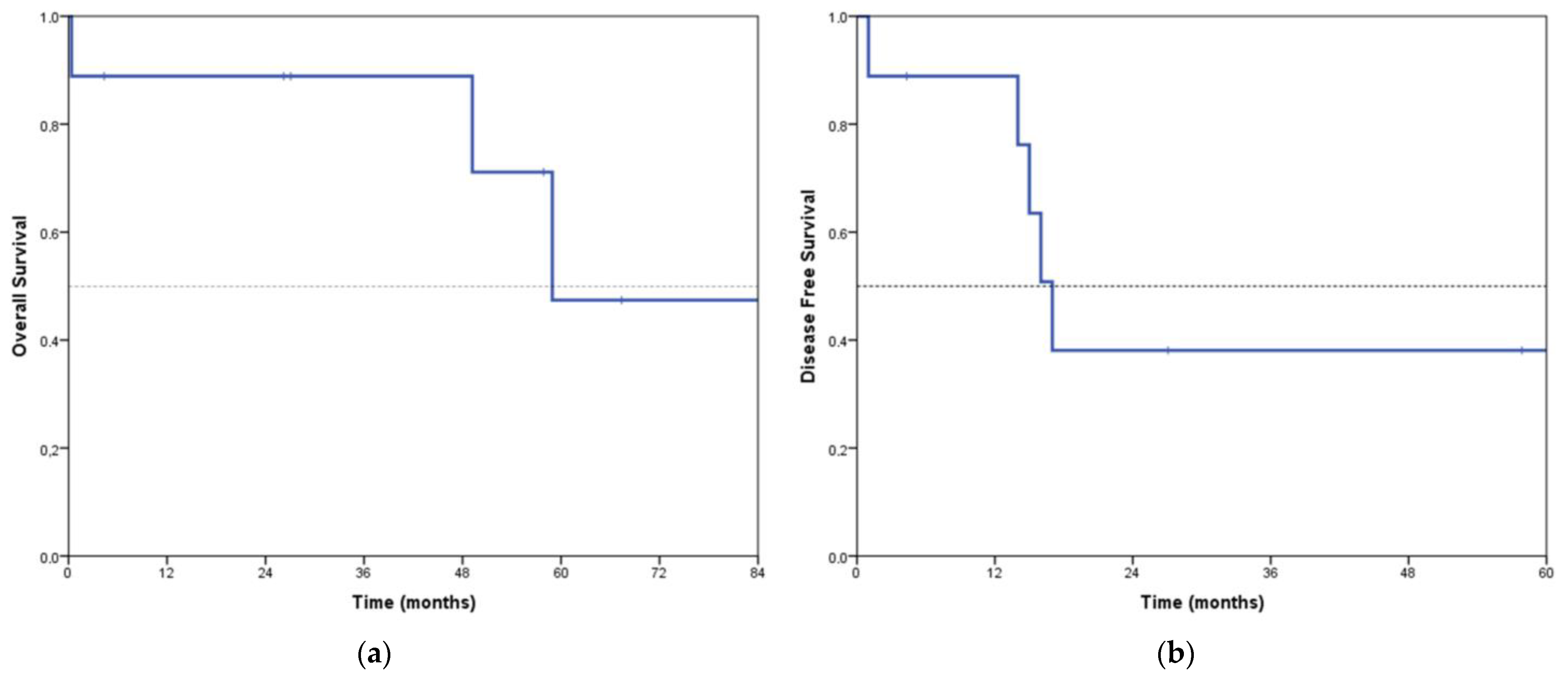
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boffetta, P. Epidemiology of peritoneal mesothelioma: A review. Ann. Oncol. 2007, 18, 985–990. [Google Scholar] [CrossRef] [PubMed]
- Moolgavkar, S.H.; Meza, R.; Turim, J. Pleural and peritoneal mesotheliomas in SEER: Age effects and temporal trends, 1973–2005. Cancer Causes Control 2009, 20, 935–944. [Google Scholar] [CrossRef] [PubMed]
- Deraco, M.; Bartlett, D.; Kusamura, S.; Baratti, D. Consensus statement on peritoneal mesothelioma. J. Surg. Oncol. 2008, 98, 268–272. [Google Scholar] [CrossRef]
- Janne, P.A.; Wozniak, A.J.; Belani, C.P.; Keohan, M.-L.; Ross, H.J.; Polikoff, J.A.; Mintzer, D.M.; Taylor, L.; Ashland, J.; Ye, Z.; et al. Open-label study of pemetrexed alone or in combination with cisplatin for the treatment of patients with peritoneal mesothelioma: Outcomes of an expanded access program. Clin. Lung Cancer 2005, 7, 40–46. [Google Scholar] [CrossRef]
- Yan, T.D.; Deraco, M.; Baratti, D.; Kusamura, S.; Elias, D.; Glehen, O.; Gilly, F.N.; Levine, E.A.; Shen, P.; Mohamed, F.; et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for malignant peritoneal mesothelioma: Multi-institutional experience. J. Clin. Oncol. 2009, 27, 6237–6242. [Google Scholar] [CrossRef]
- Greenbaum, A.; Alexander, H.R. Peritoneal mesothelioma. Transl. Lung Cancer Res. 2020, 9 (Suppl. S1), S120–S132. [Google Scholar] [CrossRef] [PubMed]
- Kusamura, S.; Kepenekian, V.; Villeneuve, L.; Lurvink, R.; Govaerts, K.; De Hingh, I.; Moran, B.; Van der Speeten, K.; Deraco, M.; Glehen, O.; et al. Peritoneal mesothelioma: PSOGI/EURACAN clinical practice guidelines for diagnosis, treatment and follow-up. Eur. J. Surg. Oncol. 2021, 47, 36–59. [Google Scholar] [CrossRef]
- Valle, M.; Van der Speeten, K.; Garofalo, A. Laparoscopic hyperthermic intraperitoneal peroperative chemotherapy (HIPEC) in the management of refractory malignant ascites: A multi-institutional retrospective analysis in 52 patients. J. Surg. Oncol. 2009, 100, 331–334. [Google Scholar] [CrossRef]
- Facchiano, E.; Risio, D.; Kianmanesh, R.; Msika, S. Laparoscopic hyperthermic intraperitoneal chemotherapy: Indications, aims, and results: A systematic review of the literature. Ann. Surg. Oncol. 2012, 19, 2946–2950. [Google Scholar] [CrossRef]
- Vogin, G.; Network, T.R.; Hettal, L.; Vignaud, J.-M.; Dartigues, P.; Goere, D.; Ferron, G.; Heyd, B.; Bereder, J.-M.; Tuech, J.-J.; et al. Well-Differentiated Papillary Mesothelioma of the Peritoneum: A Retrospective Study from the RENAPE Observational Registry. Ann. Surg. Oncol. 2019, 26, 852–860. [Google Scholar] [CrossRef]
- Noiret, B.; Renaud, F.; Piessen, G.; Eveno, C. Multicystic peritoneal mesothelioma: A systematic review of the literature. Pleura Peritoneum 2019, 4, 20190024. [Google Scholar] [CrossRef] [PubMed]
- Sugarbaker, P.H.; Chang, D. Long-term regional chemotherapy for patients with epithelial malignant peritoneal mesothelioma results in improved survival. Eur. J. Surg. Oncol. 2017, 43, 1228–1235. [Google Scholar] [CrossRef]
- Baratti, D.; Kusamura, S.; Cabras, A.D.; Bertulli, R.; Hutanu, I.; Deraco, M. Diffuse malignant peritoneal mesothelioma: Long-term survival with complete cytoreductive surgery followed by hyperthermic intraperitoneal chemotherapy (HIPEC). Eur. J. Cancer 2013, 49, 3140–3148. [Google Scholar] [CrossRef] [PubMed]
- Gomez Portilla, A.; Cendoya, I.; Muriel, J.; Olabarria, I.; Guede, N.; Moraza, N.; Fernández, E.; Martínez de Lecea, C.; Magrach, L.; Martín, E.; et al. Malignant peritoneal mesothelioma. Our experienced with triple combined therapy: Cytoreduction, intraperitoneal perioperative chemotherapy and hyperthermia. Cir. Esp. 2007, 81, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Alexander, H.R., Jr.; Bartlett, D.L.; Pingpank, J.F.; Libutti, S.K.; Royal, R.; Hughes, M.S.; Holtzman, M.; Hanna, N.; Turner, K.; Beresneva, T.; et al. Treatment factors associated with long-term survival after cytoreductive surgery and regional chemotherapy for patients with malignant peritoneal mesothelioma. Surgery 2013, 153, 779–786. [Google Scholar] [CrossRef]
- Helm, J.H.; Miura, J.T.; Glenn, J.A.; Marcus, R.K.; Larrieux, G.; Jayakrishnan, T.T.; Donahue, A.E.; Gamblin, T.C.; Turaga, K.K.; Johnston, F.M. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for malignant peritoneal mesothelioma: A systematic review and meta-analysis. Ann. Surg. Oncol. 2015, 22, 1686–1693. [Google Scholar] [CrossRef]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Seshadri, R.A.; Hemanth Raj, E. Diagnostic Laparoscopy in the Pre-operative Assessment of Patients Undergoing Cytoreductive Surgery and HIPEC for Peritoneal Surface Malignancies. Indian J. Surg. Oncol. 2016, 7, 230–235. [Google Scholar] [CrossRef]
- Passot, G.; Dumont, F.; Goéré, D.; Arvieux, C.; Rousset, P.; Regimbeau, J.; Elias, D.; Villeneuve, L.; Glehen, O.; Abba, J.; et al. Multicentre study of laparoscopic or open assessment of the peritoneal cancer index (BIG-RENAPE). Br. J. Surg. 2018, 105, 663–667. [Google Scholar] [CrossRef]
- Chua, T.C.; Yan, T.D.; Saxena, A.; Morris, D.L. Should the treatment of peritoneal carcinomatosis by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy still be regarded as a highly morbid procedure?: A systematic review of morbidity and mortality. Ann. Surg. 2009, 249, 900–907. [Google Scholar]
- Malgras, B.; Gayat, E.; Aoun, O.; Lo Dico, R.; Eveno, C.; Pautrat, K.; Delhorme, J.B.; Passot, G.; Marchal, F.; Sgarbura, O.; et al. Impact of Combination Chemotherapy in Peritoneal Mesothelioma Hyperthermic Intraperitoneal Chemotherapy (HIPEC): The RENAPE Study. Ann. Surg. Oncol. 2018, 25, 3271–3279. [Google Scholar] [CrossRef] [PubMed]
- Deraco, M.; Baratti, D.; Hutanu, I.; Bertuli, R.; Kusamura, S. The role of perioperative systemic chemotherapy in diffuse malignant peritoneal mesothelioma patients treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann. Surg. Oncol. 2013, 20, 1093–1100. [Google Scholar] [CrossRef] [PubMed]
- Kepenekian, V.; Elias, D.; Passot, G.; Mery, E.; Goere, D.; Delroeux, D.; Quenet, F.; Ferron, G.; Pezet, D.; Guilloit, J.M.; et al. Diffuse malignant peritoneal mesothelioma: Evaluation of systemic chemotherapy with comprehensive treatment through the RENAPE Database: Multi-Institutional Retrospective Study. Eur. J. Cancer 2016, 65, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Le Roy, F.; Gelli, M.; Hollebecque, A.; Honoré, C.; Boige, V.; Dartigues, P.; Benhaim, L.; Malka, D.; Ducreux, M.; Elias, D.; et al. Conversion to Complete Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy for Malignant Peritoneal Mesothelioma After Bidirectional Chemotherapy. Ann. Surg. Oncol. 2017, 24, 3640–3646. [Google Scholar] [CrossRef] [PubMed]
- Alyami, M.; Mercier, F.; Siebert, M.; Bonnot, P.E.; Laplace, N.; Villeneuve, L.; Passot, G.; Glehen, O.; Bakrin, N.; Kepenekian, V. Unresectable peritoneal metastasis treated by pressurized intraperitoneal aerosol chemotherapy (PIPAC) leading to cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Eur. J. Surg. Oncol. 2021, 47, 128–133. [Google Scholar] [CrossRef]
- Sgarbura, O.; Gourgou, S.; Tosi, D.; Bakrin, N.; Bouazza, N.; Delaine, S.; De Forges, H.; Pocard, M.; Quénet, F. MESOTIP: Phase II multicenter randomized trial evaluating the association of PIPAC and systemic chemotherapy vs. systemic chemotherapy alone as 1st-line treatment of malignant peritoneal mesothelioma. Pleura Peritoneum 2019, 4, 20190010. [Google Scholar] [CrossRef]
- Votanopoulos, K.I.; Sugarbaker, P.; Deraco, M.; Morris, D.; Glehen, O.; Elias, D.; De Simone, M.; Robella, M.; Heyd, B.; Kusamura, S.; et al. Is Cytoreductive Surgery with Hyperthermic Intraperitoneal Chemotherapy Justified for Biphasic Variants of Peritoneal Mesothelioma? Outcomes from the Peritoneal Surface Oncology Group International Registry. Ann. Surg. Oncol. 2018, 25, 667–673. [Google Scholar] [CrossRef]
- Alyami, M.; Hübner, M.; Grass, F.; Bakrin, N.; Villeneuve, L.; Laplace, N.; Passot, G.; Glehen, O.; Kepenekian, V. Pressurised intraperitoneal aerosol chemotherapy: Rationale, evidence, and potential indications. Lancet Oncol. 2019, 20, e368–e377. [Google Scholar] [CrossRef]
| Multicystic | Biphasic | Epithelioid | |
|---|---|---|---|
| N° of patients | 3 | 3 | 11 |
| Origin: our center/other centers | 1/2 | 2/1 | 2/9 |
| Age (median) | 51 | 64 | 57 |
| Sex (female/male) | 3/0 | 1/2 | 5/6 |
| Asbestos exposure | 0 | 0 | 1 |
| Previous abdominal surgeries | 1 | 0 | 1 |
| Unresectable | 0 | 3 | 2 |
| Procedures | Number |
|---|---|
| Major omentectomy | 9 |
| Appendectomy | 7 |
| Right diaphragm peritonectomy | 6 |
| Left diaphragm peritonectomy | 4 |
| Morrison peritonectomy | 4 |
| Hepatoduodenal ligament | 4 |
| Lateral parietal peritonectomy | 4 |
| Pelvic peritonectomy | 4 |
| Cholecystectomy | 4 |
| Splenectomy | 3 |
| Anterior parietal peritonectomy | 3 |
| Right hemicolectomy | 3 |
| Total hysterectomy | 1 |
| Bilateral salpingo−oophorectomy | 1 |
| Anterior resection of rectum | 1 |
| Superior recess of the omental bursa | 1 |
| Hepatic capsulectomy (partial) | 1 |
| Small bowel resection | 1 |
| Electrofulgurations * | 5 |
| Multicystic (n = 3) | Epithelioid (n = 10) | Total (n = 13) | |
|---|---|---|---|
| Staging laparoscopy | 0 | 7 | * |
| Neoadj treatment | NA | 7 | * |
| surgical PCI, median (range) | 18 (8–21) | 14 (4–25) | 15 (4–25) |
| Duration (min), median (range) | 350 (240–350) | 360 (300–510) | 360 (240–510) |
| Transfusion | 0 | 1 | 1 |
| Complic grade ≥ III | 0 | 2 | 2 (15.3%) |
| Complic any grade | 1 | 6 | 7 (53.8%) |
| Reoperation | 0 | 1 | 1 |
| Mortality | 0 | 1 | 1 (7.6%) |
| Length of stay (days), median (range) | 7 (7–13) | 11 (6–30) | 10 (6–30) |
| Adjuvant treatment | NA | 4 | * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, F.; Pereira, M.; Manzanedo, I.; Serrano, Á.; Pérez-Viejo, E. Peritoneal Mesothelioma in a High Volume Peritoneal Surface Malignancies Unit. J. Clin. Med. 2023, 12, 2288. https://doi.org/10.3390/jcm12062288
Pereira F, Pereira M, Manzanedo I, Serrano Á, Pérez-Viejo E. Peritoneal Mesothelioma in a High Volume Peritoneal Surface Malignancies Unit. Journal of Clinical Medicine. 2023; 12(6):2288. https://doi.org/10.3390/jcm12062288
Chicago/Turabian StylePereira, Fernando, Mónica Pereira, Israel Manzanedo, Ángel Serrano, and Estibalitz Pérez-Viejo. 2023. "Peritoneal Mesothelioma in a High Volume Peritoneal Surface Malignancies Unit" Journal of Clinical Medicine 12, no. 6: 2288. https://doi.org/10.3390/jcm12062288
APA StylePereira, F., Pereira, M., Manzanedo, I., Serrano, Á., & Pérez-Viejo, E. (2023). Peritoneal Mesothelioma in a High Volume Peritoneal Surface Malignancies Unit. Journal of Clinical Medicine, 12(6), 2288. https://doi.org/10.3390/jcm12062288






