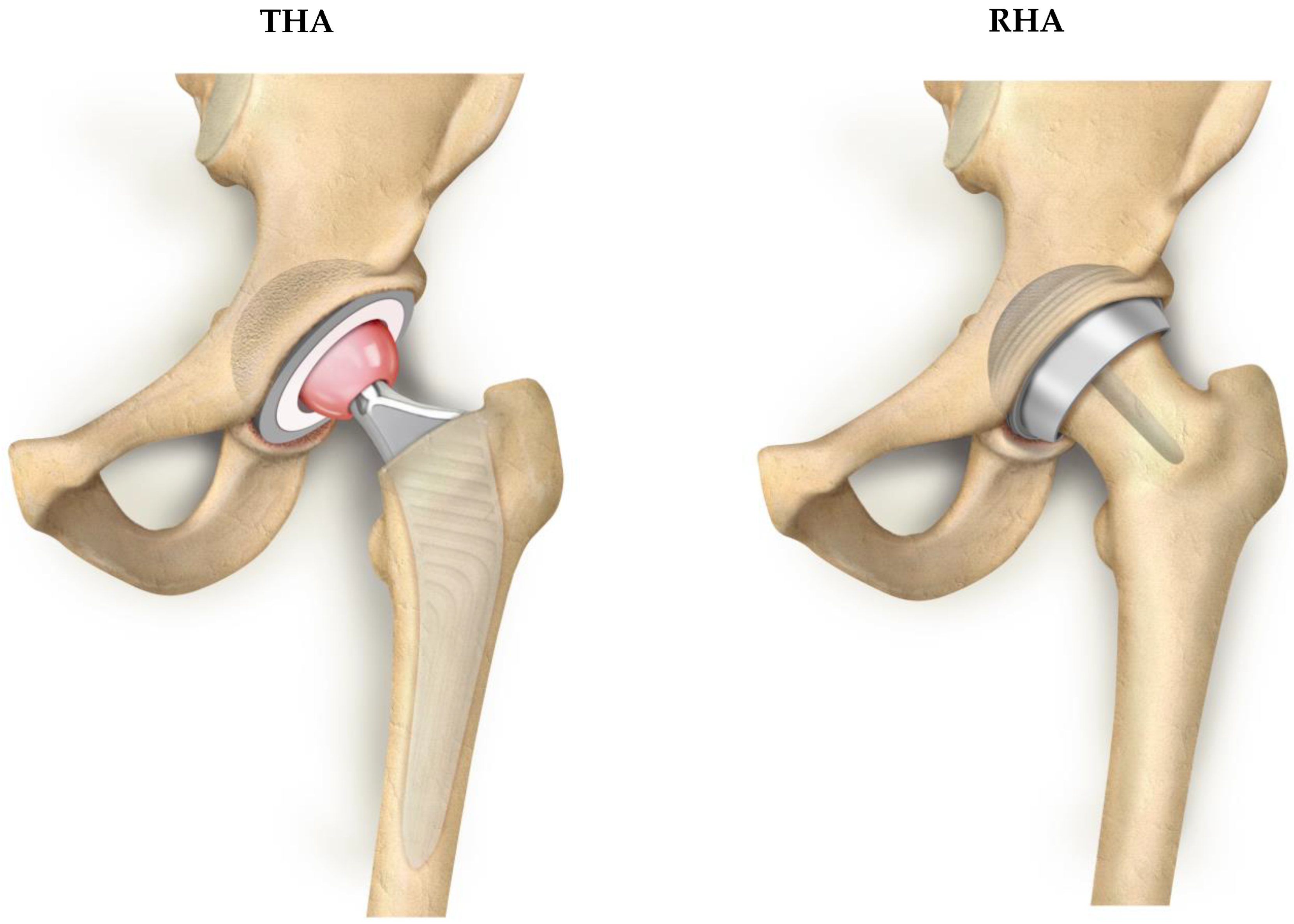
Resurfacing Hip Arthroplasty Is a Safe and Effective Alternative to Total Hip Arthroplasty in Young Patients: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Study Selection
2.3. Data Extraction
2.4. Quality Assessment
2.5. Statistical Analysis
3. Results
3.1. Literature Search
3.2. Qualitative Analysis
3.2.1. Study Characteristics
3.2.2. Main Findings
3.3. Meta-Analysis
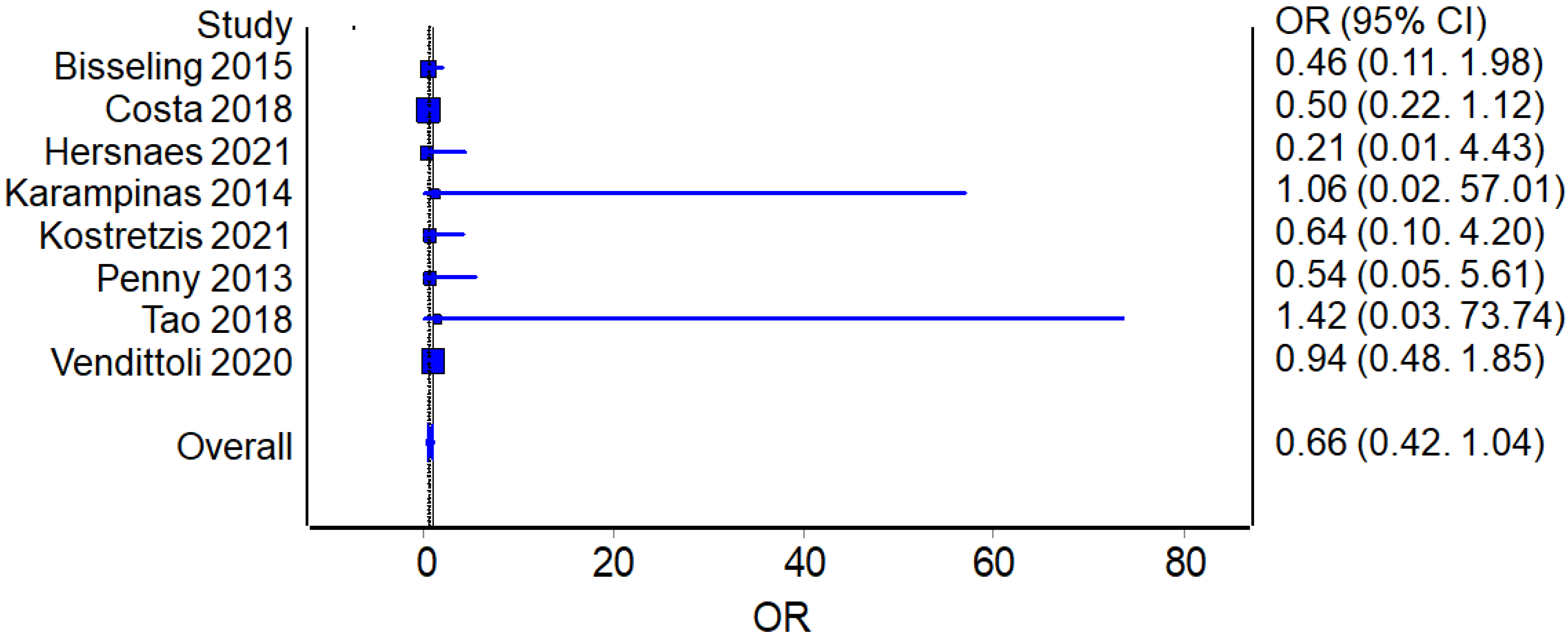
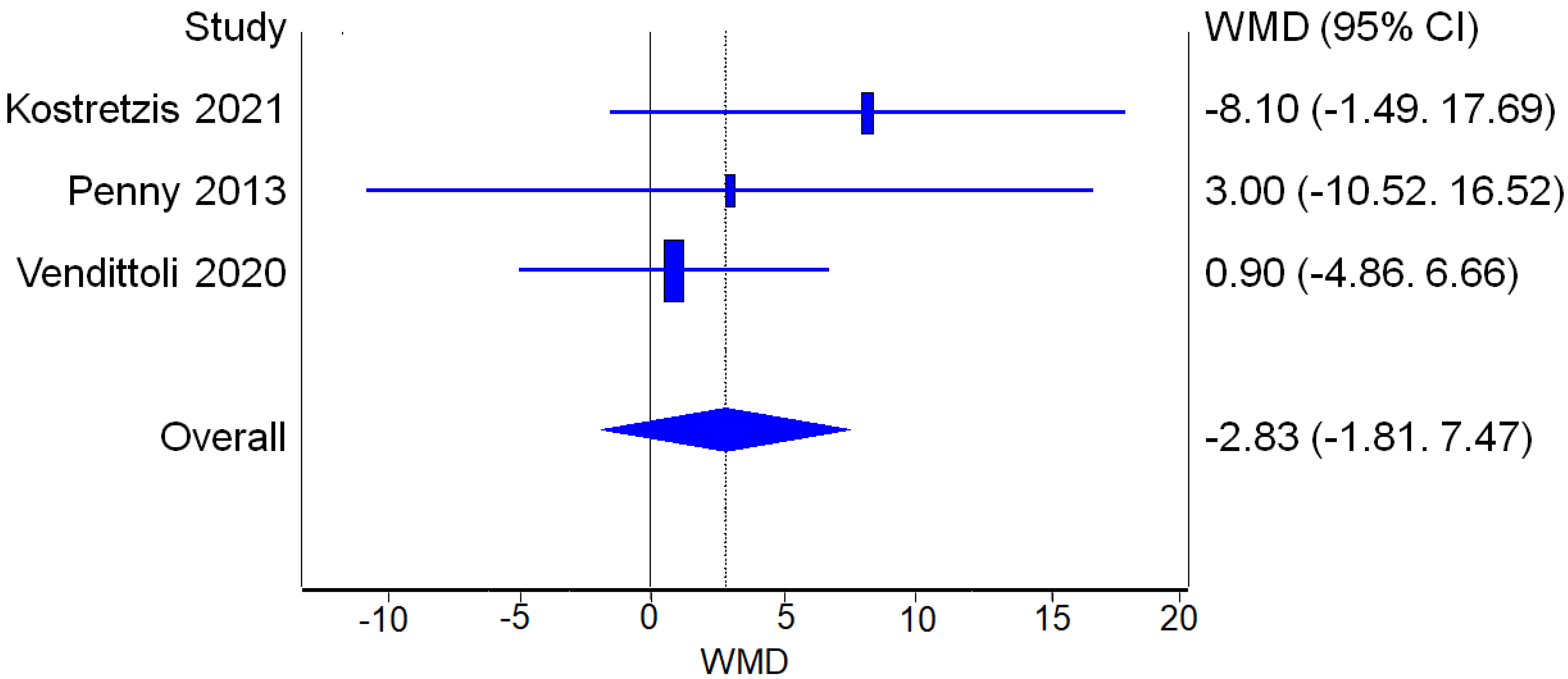
3.3.1. Complications and Revisions
Complications
Revisions
3.3.2. Perioperative Parameters
Operative Time
Blood Loss
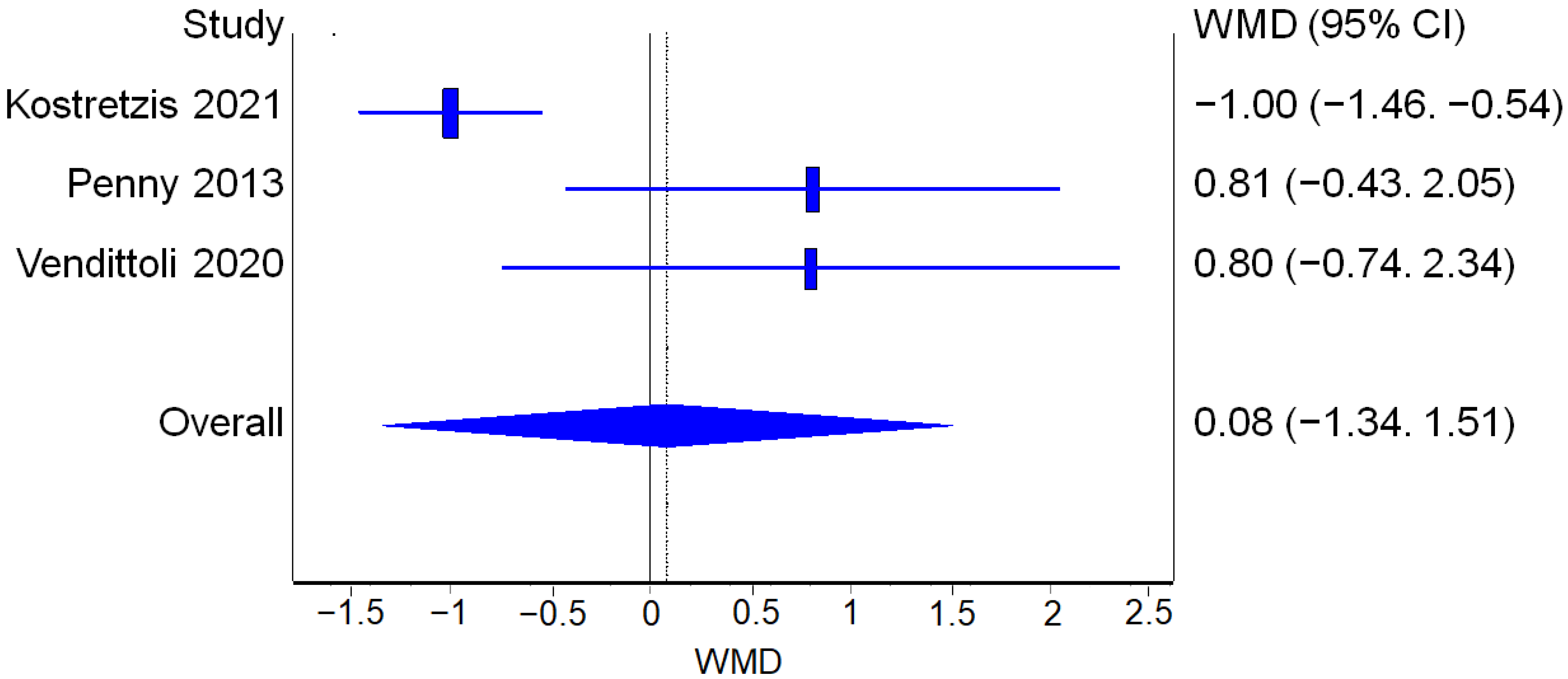
3.3.3. Functional Outcomes
WOMAC
UCLA
HHS
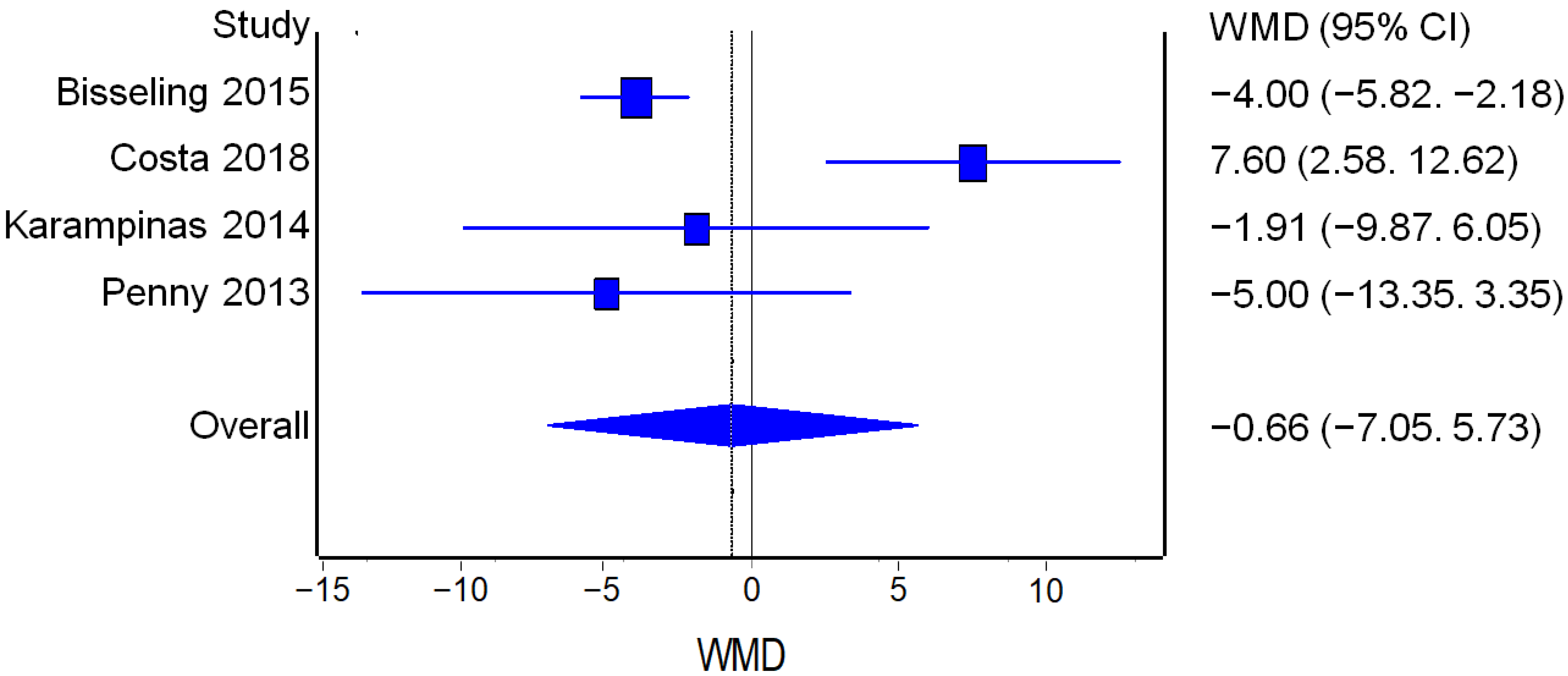
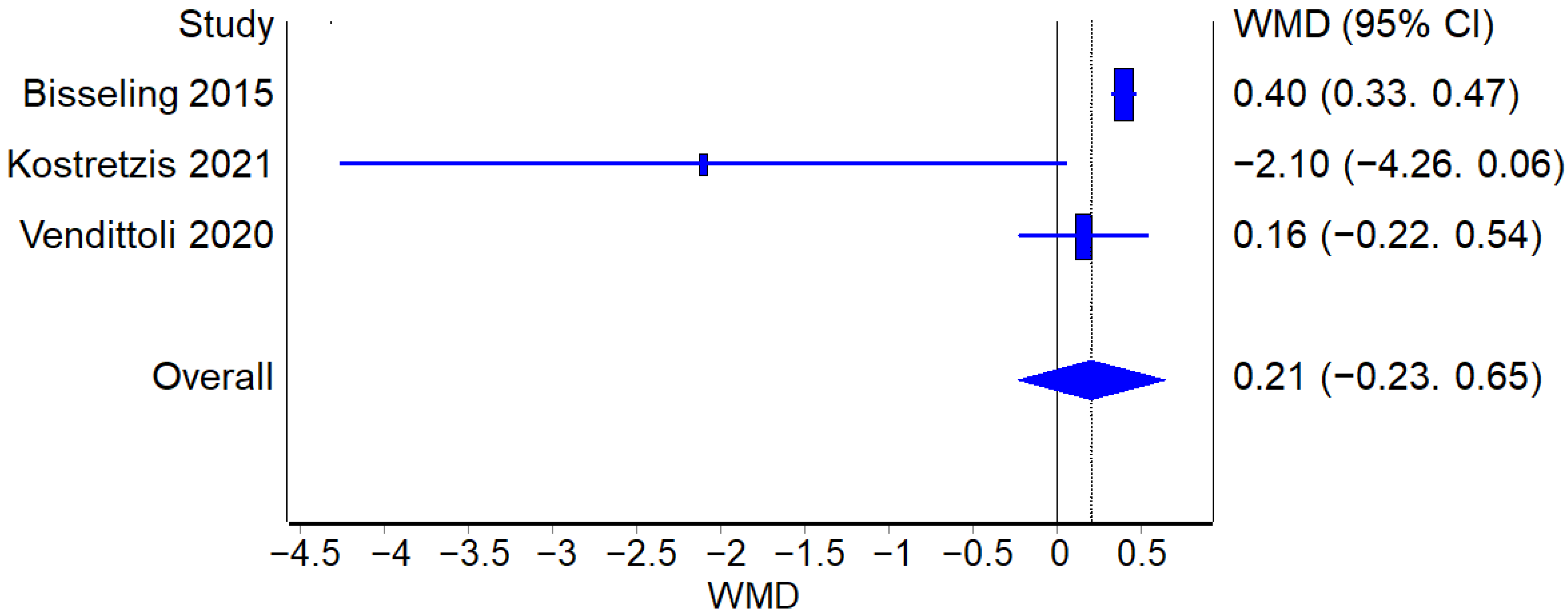
3.3.4. Metal Ions Levels
Cobalt Levels
Chromium Levels
3.4. Quality Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Learmonth, I.D.; Young, C.; Rorabeck, C. The Operation of the Century: Total Hip Replacement. Lancet 2007, 370, 1508–1519. [Google Scholar] [CrossRef]
- Lons, A.; Arnould, A.; Pommepuy, T.; Drumez, E.; Girard, J. Excellent Short-Term Results of Hip Resurfacing in a Selected Population of Young Patients. Orthop. Traumatol. Surg. Res. 2015, 101, 661–665. [Google Scholar] [CrossRef]
- Ortiz-Declet, V.R.; Iacobelli, D.A.; Yuen, L.C.; Perets, I.; Chen, A.W.; Domb, B.G. Birmingham Hip Resurfacing vs Total Hip Arthroplasty: A Matched-Pair Comparison of Clinical Outcomes. J. Arthroplasty 2017, 32, 3647–3651. [Google Scholar] [CrossRef] [PubMed]
- Girard, J. Hip Resurfacing: International Perspectives: Review Article. HSS J. 2017, 13, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Girard, J.; De Smet, K. Reproducing the Proximal Femur Anatomy Using Hip Resurfacing Implants. Pers. Hip Knee Jt. Replace. 2020, 35–44. [Google Scholar] [CrossRef]
- Girard, J.; Epinette, J.A.; Martinot, P.; Dartus, J. French Hip Resurfacing Registry: A Study of 1650 Cases. Orthop. Traumatol. Surg. Res. 2022, 108, 103087. [Google Scholar] [CrossRef] [PubMed]
- Haddad, F.S.; Konan, S.; Tahmassebi, J. A Prospective Comparative Study of Cementless Total Hip Arthroplasty and Hip Resurfacing in Patients under the Age of 55 Years. Bone Jt. J. 2015, 97-B, 617–622. [Google Scholar] [CrossRef]
- Kwon, Y.M.; Thomas, P.; Summer, B.; Pandit, H.; Taylor, A.; Beard, D.; Murray, D.W.; Gill, H.S. Lymphocyte Proliferation Responses in Patients with Pseudotumors Following Metal-on-Metal Hip Resurfacing Arthroplasty. J. Orthop. Res. 2010, 28, 444–450. [Google Scholar] [CrossRef]
- van Lingen, C.P.; Zagra, L.M.; Ettema, H.B.; Verheyen, C.C. Sequelae of Large-Head Metal-on-Metal Hip Arthroplasties: Current Status and Future Prospects. EFORT Open Rev. 2016, 1, 345–353. [Google Scholar] [CrossRef]
- Wiley, K.F.; Ding, K.; Stoner, J.A.; Teague, D.C.; Yousuf, K.M. Incidence of Pseudotumor and Acute Lymphocytic Vasculitis Associated Lesion (ALVAL) Reactions in Metal-on-Metal Hip Articulations: A Meta-Analysis. J. Arthroplasty 2013, 28, 1238–1245. [Google Scholar] [CrossRef]
- McInnes, M.D.F.; Moher, D.; Thombs, B.D.; McGrath, T.A.; Bossuyt, P.M.; Clifford, T.; Cohen, J.F.; Deeks, J.J.; Gatsonis, C.; Hooft, L.; et al. Preferred Reporting Items for a Systematic Review and Meta-Analysis of Diagnostic Test Accuracy Studies The PRISMA-DTA Statement. JAMA J. Am. Med. Assoc. 2018, 319, 388–396. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Int. J. Surg. 2010, 8, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Neyeloff, J.L.; Fuchs, S.C.; Moreira, L.B. Meta-Analyses and Forest Plots Using a Microsoft Excel Spreadsheet: Step-by-Step Guide Focusing on Descriptive Data Analysis. BMC Res. Notes 2012, 5, 52. [Google Scholar] [CrossRef]
- Costa, M.L.; Achten, J.; Parsons, N.R.; Edlin, R.P.; Foguet, P.; Prakash, U.; Griffin, D.R. Total Hip Arthroplasty versus Resurfacing Arthroplasty in the Treatment of Patients with Arthritis of the Hip Joint: Single Centre, Parallel Group, Assessor Blinded, Randomised Controlled Trial. BMJ 2012, 344, e2147. [Google Scholar] [CrossRef]
- Karampinas, P.K.; Evangelopoulos, D.S.; Vlamis, J.; Nikolopoulos, K.; Korres, D.S. Confronting Hip Resurfacing and Big Femoral Head Replacement Gait Analysis. Orthop. Rev. 2014, 6, 5221. [Google Scholar] [CrossRef]
- Rama, K.R.B.S.; Vendittoli, P.A.; Ganapathi, M.; Borgmann, R.; Roy, A.; Lavigne, M. Heterotopic Ossification After Surface Replacement Arthroplasty and Total Hip Arthroplasty. A Randomized Study. J. Arthroplasty 2009, 24, 256–262. [Google Scholar] [CrossRef]
- Vendittoli, P.A.; Ganapathi, M.; Roy, A.G.; Lusignan, D.; Lavigne, M. A Comparison of Clinical Results of Hip Resurfacing Arthroplasty and 28 Mm Metal on Metal Total Hip Arthroplasty: A Randomised Trial with 3-6 Years Follow-Up. HIP Int. 2010, 20, 1–13. [Google Scholar] [CrossRef]
- Vendittoli, P.A.; Roy, A.; Mottard, S.; Girard, J.; Lusignan, D.; Lavigne, M. Metal Ion Release from Bearing Wear and Corrosion with 28 Mm and Large-Diameter Metal-on-Metal Bearing Articulations: A Follow-up Study. J. Bone Jt. Surg. Ser. B 2010, 92, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Lavigne, M.; Therrien, M.; Nantel, J.; Roy, A.; Prince, F.; Vendittoli, P.A. The John Charnley Award: The Functional Outcome of Hip Resurfacing and Large-Head THA Is the Same: A Randomized, Double-Blind Study. Clin. Orthop. Relat. Res. 2010, 468, 326–336. [Google Scholar] [CrossRef]
- Smolders, J.M.H.; Hol, A.; Rijnberg, W.J.; van Susante, J.L.C. Metal Ion Levels and Functional Results after Either Resurfacing Hip Arthroplasty or Conventional Metal-on-Metal Hip Arthroplasty. Acta Orthop. 2011, 82, 559–566. [Google Scholar] [CrossRef]
- Costa, M.L.; Achten, J.; Foguet, P.; Parsons, N.R. Comparison of Hip Function and Quality of Life of Total Hip Arthroplasty and Resurfacing Arthroplasty in the Treatment of Young Patients with Arthritis of the Hip Joint at 5 Years. BMJ Open 2018, 8, e018849. [Google Scholar] [CrossRef]
- Konan, S.; Waugh, C.; Ohly, N.; Duncan, C.P.; Masri, B.A.; Garbuz, D.S. Mid-Term Results of a Prospective Randomised Controlled Trial Comparing Large-Head Metal-on-Metal Hip Replacement to Hip Resurfacing Using Patient-Reported Outcome Measures and Objective Functional Task-Based Outcomes. HIP Int. 2021, 31, 637–643. [Google Scholar] [CrossRef]
- Hersnaes, P.N.; Gromov, K.; Otte, K.S.; Gebuhr, P.H.; Troelsen, A. Harris Hip Score and SF-36 Following Metal-on-Metal Total Hip Arthroplasty and Hip Resurfacing—A Randomized Controlled Trial with 5-Years Follow up Including 75 Patients. BMC Musculoskelet. Disord. 2021, 22, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Penny, J.Ø.; Ovesen, O.; Varmarken, J.E.; Overgaard, S. Similar Range of Motion and Function after Resurfacing Large-Head or Standard Total Hip Arthroplasty: 2-Year Results from a Randomized Clinical Trial. Acta Orthop. 2013, 84, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Tao, R.; Liu, F.; Liu, Y.K.; Lu, Y.; Xu, H.; Cao, Y.; Zhou, Z.Y.; Wang, W. A Prospective Comparative Study of Hip Resurfacing Arthroplasty and Large-Diameter Head Metal-on-Metal Total Hip Arthroplasty in Younger Patients—A Minimum of Five Year Follow-Up. Int. Orthop. 2018, 42, 2323–2327. [Google Scholar] [CrossRef]
- Bisseling, P.; Smolders, J.M.H.; Hol, A.; van Susante, J.L.C. Metal Ion Levels and Functional Results Following Resurfacing Hip Arthroplasty versus Conventional Small-Diameter Metal-on-Metal Total Hip Arthroplasty; a 3 to 5year Follow-up of a Randomized Controlled Trial. J. Arthroplasty 2015, 30, 61–67. [Google Scholar] [CrossRef]
- Vendittoli, P.A.; Shahin, M.; Rivière, C.; Roy, A.G.; Barry, J.; Lavigne, M. Hip Resurfacing Compared with 28-Mm Metal-on-Metal Total Hip Replacement: A Randomized Study with 15 Years of Follow-Up. J. Bone Jt. Surg. Am. 2020, 102, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Vendittoli, P.A.; Rivière, C.; Roy, A.G.; Barry, J.; Lusignan, D.; Lavigne, M. Metal-on-Metal Hip Resurfacing Compared with 28-Mm Diameter Metal-on-Metal Total Hip Replacement: A Randomised Study with Six to Nine Years’ Follow-Up. Bone Jt. J. 2013, 95 B, 1464–1473. [Google Scholar] [CrossRef]
- Kostretzis, L.; Lavigne, M.; Kiss, M.O.; Shahin, M.; Barry, J.; Vendittoli, P.A. Despite Higher Revision Rate, MoM Large-Head THA Offers Better Clinical Scores than HR: 14-Year Results from a Randomized Controlled Trial Involving 48 Patients. BMC Musculoskelet. Disord. 2021, 22, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Vendittoli, P.A.; Lavigne, M.; Roy, A.G.; Lusignan, D. A Prospective Randomized Clinical Trial Comparing Metal-on-Metal Total Hip Arthroplasty and Metal-on-Metal Total Hip Resurfacing in Patients Less than 65 Years Old. HIP Int. 2006, 16, 73–81. [Google Scholar] [CrossRef]
- Garbuz, D.S.; Tanzer, M.; Greidanus, N.V.; Masri, B.A.; Duncan, C.P. The John Charnley Award: Metal-on-Metal Hip Resurfacing versus Large-Diameter Head Metal-on-Metal Total Hip Arthroplasty: A Randomized Clinical Trial. Clin. Orthop. Relat. Res. 2010, 468, 318–325. [Google Scholar] [CrossRef]
- Fouilleron, N.; Wavreille, G.; Endjah, N.; Girard, J. Running Activity after Hip Resurfacing Arthroplasty: A Prospective Study. Am. J. Sports Med. 2012, 40, 889–894. [Google Scholar] [CrossRef]
- Girard, J.; Miletic, B.; Deny, A.; Migaud, H.; Fouilleron, N. Can Patients Return to High-Impact Physical Activities after Hip Resurfacing? A Prospective Study. Int. Orthop. 2013, 37, 1019–1024. [Google Scholar] [CrossRef]
- Amstutz, H.C.; Beaulé, P.E.; Dorey, F.J.; Le Duff, M.J.; Campbell, P.A.; Gruen, T.A. Metal-on-Metal Hybrid Surface Arthroplasty. Surgical Technique. J. Bone Jt. Surg. Am. 2006, 88 Pt 2 (Suppl. S1), 234–249. [Google Scholar] [CrossRef]
- Girard, J.; Lavigne, M.; Vendittoli, P.A.; Roy, A.G. Biomechanical Reconstruction of the Hip: A Randomised Study Comparing Total Hip Resurfacing and Total Hip Arthroplasty. J. Bone Jt. Surg. Br. 2006, 88, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Amstutz, H.C.; Le Duff, M.J. The Mean Ten-Year Results of Metal-on-Metal Hybrid Hip Resurfacing Arthroplasty. Bone Jt. J. 2018, 100-B, 1424–1433. [Google Scholar] [CrossRef]
- Hellman, M.D.; Ford, M.C.; Barrack, R.L. Is There Evidence to Support an Indication for Surface Replacement Arthroplasty?: A Systematic Review. Bone Jt. J. 2019, 101-B, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.J.; Le Duff, M.J.; Yoon, J.P.; Al-Hamad, M.; Amstutz, H.C. Metal Ion Levels in Total Hip Arthroplasty versus Hip Resurfacing. J. Arthroplasty 2013, 28, 1235–1237. [Google Scholar] [CrossRef] [PubMed]
- Palazzuolo, M.; Antoniadis, A.; Delaune, L.; Tornare, I.; Wegrzyn, J. Comparison of the Long-Term Cause of Failure and Survivorship of Four Hundred and Twenty Seven Metal-on-Metal Hip Arthroplasties: Resurfacing versus Large Head Total Hip Arthroplasty. Int. Orthop. 2021, 45, 3075–3081. [Google Scholar] [CrossRef]
- Stoney, J.; Graves, S.E.; De Steiger, R.N.; Rainbird, S.; Kelly, T.L.; Hatton, A. Is the Survivorship of Birmingham Hip Resurfacing Better Than Selected Conventional Hip Arthroplasties in Men Younger Than 65 Years of Age? A Study from the Australian Orthopaedic Association National Joint Replacement Registry. Clin. Orthop. Relat. Res. 2020, 478, 2625. [Google Scholar] [CrossRef] [PubMed]
- Koff, M.F.; Gao, M.A.; Neri, J.P.; Chiu, Y.F.; Lin, B.Q.; Burge, A.J.; Su, E.; Padgett, D.E.; Potter, H.G. Adverse Local Tissue Reactions Are Common in Asymptomatic Individuals After Hip Resurfacing Arthroplasty: Interim Report from a Prospective Longitudinal Study. Clin. Orthop. Relat. Res. 2021, 479, 2633–2650. [Google Scholar] [CrossRef]
- Kumar, P.; Ksheersagar, V.; Aggarwal, S.; Jindal, K.; Dadra, A.; Kumar, V.; Patel, S. Complications and Mid to Long Term Outcomes for Hip Resurfacing versus Total Hip Replacement: A Systematic Review and Meta-Analysis. Eur. J. Orthop. Surg. Traumatol. 2022; online ahead of print. [Google Scholar] [CrossRef]
- Seppänen, M.; Karvonen, M.; Virolainen, P.; Remes, V.; Pulkkinen, P.; Eskelinen, A.; Liukas, A.; Mäkelä, K.T. Poor 10-Year Survivorship of Hip Resurfacing Arthroplasty. Acta Orthop. 2016, 87, 554–559. [Google Scholar] [CrossRef] [PubMed]
- Pailhe, R.; Matharu, G.S.; Sharma, A.; Pynsent, P.B.; Treacy, R.B. Survival and Functional Outcome of the Birmingham Hip Resurfacing System in Patients Aged 65 and Older at up to Ten Years of Follow-Up. Int. Orthop. 2014, 38, 1139. [Google Scholar] [CrossRef]
- Magan, A.; Wignadasan, W.; Kayani, B.; Radhakrishnan, G.; Ronca, F.; Haddad, F.S. A Meta-Analysis Assessing Time for Return to Sport Following Hip Resurfacing. Arch. Orthop. Trauma Surg. 2022; online ahead of print. [Google Scholar] [CrossRef]
- Common, H.; Rousseau, R.; Putman, S.; Migaud, H.; Girard, J. High-Level Judo Practice after Hip Resurfacing. Orthop. Traumatol. Surg. Res. 2020, 106, 1511–1514. [Google Scholar] [CrossRef]
- Girard, J.; Lons, A.; Pommepuy, T.; Isida, R.; Benad, K.; Putman, S. High-Impact Sport after Hip Resurfacing: The Ironman Triathlon. Orthop. Traumatol. Surg. Res. 2017, 103, 675–678. [Google Scholar] [CrossRef] [PubMed]
- Vanlommel, J.; Goldhofer, M.; Walter, W.L. Surfing After Hip Resurfacing Surgery. Clin. J. Sport Med. 2022, 32, 135–138. [Google Scholar] [CrossRef]
- Guyen, O.; Tissot, C. Suivi Des Patients Avec Arthroplastie de Hanche Métal-Métal et Stratégie de Prise En Charge Des Complications. Rev. Med. Suisse 2016, 12, 2156–2163. [Google Scholar]
- Pulik, Ł.; Romaniuk, K.; Jaśkiewicz, K.; Wojtyński, P.; Łȩgosz, P.; Małdyk, P. An Update on Joint-Specific Outcome Measures in Total Hip Replacement. Reumatologia 2020, 58, 107–115. [Google Scholar] [CrossRef]
- Stratford, P.W.; Kennedy, D.M. Does Parallel Item Content on WOMAC’s Pain and Function Subscales Limit Its Ability to Detect Change in Functional Status? BMC Musculoskelet. Disord. 2004, 5, 1–9. [Google Scholar] [CrossRef]





| Author | Year | Journal | Study Type | Treatment Group | N° pts | M | W | Age | BMI |
|---|---|---|---|---|---|---|---|---|---|
| Bisseling P et al. [27] | 2015 | The Journal of arthroplasty | RCT | RHA | 42 | 21 | 17 | 57.5 | 26.1 |
| THA | 42 | 21 | 12 | 59.2 | 28 | ||||
| Costa ML et al. [22] | 2018 | BMJ open | RCT | RHA | 60 | 36 | 24 | 56.5 | 28.4 |
| THA | 62 | 35 | 27 | 56.7 | 28.9 | ||||
| Costa ML et al. [15] | 2012 | BMJ (Clinical research ed) | RCT | RHA | 60 | 38 | 22 | 56.3 | 28.6 |
| THA | 66 | 36 | 30 | 56.6 | 28.7 | ||||
| Garbuz DS et al. [32] | 2010 | Clinical orthopaedics and related research | RCT | RHA | 48 | 43 | 5 | 51.5 | 28.3 |
| THA | 56 | 50 | 6 | 52 | 28.2 | ||||
| Hersnaes PN et al. [24] | 2021 | BMC musculoskeletal disorders | RCT | RHA | 36 | 26 | 10 | 59.4 | 27.45 |
| THA | 39 | 26 | 13 | 61.9 | 28.4 | ||||
| Karampinas PK et al. [16] | 2014 | Orthopedic reviews | RCT | RHA | 20 | 7 | 8 | 50.5 | 31 |
| THA | 21 | 11 | 5 | 50.7 | 31.6 | ||||
| Konan S et al. [23] | 2021 | Hip international: the journal of clinical and experimental research on hip pathology and therapy | RCT | RHA | 48 | 43 | 5 | 51.5 | 28.3 |
| THA | 56 | 50 | 6 | 52 | 28.2 | ||||
| Kostretzis L et al. [30] | 2021 | BMC musculoskeletal disorders | RCT | RHA | 24 | 14 | 10 | 50 | 28 |
| THA | 24 | 15 | 9 | 50 | 28 | ||||
| Lavigne M et al. [20] | 2010 | Clinical orthopaedics and related research | RCT | RHA | 24 | 14 | 10 | 49.6 | 27.9 |
| THA | 24 | 15 | 9 | 49.8 | 27.8 | ||||
| Penny J et al. [25] | 2013 | Acta orthopaedica | RCT | RHA | 20 | 12 | 8 | 57 | 28 |
| THA | 34 | 24 | 10 | 56 | 27 | ||||
| Rama KR et al. [17] | 2009 | The Journal of Arthroplasty | RCT | RHA | 109 | 65 | 38 | 50 | 27.3 |
| THA | 100 | 66 | 31 | 50.3 | 29.7 | ||||
| Smolders JM et al. [21] | 2011 | Acta orthopaedica | RCT | RHA | 42 | 21 | 17 | 58 | 26 |
| THA | 42 | 21 | 12 | 59 | 28 | ||||
| Tao R et al. [26] | 2018 | International orthopaedics | RCT | RHA | 28 | 19 | 9 | 43 | 21.5 |
| THA | 40 | 28 | 12 | 47 | 21.8 | ||||
| Vendittoli PA et al. [31] | 2006 | Hip international: the journal of clinical and experimental research on hip pathology and therapy | RCT | RHA | 109 | 67 | 40 | 49.1 | 27.2 |
| THA | 100 | 70 | 33 | 50.6 | 29.6 | ||||
| Vendittoli PA et al. [18] | 2010 | Hip international: the journal of clinical and experimental research on hip pathology and therapy | RCT | RHA | 109 | 69 | 40 | 49.2 | 27 |
| THA | 100 | 68 | 32 | 51 | 30 | ||||
| Vendittoli PA et al. [19] | 2010 | The Journal of bone and joint surgery. British volume | RCT | RHA | 109 | 42 | 22 | 49.3 | 27.1 |
| THA | 100 | 33 | 20 | 51 | 29.2 | ||||
| Vendittoli PA et al. [29] | 2013 | Bone & Joint Journal | RCT | RHA | 109 | 66 | 38 | 49.2 | 27 |
| THA | 100 | 67 | 32 | 51 | 30 | ||||
| Vendittoli PA et al. [28] | 2020 | Journal of Bone and Joint Surgery-American Volume | RCT | RHA | 109 | 66 | 38 | 48.9 | 26.6 |
| THA | 100 | 67 | 32 | 50.7 | 30 |
| Series of Patients | Treatment Type | Complications | Revisions | Operative Time (min) | Blood Loss (ml) | Incision Lenght (cm) | WOMAC Pre-op | WOMAC Post-op | UCLA Pre-op | UCLA Post-op | HHS Pre-op | HHS Post-op | Cobalt Level Pre-op | Cobalt Level Post-op | Chromium Level Pre-op | Chromium Level Post-op |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bisseling P et al. [21,27] | RHA | 3 | 3 | 77.3 ± 11.2 | NR | NR | NR | NR | 5 ± 0.75 | 7 ± 0.25 | 57 ± 4 | 98 ± 0.5 | 0.1 ± 0.1 | 1.3 ± 0.175 | 0.1 ± 0.1 | 0.9 ± 0.225 |
| THA | 6 | 3 | 55.6 ± 11.8 | NR | NR | NR | NR | 4 ± 1 | 7 ± 0.5 | 53 ± 3.75 | 98 ± 0.5 | 0.1 ± 0.1 | 0.9 ± 0.125 | 0.1 ± 0.1 | 0.1 ± 0.175 | |
| Costa ML et al. [15,22] | RHA | 13 | 1 | NR | NR | NR | NR | NR | NR | NR | 48.6 ± 14.2 | 88.4 ± 2.2 | NR | NR | NR | NR |
| THA | 22 | 3 | NR | NR | NR | NR | NR | NR | NR | 50.1 ± 13.5 | 82.3 ± 4.8 | NR | NR | NR | NR | |
| Hersnaes PN et al. [24] | RHA | 0 | 6 | NR | NR | NR | NR | NR | NR | NR | NR | 97.66 ± 5.5 | NR | 0.92 ± 0.21 | NR | 1.21 ± 0.53 |
| THA | 2 | 0 | NR | NR | NR | NR | NR | NR | NR | NR | 99.3 ± 1.52 | NR | 1.67 ± 0.36 | NR | 1.36 ± 0.53 | |
| Karampinas PK et al. [16] | RHA | 0 | NR | NR | NR | NR | 72.36 ± 10.16 | 94.55 ± 3.01 | 4.07 ± 1.49 | 8.13 ± 1.14 | 60.3 ± 39.94 | 95.6 ± 71.95 | NR | NR | NR | NR |
| THA | 0 | NR | NR | NR | NR | 65.58 ± 10.89 | 93.35 ± 34.79 | 3.5 ± 1.15 | 6.75 ± 1.13 | 56.5 ± 11.88 | 93.7 ± 53.61 | NR | NR | NR | NR | |
| Konan S et al. [23,32] | RHA | NR | 1 | NR | NR | NR | NR | 88.61 ± 3.4 | NR | 6.5 ± 1.9 | NR | NR | NR | NR | NR | NR |
| THA | NR | 7 | NR | NR | NR | NR | 88 ± 15.7 | NR | 5.9 ± 1.7 | NR | NR | NR | NR | NR | NR | |
| Kostretzis L et al. [20,30] | RHA | 2 | 2 | NR | NR | NR | 46.5 ± 14.9 | 85 ± 16 | NR | 7.2 ± 1.8 | NR | NR | NR | 1.7 ± 2 | NR | 1.4 ± 1.1 |
| THA | 3 | 5 | NR | NR | NR | 54.31 ± 4.5 | 94 ± 7.8 | NR | 6.7 ± 1.8 | NR | NR | NR | 3.8 ± 3.2 | NR | 1.9 ± 1 | |
| Penny J et al. [25] | RHA | 1 | 1 | 113 ± 15 | 625 ± 467 | 24 ± 2.8 | 50 ± 21 | 81 ± 3 | 5.8 ± 2.2 | 7.3 ± 1.8 | 63 ± 10 | 93 ± 10 | NR | NR | NR | NR |
| THA | 3 | 0 | 83 ± 12 | 753 ± 315 | 15 ± 2.6 | 55 ± 16 | 101 ± 8 | 6.3 ± 1.8 | 7 ± 2 | 56 ± 9 | 91 ± 14 | NR | NR | NR | NR | |
| Tao R et al. [26] | RHA | 0 | 0 | 98 ± 12 | 353 ± 79 | NR | NR | NR | NR | NR | NR | 90.4 ± 2,4 | NR | NR | NR | NR |
| THA | 0 | 1 | 79 ± 9 | 429 ± 109 | NR | NR | NR | NR | NR | NR | 90.8 ± 5.1 | NR | NR | NR | NR | |
| Vendittoli PA et al. [17,18,19,28,29,31] | RHA | 21 | 9 | 101 ± 18.1 | 529 ± 262.7 | 17.2 ± 3.4 | 52.7 ± 16.2 | 10.7 ± 16.2 | NR | 6.3 ± 4.6 | NR | NR | 0.16 ± 0.16 | 0.92 ± 0.87 | 1.02 ± 0.64 | 2.09 ± 1.93 |
| THA | 21 | 5 | 87 ± 24.1 | 543 ± 467.2 | 15.1 ± 5 | 55 ± 18.9 | 8.81 ± 1.8 | NR | 6.4 ± 4.6 | NR | NR | 0.2 ± 0.26 | 0.76 ± 0.87 | 1.05 ± 0.82 | 1.42 ± 0.74 |
| Study | D1 | D2 | D3 | D4 | D5 | Overall |
|---|---|---|---|---|---|---|
| Bisseling P et al., 2015 [27] | ||||||
| Costa ML et al., 2012 [15] | ||||||
| Costa ML et al., 2018 [22] | ||||||
| Garbuz DS et al., 2010 [32] | ||||||
| Hersnaes PN et al., 2021 [24] | ||||||
| Karampinas PK et al., 2014 [16] | ||||||
| Konan S et al., 2021 [23] | ||||||
| Kostretzis L et al., 2021 [30] | ||||||
| Lavigne M et al., 2010 [20] | ||||||
| Penny J et al., 2013 [25] | ||||||
| Rama KR et al., 2009 [17] | ||||||
| Smolders JM et al., 2011 [21] | ||||||
| Tao R et al., 2018 [26] | ||||||
| Vendittoli PA et al., 2006 [31] | ||||||
| Vendittoli PA et al., 2010 [18] | ||||||
| Vendittoli PA et al., 2013 [19] | ||||||
| Vendittoli PA et al., 2020 [29] | ||||||
| Vendittoli PA et al., 2010 [28] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palazzuolo, M.; Bensa, A.; Bauer, S.; Blakeney, W.G.; Filardo, G.; Riegger, M. Resurfacing Hip Arthroplasty Is a Safe and Effective Alternative to Total Hip Arthroplasty in Young Patients: A Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 12, 2093. https://doi.org/10.3390/jcm12062093
Palazzuolo M, Bensa A, Bauer S, Blakeney WG, Filardo G, Riegger M. Resurfacing Hip Arthroplasty Is a Safe and Effective Alternative to Total Hip Arthroplasty in Young Patients: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2023; 12(6):2093. https://doi.org/10.3390/jcm12062093
Chicago/Turabian StylePalazzuolo, Michele, Alessandro Bensa, Stefan Bauer, William G. Blakeney, Giuseppe Filardo, and Martin Riegger. 2023. "Resurfacing Hip Arthroplasty Is a Safe and Effective Alternative to Total Hip Arthroplasty in Young Patients: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 12, no. 6: 2093. https://doi.org/10.3390/jcm12062093
APA StylePalazzuolo, M., Bensa, A., Bauer, S., Blakeney, W. G., Filardo, G., & Riegger, M. (2023). Resurfacing Hip Arthroplasty Is a Safe and Effective Alternative to Total Hip Arthroplasty in Young Patients: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 12(6), 2093. https://doi.org/10.3390/jcm12062093