Value of Right and Left Ventricular T1 and T2 Blood Pool Mapping in Patients with Chronic Thromboembolic Hypertension before and after Balloon Pulmonary Angioplasty
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. CMR Imaging
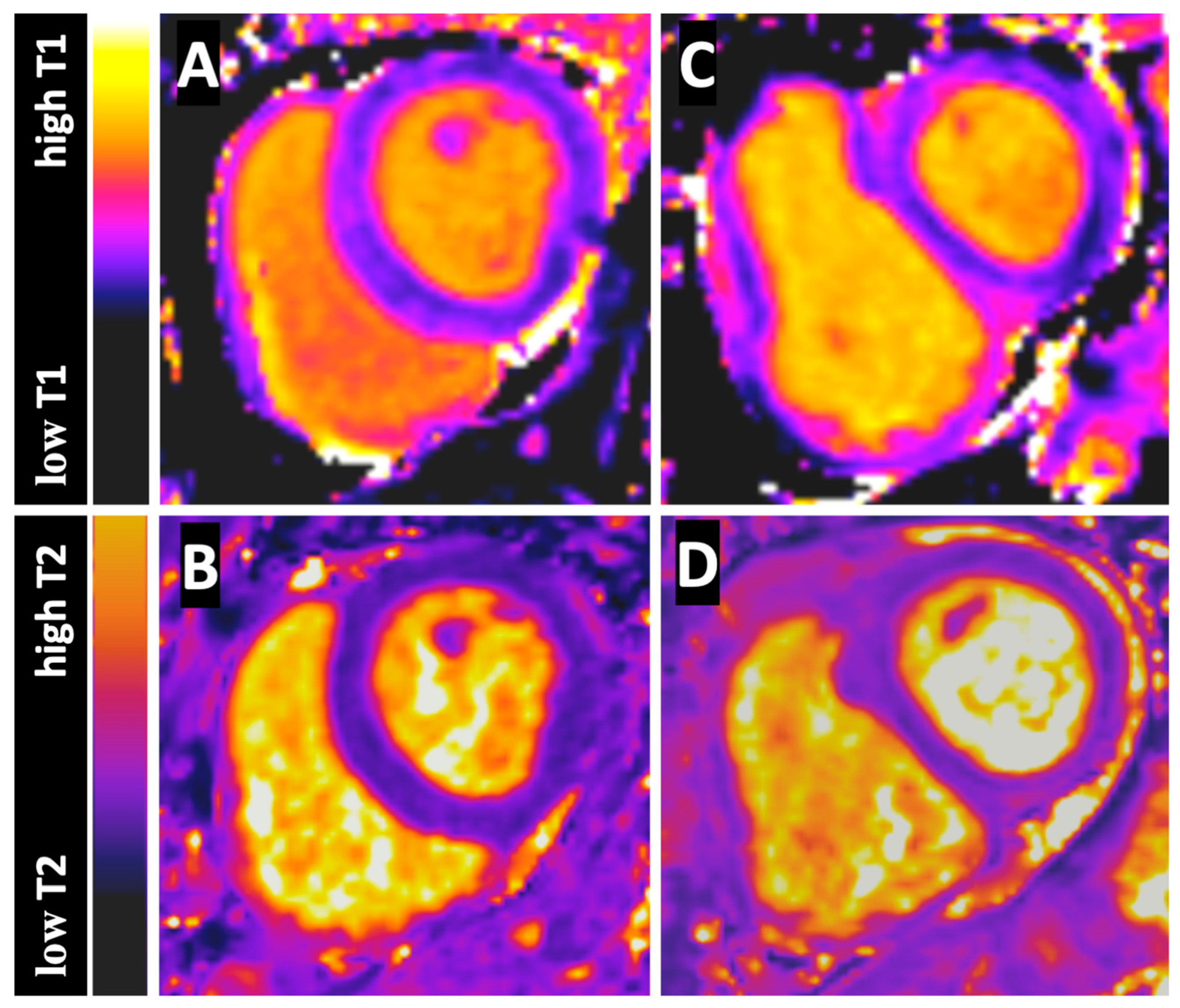
2.3. CMR Analysis
2.4. Right Heart Catheterization
2.5. Statistical Analysis
3. Results
4. Discussion
- (1)
- LV and RV native T1 and T2 blood pool values measured on basal SA sections are reproducibly in both control subjects and CTEPH patients.
- (2)
- RVT1 and RVT2 values are lower compared to LVT1 and LVT2 values in both control subjects and CTEPH patients.
- (3)
- Moreover, RVT2 blood pool values are significantly reduced compared to control subjects in CTEPH patients, whereas LVT2 blood pool values are similar.
- (4)
- Interestingly, RVT2 blood pool values show significant correlations to measures of pulmonary hemodynamic (mPAP and PVR) and correlates of oxygen saturation (CI) before and even after BPA.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BPA | balloon pulmonary angioplasty |
| CMR | cardiac magnetic resonance imaging |
| CTEPH | chronic thromboembolic pulmonary hypertension |
| ECV | extracellular volume |
| LV | left ventricle |
| mPAP | mean pulmonary arterial pressure |
| PEA | pulmonary endarterectomy |
| PH | pulmonary hypertension |
| PVR | pulmonary vascular resistance |
| ROI | region of interest |
| RV | right ventricle |
| RVT1 | right ventricular T1 blood pool value |
| RVT2 | right ventricular T2 blood pool value |
| LVT1 | left ventricular T1 blood pool value |
| LVT2 | left ventricular T2 blood pool value |
| RVT1/LVT1 | right to left ventricular T1 blood pool ratio |
| RVT2/LVT2 | right to left ventricular T2 blood pool ratio |
| SD | standard deviation |
References
- Ferreira, V.M.; Schulz-Menger, J.; Holmvang, G.; Kramer, C.M.; Carbone, I.; Sechtem, U.; Kindermann, I.; Gutberlet, M.; Cooper, L.T.; Liu, P.; et al. Cardiovascular Magnetic Resonance in Nonischemic Myocardial Inflammation: Expert Recommendations. J. Am. Coll. Cardiol. 2018, 72, 3158–3176. [Google Scholar] [CrossRef]
- Reiter, U.; Reiter, G.; Kovacs, G.; Adelsmayr, G.; Greiser, A.; Olschewski, H.; Fuchsjäger, M. Native myocardial T1 mapping in pulmonary hypertension: Correlations with cardiac function and hemodynamics. Eur. Radiol. 2017, 27, 157–166. [Google Scholar] [CrossRef]
- Asano, R.; Ogo, T.; Morita, Y.; Kotoku, A.; Aoki, T.; Hirakawa, K.; Nakayama, S.; Ueda, J.; Tsuji, A.; Waddingham, M.T.; et al. Prognostic value of right ventricular native T1 mapping in pulmonary arterial hypertension. PLoS ONE 2021, 16, e0260456. [Google Scholar] [CrossRef] [PubMed]
- Spruijt, O.A.; Vissers, L.; Bogaard, H.-J.; Hofman, M.B.M.; Vonk-Noordegraaf, A.; Marcus, J.T. Increased native T1-values at the interventricular insertion regions in precapillary pulmonary hypertension. Int. J. Cardiovasc. Imaging 2016, 32, 451–459. [Google Scholar] [CrossRef] [PubMed]
- García-Álvarez, A.; García-Lunar, I.; Pereda, D.; Fernández-Jimenez, R.; Sánchez-González, J.; Mirelis, J.G.; Nuño-Ayala, M.; Sánchez-Quintana, D.; Fernández-Friera, L.; García-Ruiz, J.M.; et al. Association of Myocardial T1-Mapping CMR With Hemodynamics and RV Performance in Pulmonary Hypertension. JACC Cardiovasc. Imaging 2015, 8, 76–82. [Google Scholar] [CrossRef]
- Chen, Y.; Yun, H.; Jin, H.; Kong, D.H.; Long, Y.L.; Fu, C.X.; Yang, S.; Zeng, M.S. Association of native T1 times with biventricular function and hemodynamics in precapillary pulmonary hypertension. Int. J. Cardiovasc. Imaging 2017, 33, 1179–1189. [Google Scholar] [CrossRef]
- Roller, F.C.; Wiedenroth, C.; Breithecker, A.; Liebetrau, C.; Mayer, E.; Schneider, C.; Rolf, A.; Hamm, C.; Krombach, G.A. Native T1 mapping and extracellular volume fraction measurement for assessment of right ventricular insertion point and septal fibrosis in chronic thromboembolic pulmonary hypertension. Eur. Radiol. 2017, 27, 1980–1991. [Google Scholar] [CrossRef] [PubMed]
- Habert, P.; Capron, T.; Hubert, S.; Bentatou, Z.; Bartoli, A.; Tradi, F.; Renard, S.; Rapacchi, S.; Guye, M.; Bernard, M.; et al. Quantification of right ventricular extracellular volume in pulmonary hypertension using cardiac magnetic resonance imaging. Diagn. Interv. Imaging 2020, 101, 311–320. [Google Scholar] [CrossRef]
- Roller, F.C.; Kriechbaum, S.; Breithecker, A.; Liebetrau, C.; Haas, M.; Schneider, C.; Rolf, A.; Guth, S.; Mayer, E.; Hamm, C.; et al. Correlation of native T1 mapping with right ventricular function and pulmonary haemodynamics in patients with chronic thromboembolic pulmonary hypertension before and after balloon pulmonary angioplasty. Eur. Radiol. 2018, 29, 1565–1573. [Google Scholar] [CrossRef]
- Guo, J.; Wang, L.; Wang, J.; Wan, K.; Gong, C.; Chen, X.; Guo, J.; Xu, Y.; He, J.; Yin, L.; et al. Prognostic Value of Hepatic Native T1 and Extracellular Volume Fraction in Patients with Pulmonary Arterial Hypertension. J. Am. Heart Assoc. 2022, 11, e026254. [Google Scholar] [CrossRef]
- Emrich, T.; Bordonaro, V.; Schoepf, U.J.; Petrescu, A.; Bs, G.Y.; Halfmann, M.; Schoeler, T.; Decker, J.; Abidoye, I.; Emrich, A.L.; et al. Right/Left Ventricular Blood Pool T2 Ratio as an Innovative Cardiac MRI Screening Tool for the Identification of Left-to-Right Shunts in Patients with Right Ventricular Disease. J. Magn. Reson. Imaging 2022, 55, 1452–1458. [Google Scholar] [CrossRef] [PubMed]
- Varghese, J.; Potter, L.C.; LaFountain, R.; Pan, X.; Raman, S.V.; Ahmad, R.; Simonetti, O.P. CMR-based blood oximetry via multi-parametric estimation using multiple T2 measurements. J. Cardiovasc. Magn. Reson. 2017, 19, 88. [Google Scholar] [CrossRef]
- Saini, B.S.; Darby, J.R.T.; Portnoy, S.; Sun, L.; Amerom, J.; Lock, M.C.; Soo, J.Y.; Holman, S.L.; Perumal, S.R.; Kingdom, J.C.; et al. Normal human and sheep fetal vessel oxygen saturations by T2 magnetic resonance imaging. J. Physiol. 2020, 598, 3259–3281. [Google Scholar] [CrossRef]
- Thavendiranathan, P.; Walls, M.; Giri, S.; Verhaert, D.; Rajagopalan, S.; Moore, S.; Simonetti, O.P.; Raman, S.V. Improved Detection of Myocardial Involvement in Acute Inflammatory Cardiomyopathies Using T2 Mapping. Circ. Cardiovasc. Imaging 2012, 5, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Hinkle, D.E.; Wiersma, W.; Jurs, S.G. Applied Statistics for the Behavioral Sciences, 5th ed.; Houghton Mifflin: Boston, MA, USA, 2003. [Google Scholar]
- Bull, S.; White, S.K.; Piechnik, S.K.; Flett, A.S.; Ferreira, V.; Loudon, M.; Francis, J.M.; Karamitsos, T.; Prendergast, B.D.; Robson, M.D.; et al. Human non-contrast T1 values and correlation with histology in diffuse fibrosis. Heart 2013, 99, 932–937. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-P.; Lee, W.; Lee, J.M.; Park, E.-A.; Kim, H.-K.; Kim, Y.-J.; Sohn, D.-W. Assessment of Diffuse Myocardial Fibrosis by Using MR Imaging in Asymptomatic Patients with Aortic Stenosis. Radiology 2015, 274, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Dass, S.; Suttie, J.J.; Piechnik, S.K.; Ferreira, V.M.; Holloway, C.J.; Banerjee, R.; Mahmod, M.; Cochlin, L.; Karamitsos, T.D.; Robson, M.D.; et al. Myocardial Tissue Characterization Using Magnetic Resonance Noncontrast T1 Mapping in Hypertrophic and Dilated Cardiomyopathy. Circ. Cardiovasc. Imaging 2012, 5, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Karamitsos, T.D.; Piechnik, S.K.; Banypersad, S.M.; Fontana, M.; Ntusi, N.B.; Ferreira, V.M.; Whelan, C.J.; Myerson, S.G.; Robson, M.D.; Hawkins, P.N.; et al. Noncontrast T1 Mapping for the Diagnosis of Cardiac Amyloidosis. JACC Cardiovasc. Imaging 2013, 6, 488–497. [Google Scholar] [CrossRef]
- Fontana, M.; Banypersad, S.M.; Treibel, T.A.; Maestrini, V.; Sado, D.M.; White, S.K.; Pica, S.; Castelletti, S.; Piechnik, S.K.; Robson, M.D.; et al. Native T1 Mapping in Transthyretin Amyloidosis. JACC Cardiovasc. Imaging 2014, 7, 157–165. [Google Scholar] [CrossRef]
- Sado, D.M.; Maestrini, V.; Piechnik, S.K.; Banypersad, S.M.; White, S.K.; Flett, A.S.; Robson, M.D.; Neubauer, S.; Ariti, C.; Arai, A.; et al. Noncontrast myocardial T1 mapping using cardiovascular magnetic resonance for iron overload. J. Magn. Reson. Imaging 2015, 41, 1505–1511. [Google Scholar] [CrossRef]
- Roller, F.C.; Fuest, S.; Meyer, M.; Harth, S.; Gündüz, D.; Bauer, P.; Schneider, C.; Rolfs, A.; Krombach, G.A.; Tanislav, C. Assessment of Cardiac Involvement in Fabry Disease (FD) with Native T1 Mapping. Rofo 2019, 191, 932–939. [Google Scholar] [CrossRef] [PubMed]
- Messroghli, D.R.; Moon, J.C.; Ferreira, V.M.; Grosse-Wortmann, L.; He, T.; Kellman, P.; Mascherbauer, J.; Nezafat, R.; Salerno, M.; Schelbert, E.B.; et al. Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: A consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI). J. Cardiovasc. Magn. Reson. 2017, 19, 1–24, Erratum in: J. Cardiovasc. Magn. Reson. 2018, 20, 9. [Google Scholar] [CrossRef]
- Thulborn, K.R.; Waterton, J.C.; Matthews, P.M.; Radda, G.K. Oxygenation dependence of the transverse relaxation time of water protons in whole blood at high field. Biochim. Et Biophys. Acta 1982, 714, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Soto, A.E.; Abdulmalik, O.; Langham, M.C.; Schwartz, N.; Lee, H.; Wehrli, F.W. T2-prepared balanced steady-state free precession (bSSFP) for quantifying whole-blood oxygen saturation at 1.5T. Magn. Reson. Med. 2018, 79, 1893–1900. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.-L.; Qin, Q.; Zhao, X.; Duong, T. Blood longitudinal (T1) and transverse (T2) relaxation time constants at 11.7 Tesla. Magn. Reson. Mater. Phys. Biol. Med. 2012, 25, 245–249. [Google Scholar] [CrossRef]
- Liu, P.; Chalak, L.F.; Krishnamurthy, L.C.; Mir, I.; Peng, S.-L.; Huang, H.; Lu, H. T1 and T2 values of human neonatal blood at 3 Tesla: Dependence on hematocrit, oxygenation, and temperature. Magn. Reson. Med. 2016, 75, 1730–1735. [Google Scholar] [CrossRef]

| n = 26 | |
|---|---|
| Age, years | 64.8 ± 12.8 |
| Female gender | 15 (57.7) |
| Body mass index (BMI), kg/m² | 24.7 ± 2.9 |
| Diabetes mellitus | 3 (11.5) |
| Arterial hypertension | 16 (61.5) |
| Smoking | 9 (34.6) |
| Coronary artery disease (CAD) | 5 (19) |
| Atrial fibrillation | 2 (7.7) |
| Chronic kidney failure | 5 (19.2) |
| Glomerular filtration rate, mL/min | 78 ± 20 |
| Creatinine, µmol/L | 0.95 ± 0.27 |
| Cancer history | 5 (19.2) |
| Chronic obstructive pulmonary disease (COPD) | 1 (3.8) |
| History acute pulmonary embolism | 22 (84.6) |
| LV T1 | RV T1 | p | LV T2 | RV T2 | p | |
|---|---|---|---|---|---|---|
| CTEPH | 1493.1 ± 92.0 | 1460.2 ± 100.7 | 0.0063 | 237.4 ± 50.1 | 172.2 ± 35.8 | <0.0001 |
| Controls | 1465.0 ± 64.5 | 1431.2 ± 71.7 | 0.0002 | 233.0 ± 38.4 | 199.8 ± 35.2 | <0.0001 |
| CTEPH | Controls | p | |
|---|---|---|---|
| LV Native T1 time (ms) | 1493.1 ± 92.0 | 1465.0 ± 64.5 | 0.2827 |
| RV Native T1 time (ms) | 1460.2 ± 100.7 | 1431.2 ± 71.7 | 0.3122 |
| LV T2 time (ms) | 237.4 ± 50.1 | 233.0 ± 38.4 | 0.8270 |
| RV T2 time (ms) | 172.2 ± 35.8 | 199.8 ± 35.2 | 0.0065 |
| RVT1/LVT1 | 0.98 ± 0.03 | 0.98 ± 0.03 | 0.8809 |
| RVT2/LVT2 | 0.72 ± 0.11 | 0.87 ± 0.13 | 0.0006 |
| CTEPH Pre-BPA | CTEPH Post-BPA | p | |
|---|---|---|---|
| RVEF | 36.1 ± 11.6 | 50.0 ± 7.8 | 0.0001 |
| mPAP | 40.8 ± 7.3 | 31.3 ± 5.9 | 0.0001 |
| PVR | 532.2 ± 186.3 | 347.2 ± 104.2 | 0.0001 |
| CI | 2.6 ± 0.8 | 2.8 ± 0.7 | 0.08 |
| CTEPH Pre-BPA | CTEPH Post-BPA | p | |
|---|---|---|---|
| LV Native T1 time (ms) | 1493.1 ± 92.0 | 1518.3 ± 105.6 | 0.1482 |
| RV Native T1 time (ms) | 1460.2 ± 100.7 | 1480.4 ± 104.7 | 0.1375 |
| LV T2 time (ms) | 237.4 ± 50.1 | 253.4 ± 40.0 | 0.0678 |
| RV T2 time (ms) | 172.2 ± 35.8 | 196.7 ± 38.6 | 0.0048 |
| RVT1/LVT1 | 0.98 ± 0.03 | 0.98 ± 0.03 | 0.6528 |
| RVT2/LVT2 | 0.72 ± 0.11 | 0.78 ± 0.11 | 0.0036 |
| R | CI 95% | p Value | |
|---|---|---|---|
| RVT2 to CI | |||
| Pre-BPA | 0.5155 | 0.1483 to 0.7578 | 0.0070 |
| Post-BPA | 0.4769 | 0.09784 to 0.7351 | 0.0138 |
| RVT2 to mPAP | |||
| Pre-BPA | −0.2541 | −0.5919 to 0.1596 | 0.2104 |
| Post-BPA | −0.2585 | −0.5950 to −0.1550 | 0.2022 |
| RVT2 to PVR | |||
| Pre-BPA | −0.4571 | −0.7232 to −0.07270 | 0.0189 |
| Post-BPA | −0.4396 | −0.7126 to −0.05092 | 0.0246 |
| R | CI 95% | p Value | |
|---|---|---|---|
| T2 Ratio to CI | |||
| Pre-BPA | 0.06735 | −0.3393 to 0.4528 | 0.7437 |
| Post-BPA | 0.07223 | −0.7061 to −0.03788 | 0.7259 |
| T2 Ratio to mPAP | |||
| Pre-BPA | −0.1835 | −0.5416 to 0.2309 | 0.3965 |
| Post-BPA | −0.4290 | −0.7061 to −0.03788 | 0.0288 |
| T2 Ratio to PVR | |||
| Pre-BPA | −0.2109 | −0.5614 to 0.2037 | 0.3010 |
| Post-BPA | −0.4326 | −0.7084 to −0.04234 | 0.0273 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roller, F.C.; Schüßler, A.; Kremer, N.; Harth, S.; Kriechbaum, S.D.; Wiedenroth, C.B.; Guth, S.; Breithecker, A.; Richter, M.; Tello, K.; et al. Value of Right and Left Ventricular T1 and T2 Blood Pool Mapping in Patients with Chronic Thromboembolic Hypertension before and after Balloon Pulmonary Angioplasty. J. Clin. Med. 2023, 12, 2092. https://doi.org/10.3390/jcm12062092
Roller FC, Schüßler A, Kremer N, Harth S, Kriechbaum SD, Wiedenroth CB, Guth S, Breithecker A, Richter M, Tello K, et al. Value of Right and Left Ventricular T1 and T2 Blood Pool Mapping in Patients with Chronic Thromboembolic Hypertension before and after Balloon Pulmonary Angioplasty. Journal of Clinical Medicine. 2023; 12(6):2092. https://doi.org/10.3390/jcm12062092
Chicago/Turabian StyleRoller, Fritz C., Armin Schüßler, Nils Kremer, Sebastian Harth, Steffen D. Kriechbaum, Christoph B. Wiedenroth, Stefan Guth, Andreas Breithecker, Manuel Richter, Khodr Tello, and et al. 2023. "Value of Right and Left Ventricular T1 and T2 Blood Pool Mapping in Patients with Chronic Thromboembolic Hypertension before and after Balloon Pulmonary Angioplasty" Journal of Clinical Medicine 12, no. 6: 2092. https://doi.org/10.3390/jcm12062092
APA StyleRoller, F. C., Schüßler, A., Kremer, N., Harth, S., Kriechbaum, S. D., Wiedenroth, C. B., Guth, S., Breithecker, A., Richter, M., Tello, K., Seeger, W., Mayer, E., & Krombach, G. A. (2023). Value of Right and Left Ventricular T1 and T2 Blood Pool Mapping in Patients with Chronic Thromboembolic Hypertension before and after Balloon Pulmonary Angioplasty. Journal of Clinical Medicine, 12(6), 2092. https://doi.org/10.3390/jcm12062092






