Do Cortisol and Dehydroepiandrosterone Influence Motivational Factors for Non-Suicidal Self-Injury in Female Adolescents?
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Clinical Assessment
- -
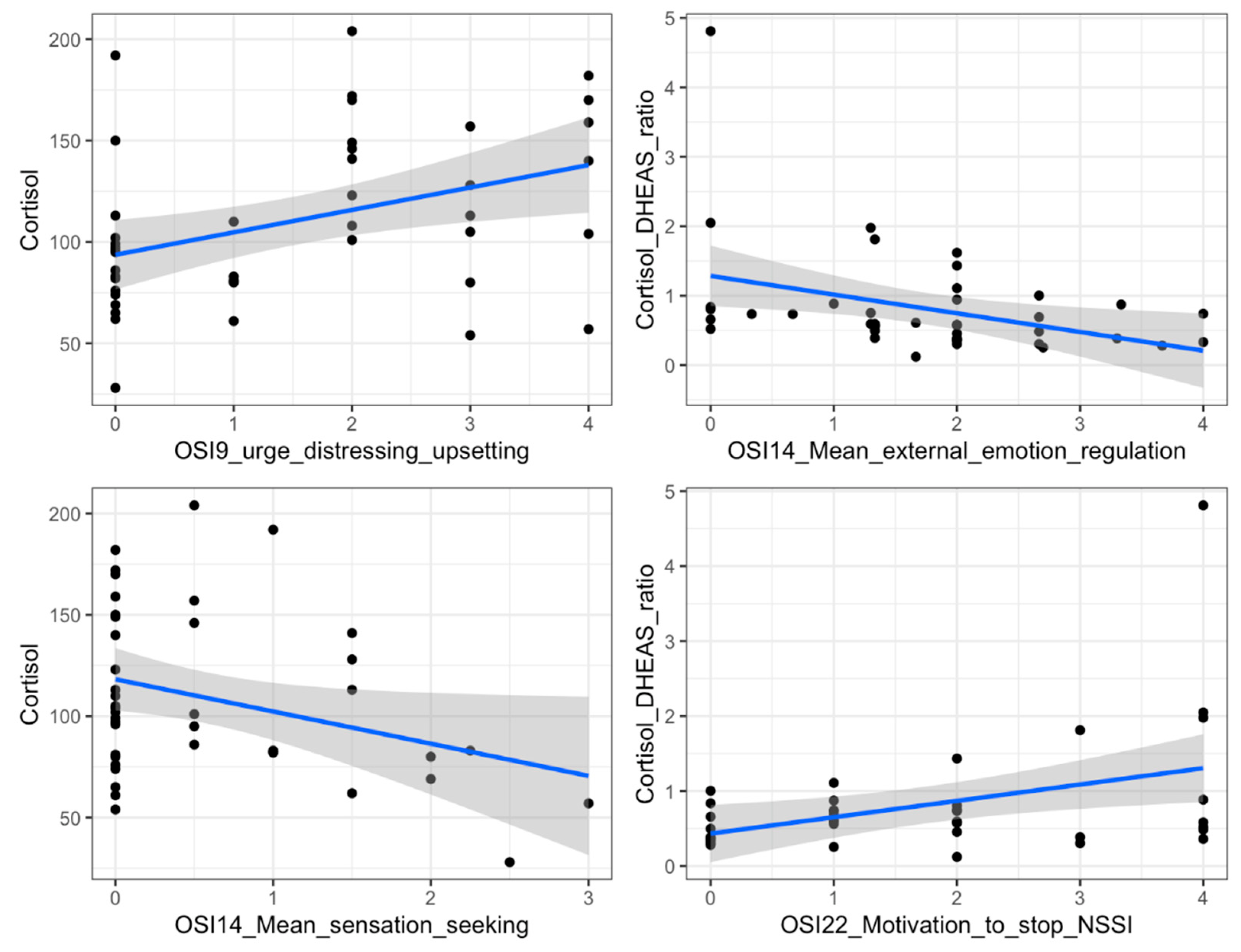
- No. 9 (“When you get the urge to hurt yourself: The urge is distressing/upsetting; The urge is comforting; The urge is intrusive/invasive”), where for each of the three possible urges for each subject the scores range from 0 (not at all) to 4 (extremely);
- -
- No. 14 (“Why do you think you started and if you continue, why do you still self-injure without meaning to kill yourself?”), where the scores range from 0 (never a reason) to 4 (always a reason), and are subsequently scored in terms of IER (the total score, variable between 0 and 24, is the sum of the scores of subitems 4, 6, 9, 14, 16, 18), SI (the total score, variable between 0 and 28, is the sum of the scores of subitems 3, 9, 10, 11, 13, 15, 21), EER (the total score, variable between 0 and 12, is the sum of the scores of subitems 1, 12, 20), SS (the total score, variable between 0 and 16, is the sum of the scores of subitems 2, 7, 22, 23);
- -
- No. 22 (“How motivated are you at this time to stop self-injuring?”), where scores range from 0 (not motivated at all) to 4 (extremely motivated).
2.3. Laboratory Analysis
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pattison, E.M.; Kahan, J. The Deliberate Self-Harm Syndrome. Am. J. Psychiatry 1983, 140, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Favazza, A.R. The Coming of Age of Self-Mutilation. J. Nerv. Ment. Dis. 1998, 186, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Lloyd-Richardson, E.E.; Perrine, N.; Dierker, L.; Kelley, M.L. Characteristics and Functions of Non-Suicidal Self-Injury in a Community Sample of Adolescents. Psychol. Med. 2007, 37, 1183. [Google Scholar] [CrossRef] [PubMed]
- Zetterqvist, M.; Lundh, L.G.; Dahlström, Ö.; Svedin, C.G. Prevalence and Function of Non-Suicidal Self-Injury (NSSI) in a Community Sample of Adolescents, Using Suggested DSM-5 Criteria for a Potential NSSI Disorder. J. Abnorm. Child Psychol. 2013, 41, 759–773. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, M.; Terrinoni, A.; Williams, R. Non-Suicidal Self-Injury (Nssi) in Adolescent Inpatients: Assessing Personality Features and Attitude toward Death. Child Adolesc. Psychiatry Ment. Health 2012, 6, 1–8. [Google Scholar] [CrossRef]
- Koenig, J.; Rinnewitz, L.; Warth, M.; Hillecke, T.K.; Brunner, R.; Resch, F.; Kaess, M. Psychobiological Response to Pain in Female Adolescents with Nonsuicidal Self-Injury. J. Psychiatry Neurosci. 2017, 42, 189. [Google Scholar] [CrossRef]
- Kapur, N.; Cooper, J.; O’Connor, R.C.; Hawton, K. Non-Suicidal Self-Injury v. Attempted Suicide: New Diagnosis or False Dichotomy? Br. J. Psychiatry 2013, 202, 326–328. [Google Scholar] [CrossRef]
- Plener, P.L.; Libal, G.; Keller, F.; Fegert, J.M.; Muehlenkamp, J.J. An International Comparison of Adolescent Non-Suicidal Self-Injury (NSSI) and Suicide Attempts: Germany and the USA. Psychol. Med. 2009, 39, 1549–1558. [Google Scholar] [CrossRef]
- Bachmann, S. Epidemiology of Suicide and the Psychiatric Perspective. Int. J. Environ Res. Public Health 2018, 15, 1425. [Google Scholar] [CrossRef]
- Muehlenkamp, J.J.; Gutierrez, P.M. An Investigation of Differences between Self-Injurious Behavior and Suicide Attempts in a Sample of Adolescents. Suicide Life Threat Behav. 2004, 34, 12–23. [Google Scholar] [CrossRef]
- Glenn, C.R.; Klonsky, E.D. Nonsuicidal Self-Injury Disorder: An Empirical Investigation in Adolescent Psychiatric Patients. J. Clin. Child Adolesc. Psychol. 2013, 42, 496–507. [Google Scholar] [CrossRef]
- Groschwitz, R.C.; Plener, P.L.; Kaess, M.; Schumacher, T.; Stoehr, R.; Boege, I. The Situation of Former Adolescent Self-Injurers as Young Adults: A Follow-up Study. BMC Psychiatry 2015, 15, 1–9. [Google Scholar] [CrossRef]
- Ivey-Stephenson, A.Z.; Demissie, Z.; Crosby, A.E.; Stone, D.M.; Gaylor, E.; Wilkins, N.; Lowry, R.; Brown, M. Suicidal Ideation and Behaviors Among High School Students—Youth Risk Behavior Survey, United States. 2019. MMWR Suppl. 2020, 69, 47–55. [Google Scholar] [CrossRef]
- Jefsen, O.H.; Rohde, C.; Nørremark, B.; Østergaard, S.D. COVID-19-related Self-harm and Suicidality among Individuals with Mental Disorders. Acta Psychiatr. Scand. 2020, 142, 152. [Google Scholar] [CrossRef]
- Johns, M.M.; Lowry, R.; Haderxhanaj, L.T.; Rasberry, C.N.; Robin, L.; Scales, L.; Stone, D.; Suarez, N.A. Trends in Violence Victimization and Suicide Risk by Sexual Identity Among High School Students—Youth Risk Behavior Survey, United States. 2015–2019. MMWR Suppl. 2020, 69, 19. [Google Scholar] [CrossRef]
- Zetterqvist, M. The DSM-5 Diagnosis of Nonsuicidal Self-Injury Disorder: A Review of the Empirical Literature. Child Adolesc Psychiatry Ment. Health 2015, 9, 1–13. [Google Scholar] [CrossRef]
- Klonsky, E.D.; Victor, S.E.; Saffer, B.Y. Nonsuicidal Self-Injury: What We Know, and What We Need to Know. Can. J. Psychiatry 2014, 59, 565. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5-TR; American Psychiatric Association Publishing: Washington, DC, USA, 2022. [Google Scholar]
- MacIejewski, D.F.; Creemers, H.E.; Lynskey, M.T.; Madden, P.A.F.; Heath, A.C.; Statham, D.J.; Martin, N.G.; Verweij, K.J.H. Overlapping Genetic and Environmental Influences on Nonsuicidal Self-Injury and Suicidal Ideation: Different Outcomes, Same Etiology? JAMA Psychiatry 2014, 71, 699–705. [Google Scholar] [CrossRef]
- Russell, A.E.; Hemani, G.; Jones, H.J.; Ford, T.; Gunnell, D.; Heron, J.; Joinson, C.; Moran, P.; Relton, C.; Suderman, M.; et al. An Exploration of the Genetic Epidemiology of Non-Suicidal Self-Harm and Suicide Attempt. BMC Psychiatry 2021, 21, 207. [Google Scholar] [CrossRef]
- Juruena, M.F.; Eror, F.; Cleare, A.J.; Young, A.H. The Role of Early Life Stress in HPA Axis and Anxiety. Adv. Exp. Med. Biol. 2020, 1191, 141–153. [Google Scholar] [CrossRef]
- Kaess, M.; Hille, M.; Parzer, P.; Maser-Gluth, C.; Resch, F.; Brunner, R. Alterations in the Neuroendocrinological Stress Response to Acute Psychosocial Stress in Adolescents Engaging in Nonsuicidal Self-Injury. Psychoneuroendocrinology 2012, 37, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Nock, M.K. Why Do People Hurt Themselves?: New Insights into the Nature and Functions of Self-Injury. Curr. Dir. Psychol. Sci. 2009, 18, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Cyders, M.A.; Smith, G.T. Emotion-Based Dispositions to Rash Action: Positive and Negative Urgency. Psychol. Bull. 2008, 134, 807. [Google Scholar] [CrossRef] [PubMed]
- Cyders, M.A.; Smith, G.T. Mood-Based Rash Action and Its Components: Positive and Negative Urgency. Pers. Individ. Dif. 2007, 43, 839–850. [Google Scholar] [CrossRef]
- Anestis, M.D.; Joiner, T.E. Examining the Role of Emotion in Suicidality: Negative Urgency as an Amplifier of the Relationship between Components of the Interpersonal-Psychological Theory of Suicidal Behavior and Lifetime Number of Suicide Attempts. J. Affect Disord. 2011, 129, 261–269. [Google Scholar] [CrossRef]
- Beauchaine, T.P. Physiological Markers of Emotional and Behavioral Dysregulation in Externalizing Psychopathology. Monogr. Soc. Res. Child Dev. 2012, 77, 79. [Google Scholar] [CrossRef]
- Pizzie, R.G.; Kraemer, D.J.M. The Association Between Emotion Regulation, Physiological Arousal, and Performance in Math Anxiety. Front. Psychol. 2021, 12, 1465. [Google Scholar] [CrossRef]
- Jones, E.J.; Rohleder, N.; Schreier, H.M.C. Neuroendocrine Coordination and Youth Behavior Problems: A Review of Studies Assessing Sympathetic Nervous System and Hypothalamic-Pituitary Adrenal Axis Activity Using Salivary Alpha Amylase and Salivary Cortisol. Horm. Behav. 2020, 122, 104750. [Google Scholar] [CrossRef]
- Kamin, H.S.; Kertes, D.A. Cortisol and DHEA in Development and Psychopathology. Horm. Behav. 2017, 89, 69–85. [Google Scholar] [CrossRef]
- Nicolaides, N.C.; Kyratzi, E.; Lamprokostopoulou, A.; Chrousos, G.P.; Charmandari, E. Stress, the Stress System and the Role of Glucocorticoids. Neuroimmunomodulation 2015, 22, 6–19. [Google Scholar] [CrossRef]
- Campbell, B.C. Adrenarche and Middle Childhood. Hum Nat. 2011, 22, 327–349. [Google Scholar] [CrossRef]
- Farooqi, N.A.I.; Scotti, M.; Lew, J.M.; Botteron, K.N.; Karama, S.; McCracken, J.T.; Nguyen, T.V. Role of DHEA and Cortisol in Prefrontal-Amygdalar Development and Working Memory. Psychoneuroendocrinology 2018, 98, 86. [Google Scholar] [CrossRef]
- Van Niekerk, J.K.; Huppert, F.A.; Herbert, J. Salivary Cortisol and DHEA: Association with Measures of Cognition and Well-Being in Normal Older Men, and Effects of Three Months of DHEA Supplementation. Psychoneuroendocrinology 2001, 26, 591–612. [Google Scholar] [CrossRef]
- Poon, J.A.; Turpyn, C.C.; Hansen, A.; Jacangelo, J.; Chaplin, T.M. Adolescent Substance Use & Psychopathology: Interactive Effects of Cortisol Reactivity and Emotion Regulation. Cognit. Ther. Res. 2016, 40, 368–380. [Google Scholar] [CrossRef]
- Angold, A. Adolescent Depression, Cortisol and DHEA. Psychol. Med. 2003, 33, 573–581. [Google Scholar] [CrossRef]
- Pajer, K.; Tabbah, R.; Gardner, W.; Rubin, R.T.; Kenneth Czambel, R.; Wang, Y. Adrenal Androgen and Gonadal Hormone Levels in Adolescent Girls with Conduct Disorder. Psychoneuroendocrinology 2006, 31, 1245–1256. [Google Scholar] [CrossRef]
- Hernández-Díaz, Y.; González-Castro, T.B.; Tovilla-Zárate, C.A.; Juárez-Rojop, I.E.; López-Narváez, M.L.; Pérez-Hernández, N.; Rodríguez-Pérez, J.M.; Genis-Mendoza, A.D.; Nicolini, H. The Role of Peripheral Cortisol Levels in Suicide Behavior: A Systematic Review and Meta-Analysis of 30 Studies. Psychiatry Res. 2020, 293, 113448. [Google Scholar] [CrossRef]
- Gmitrowicz, A.; Kołodziej-Maciejewska, H. Dysfunkcja osi podwzgórzowo-przysadkowo-nadnerczowej u młodocianych po próbach samobójczych [Dysfunction of the hypothalamic-pituitary-adrenal axis in adolescents after a suicide attempt]. Psychiatr. Pol. 2001, 35, 803–818. [Google Scholar]
- Peng, B.; Li, J.; Liu, H.; Fang, H.; Zhao, W.; Chen, G.; Xiu, M.; Zhang, Y. Childhood Maltreatment Low Serum Cortisol Levels, and Non-Suicidal Self-Injury in Young Adults With Major Depressive Disorders. Front. Pediatr. 2022, 10, 822046. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar] [CrossRef]
- Martin, J.; Cloutier, P.; Levesque, C.; Bureau, J.-F. Psychometric Properties of the Functions and Addictive Features Scales of the Ottawa Self-Injury Inventory: A Preliminary Investigation Using a University Sample Mental Health in Pediatric ED View Project Emotionally Focused Therapy: Creating Connection Also in Spanish Language? View Project. Artic. Psychol. Assess. 2013, 25, 1013–1018. [Google Scholar] [CrossRef]
- Nixon, M.K.; Levesque, C.; Preyde, M.; Vanderkooy, J.; Cloutier, P.F. The Ottawa Self-Injury Inventory: Evaluation of an Assessment Measure of Nonsuicidal Self-Injury in an Inpatient Sample of Adolescents. Child Adolesc. Psychiatry Ment. Health 2015, 9, 26. [Google Scholar] [CrossRef] [PubMed]
- van der Voorn, B.; Hollanders, J.J.; Ket, J.C.F.; Rotteveel, J.; Finken, M.J.J. Gender-Specific Differences in Hypothalamus-Pituitary-Adrenal Axis Activity during Childhood: A Systematic Review and Meta-Analysis. Biol. Sex Differ. 2017, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bresin, K.; Schoenleber, M. Gender Differences in the Prevalence of Nonsuicidal Self-Injury: A Meta-Analysis. Clin. Psychol. Rev. 2015, 38, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Lockwood, J.; Daley, D.; Townsend, E.; Sayal, K. Impulsivity and Self-Harm in Adolescence: A Systematic Review. Eur. Child Adolesc. Psychiatry 2017, 26, 387–402. [Google Scholar] [CrossRef] [PubMed]
- Dorn, L.D. Measuring Puberty. J. Adolesc. Health 2006, 39, 625–626. [Google Scholar] [CrossRef]
- Marshall, W.A.; Tanner, J.M. Variations in the Pattern of Pubertal Changes in Boys. Arch. Dis. Child 1970, 45, 13. [Google Scholar] [CrossRef]
- Marshall, W.A.; Tanner, J.M. Variations in Pattern of Pubertal Changes in Girls. Arch. Dis. Child 1969, 44, 291. [Google Scholar] [CrossRef]
- Ordaz, S.; Luna, B. Sex Differences in Physiological Reactivity to Acute Psychosocial Stress in Adolescence. Psychoneuroendocrinology 2012, 37, 1135. [Google Scholar] [CrossRef]
- McKlveen, J.M.; Myers, B.; Flak, J.N.; Bundzikova, J.; Solomon, M.B.; Seroogy, K.B.; Herman, J.P. Role of Prefrontal Cortex Glucocorticoid Receptors in Stress and Emotion. Biol. Psychiatry 2013, 74, 672. [Google Scholar] [CrossRef]
- VanderVeen, J.D.; Plawecki, M.H.; Millward, J.B.; Hays, J.; Kareken, D.A.; O’Connor, S.; Cyders, M.A. Negative Urgency, Mood Induction, and Alcohol Seeking Behaviors. Drug Alcohol. Depend. 2016, 165, 151. [Google Scholar] [CrossRef]
- Turner, B.J.; Cobb, R.J.; Gratz, K.L.; Chapman, A.L. The Role of Interpersonal Conflict and Perceived Social Support in Nonsuicidal Self-Injury in Daily Life. J. Abnorm. Psychol. 2016, 125, 588–598. [Google Scholar] [CrossRef]
- Andrewes, H.E.; Hulbert, C.; Cotton, S.M.; Betts, J.; Chanen, A.M. An Ecological Momentary Assessment Investigation of Complex and Conflicting Emotions in Youth with Borderline Personality Disorder. Psychiatry Res. 2017, 252, 102–110. [Google Scholar] [CrossRef]
- Humber, N.; Webb, R.; Piper, M.; Appleby, L.; Shaw, J. A National Case–Control Study of Risk Factors for Suicide among Prisoners in England and Wales [Corrected]. Soc. Psychiatry Psychiatr. Epidemiol. 2013, 48, 1177–1185. [Google Scholar] [CrossRef]
- Nock, M.K.; Prinstein, M.J. A Functional Approach to the Assessment of Self-Mutilative Behavior. J. Consult. Clin. Psychol. 2004, 72, 885–890. [Google Scholar] [CrossRef]
- Koenig, J.; Rinnewitz, L.; Warth, M.; Kaess, M. Autonomic Nervous System and Hypothalamic-Pituitary-Adrenal Axis Response to Experimentally Induced Cold Pain in Adolescent Non-Suicidal Self-Injury--Study Protocol. BMC Psychiatry 2015, 15. [Google Scholar] [CrossRef]
- Matera, E.; Margari, M.; Serra, M.; Petruzzelli, M.G.; Gabellone, A.; Piarulli, F.M.; Pugliese, A.; Ritatassiello, A.; Croce, F.; Renna, C.; et al. Non-Suicidal Self-Injury: An Observational Study in a Sample of Adolescents and Young Adults. Brain Sci. 2021, 11, 974. [Google Scholar] [CrossRef]
- Reuter, M. Impact of Cortisol on Emotions under Stress and Nonstress Conditions: A Pharmacopsychological Approach. Neuropsychobiology 2002, 46, 41–48. [Google Scholar] [CrossRef]
- Het, S.; Wolf, O.T. Mood Changes in Response to Psychosocial Stress in Healthy Young Women: Effects of Pretreatment with Cortisol. Behav. Neurosci. 2007, 121, 11–20. [Google Scholar] [CrossRef]
- Het, S.; Schoofs, D.; Rohleder, N.; Wolf, O.T. Stress-Induced Cortisol Level Elevations Are Associated with Reduced Negative Affect after Stress: Indications for a Mood-Buffering Cortisol Effect. Psychosom. Med. 2012, 74, 23–32. [Google Scholar] [CrossRef]
- Soravia, L.M.; Heinrichs, M.; Aerni, A.; Maroni, C.; Schelling, G.; Ehlert, U.; Roozendaal, B.; de Quervain, D.J.F. Glucocorticoids Reduce Phobic Fear in Humans. Proc. Natl. Acad. Sci. USA 2006, 103, 5585. [Google Scholar] [CrossRef]
- McEwen, B.S.; Seeman, T. Protective and Damaging Effects of Mediators of Stress. Elaborating and Testing the Concepts of Allostasis and Allostatic Load. Ann. NY Acad. Sci. 1999, 896, 30–47. [Google Scholar] [CrossRef] [PubMed]
- Kliewer, W. Victimization and Biological Stress Responses in Urban Adolescents: Emotion Regulation as a Moderator. J. Youth Adolesc. 2016, 45, 1812–1823. [Google Scholar] [CrossRef] [PubMed]
- Cahill, L.; van Stegeren, A. Sex-Related Impairment of Memory for Emotional Events with β-Adrenergic Blockade. Neurobiol. Learn Mem. 2003, 79, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Canli, T.; Desmond, J.E.; Zhao, Z.; Gabrieli, J.D.E. Sex Differences in the Neural Basis of Emotional Memories. Proc. Natl. Acad. Sci. USA 2002, 99, 10789. [Google Scholar] [CrossRef] [PubMed]
- Bradley, M.M.; Codispoti, M.; Sabatinelli, D.; Lang, P.J. Emotion and Motivation II: Sex Differences in Picture Processing. Emotion 2001, 1, 300–319. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.E. Tend and Befriend: Biobehavioral Bases of Affiliation under Stress. Curr. Dir. Psychol. Sci. 2006, 15, 273–277. [Google Scholar] [CrossRef]
- Okabe, S.; Kitano, K.; Nagasawa, M.; Mogi, K.; Kikusui, T. Testosterone Inhibits Facilitating Effects of Parenting Experience on Parental Behavior and the Oxytocin Neural System in Mice. Physiol. Behav. 2013, 118, 159–164. [Google Scholar] [CrossRef]
- Young Kuchenbecker, S.; Pressman, S.D.; Celniker, J.; Grewen, K.M.; Sumida, K.D.; Jonathan, N.; Everett, B.; Slavich, G.M. Oxytocin, Cortisol, and Cognitive Control during Acute and Naturalistic Stress. Stress 2021, 24, 370. [Google Scholar] [CrossRef]
- Kinner, V.L.; Het, S.; Wolf, O.T. Emotion Regulation: Exploring the Impact of Stress and Sex. Front. Behav. Neurosci. 2014, 8, 1–8. [Google Scholar] [CrossRef]
- Compton, R.J.; Hofheimer, J.; Kazinka, R. Stress Regulation and Cognitive Control: Evidence Relating Cortisol Reactivity and Neural Responses to Errors. Cogn. Affect Behav. Neurosci. 2013, 13, 152. [Google Scholar] [CrossRef]
- Mikkelsen, M.B.; Tramm, G.; Zachariae, R.; Gravholt, C.H.; O’Toole, M.S. A Systematic Review and Meta-Analysis of the Effect of Emotion Regulation on Cortisol. Compr. Psychoneuroendocrinol 2021, 5, 100020. [Google Scholar] [CrossRef]
- Turner, B.J.; Chapman, A.L.; Gratz, K.L. Why Stop Self-Injuring? Development of the Reasons to Stop Self-Injury Questionnaire. Behav. Modif. 2014, 38, 69–106. [Google Scholar] [CrossRef]
- Cicchetti, D.; Rogosch, F.A. Personality, Adrenal Steroid Hormones, and Resilience in Maltreated Children: A Multilevel Perspective. Dev. Psychopathol. 2007, 19, 787–809. [Google Scholar] [CrossRef]
- Petros, N.; Opacka-Juffry, J.; Huber, J.H. Psychometric and Neurobiological Assessment of Resilience in a Non-Clinical Sample of Adults. Psychoneuroendocrinology 2013, 38, 2099–2108. [Google Scholar] [CrossRef]
- Baetens, I.; Decruy, C.; Vatandoost, S.; Vanderhaegen, B.; Kiekens, G. School-Based Prevention Targeting Non-Suicidal Self-Injury: A Pilot Study. Front. Psychiatry 2020, 11, 437. [Google Scholar] [CrossRef]
- Muehlenkamp, J.J.; Barent, A.E.; Ae, W.W.; Mcdade, M. Preventing Non-Suicidal Self-Injury in Adolescents: The Signs of Self-Injury Program. J. Youth Adolesc. 2010, 39, 306–314. [Google Scholar] [CrossRef]
- Cipriano, A.; Aprea, C.; Bellone, L.; Cotrufo, P.; Cella, S. Non-Suicidal Self-Injury: A School-Based Peer Education Program for Adolescents During COVID-19 Pandemic. Front. Psychiatry 2022, 12, 2516. [Google Scholar] [CrossRef]
| DSM-5 Diagnosis | Frequency | Percent |
|---|---|---|
| Depressive disorders | 30 | 69.77 |
| Anxiety disorders | 15 | 34.88 |
| Personality disorders | 15 | 34.88 |
| Neurodevelopmental disorders | 14 | 32.56 |
| Nutrition and eating disorders | 11 | 25.58 |
| Substance-related and addictive disorders | 10 | 23.26 |
| Bipolar disorders | 6 | 13.95 |
| Traumatic and stressful events related disorders | 4 | 9.30 |
| Schizophrenia spectrum disorders | 3 | 6.98 |
| Disruptive behavior, impulse control, and conduct disorders | 3 | 6.98 |
| Somatic symptoms and related disorders | 2 | 4.65 |
| Mean | SD | Range | Minimum | Maximum | |
|---|---|---|---|---|---|
| Cortisol (μg/L) | 110.40 | 41.71 | 176.00 | 28.00 | 204.00 |
| dehydroepiandrosterone sulfate DHEA-s (μg/dL) | 176.01 | 78.26 | 430.20 | 15.80 | 446.00 |
| Cortisol/DHEA-s ratio | 0.86 | 0.82 | 4.69 | 0.12 | 4.81 |
| Mean | SD | Range | Minimum | Maximum | |
|---|---|---|---|---|---|
| OSI9_urge_distressing_upsetting | 1.51 | 1.49 | 4.00 | 0.00 | 4.00 |
| OSI9_urge_conforting | 1.81 | 1.42 | 4.00 | 0.00 | 4.00 |
| OSI9_urge_intrusive_invasive | 1.98 | 1.52 | 4.00 | 0.00 | 4.00 |
| OSI14_Mean_external_emotion_regulation | 1.74 | 1.08 | 4.00 | 0.00 | 4.00 |
| OSI14_Mean_sensation_seeking | 0.58 | 0.84 | 3.00 | 0.00 | 3.00 |
| OSI22_Motivation_to_stop_NSSI | 1.74 | 1.48 | 4.00 | 0.00 | 4.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piarulli, F.M.; Margari, A.; Margari, F.; Matera, E.; Croce, F.; Furente, F.; Gabellone, A.; Petruzzelli, M.G. Do Cortisol and Dehydroepiandrosterone Influence Motivational Factors for Non-Suicidal Self-Injury in Female Adolescents? J. Clin. Med. 2023, 12, 1924. https://doi.org/10.3390/jcm12051924
Piarulli FM, Margari A, Margari F, Matera E, Croce F, Furente F, Gabellone A, Petruzzelli MG. Do Cortisol and Dehydroepiandrosterone Influence Motivational Factors for Non-Suicidal Self-Injury in Female Adolescents? Journal of Clinical Medicine. 2023; 12(5):1924. https://doi.org/10.3390/jcm12051924
Chicago/Turabian StylePiarulli, Francesco Maria, Anna Margari, Francesco Margari, Emilia Matera, Federica Croce, Flora Furente, Alessandra Gabellone, and Maria Giuseppina Petruzzelli. 2023. "Do Cortisol and Dehydroepiandrosterone Influence Motivational Factors for Non-Suicidal Self-Injury in Female Adolescents?" Journal of Clinical Medicine 12, no. 5: 1924. https://doi.org/10.3390/jcm12051924
APA StylePiarulli, F. M., Margari, A., Margari, F., Matera, E., Croce, F., Furente, F., Gabellone, A., & Petruzzelli, M. G. (2023). Do Cortisol and Dehydroepiandrosterone Influence Motivational Factors for Non-Suicidal Self-Injury in Female Adolescents? Journal of Clinical Medicine, 12(5), 1924. https://doi.org/10.3390/jcm12051924






