Type 2 Diabetes Prevention Programs—From Proof-of-Concept Trials to National Intervention and Beyond
Abstract
1. Introduction
2. First RCTs Considered as the Proof-of-Concept Trials on T2D Prevention
3. Long-Term Effects of Preventive Interventions in the T2D Prevention RCTs
4. Lifestyle Outcomes
5. Translation of the Findings from the RCTs to Real-Life Settings
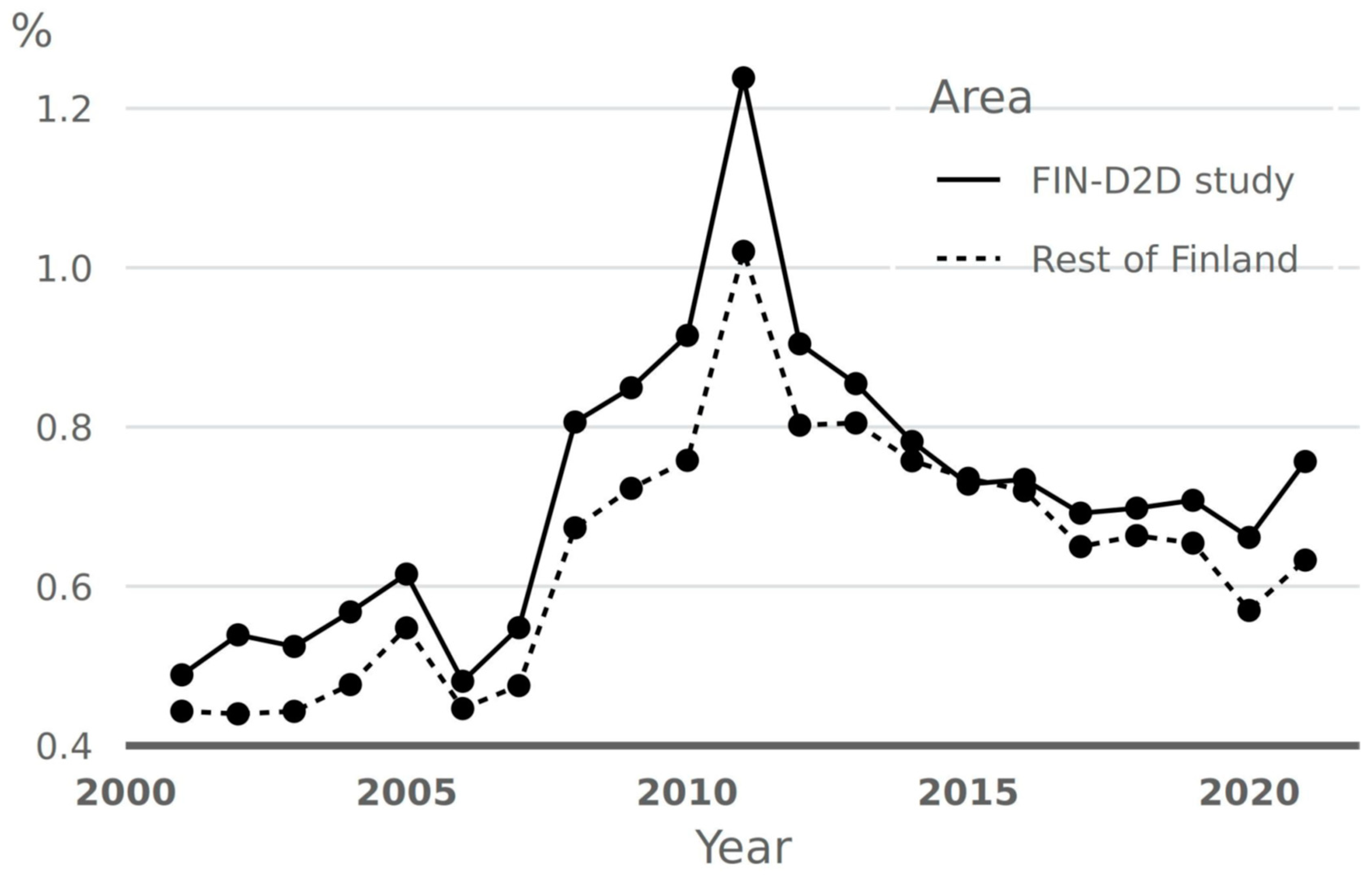
5.1. Implementation Strategies in Finland
5.2. Development of National Programs in the US
5.3. Dissemination of Experiences from the Proof-of-Concept Trials to Other European Countries
5.4. Prevention of T2D in Low- and Middle-Income Countries
5.5. T2D Prevention in Youth
5.6. Challenges to Implement T2D Prevention in High-Risk People in Real-Life
5.7. Precision Medicine in the Prevention of T2D
5.8. Can High-Risk Strategy Be Implemented without a Simultaneous Population Strategy, and What Is Their Cost-Effectiveness?
5.9. Who Are the Main Stakeholders in National Interventions to Prevent T2D, and What Resources Are Needed?
5.10. Social Determinants of Health in T2D Prevention
5.11. Global Treaty for T2D Prevention Is Necessary
- -
- Information on the health consequences of T2D and measures at the appropriate governmental level to protect all persons from exposure to risk factors for T2D;
- -
- Strong political commitment is necessary to develop and support initiatives at the national, regional, and international levels, as well as comprehensive multisectoral measures;
- -
- International cooperation, particularly transfer of technology, knowledge, financial assistance, and provision of related expertise, taking into consideration local culture, as well as social, economic, political, and legal factors;
- -
- Comprehensive multisectoral measures in accordance with evidence-based public health principles;
- -
- Technical and financial assistance addressed in the context of nationally developed strategies for sustainable development;
- -
- The participation of civil society is essential in achieving the objective of the treaty.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Joslin, E. The prevention of diabetes mellitus. JAMA 1921, 76, 79–84. [Google Scholar]
- Hamman, R. Prevention of Type 2 diabetes. In The Evidence for Diabetes Care; Williams, R., Herman, W., Kinmonth, A.L., Wareham, N.J., Eds.; John Wiley & Sons: Chichester, NY, USA, 2002; pp. 75–176. [Google Scholar]
- National Diabetes Data Group. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes 1979, 28, 1039–1057. [Google Scholar] [CrossRef] [PubMed]
- WHO. World Health Organization Expert Committee on Diabetes Mellitus. Second Report. WHO Tech Rep Ser 646; WHO: Geneva, Switzerland, 1980.
- Tuomilehto, J.; Wolf, E. Primary prevention of diabetes mellitus. Diabetes Care 1987, 10, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.R.; Li, G.W.; Hu, Y.H.; Wang, J.-X.; Yang, W.-Y.; An, Z.-X.; Hu, Z.-X.; Lin, J.; Xiao, J.-Z.; Cao, H.-B.; et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance: The Da Qing IGT and Diabetes Study. Diabetes Care 1997, 20, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Tuomilehto, J.; Lindström, J.; Eriksson, J.G.; Valle, T.T.; Hämäläinen, H.; Ilanne-Parikka, P.; Keinänen-Kiukaanniemi, S.; Laakso, M.; Louheranta, A.; Rastas, M.; et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N. Engl. J. Med. 2001, 344, 1343–1350. [Google Scholar] [CrossRef] [PubMed]
- Knowler, W.C.; Barrett-Connor, E.; Fowler, S.E.; Hamman, R.F.; Lachin, J.M.; Walker, E.A.; Nathan, D.M.; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 2002, 346, 393–403. [Google Scholar]
- Kosaka, K.; Noda, M.; Kuzuya, T. Prevention of type 2 diabetes by lifestyle intervention: A Japanese trial in IGT males. Diabetes Res. Clin. Pract. 2005, 67, 152–162. [Google Scholar] [CrossRef]
- Ramachandran, A.; Snehalatha, C.; Mary, S.; Mukesh, B.; Bhaskar, A.D.; Vijay, V.; Indian Diabetes Prevention Programme (IDPP). The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1). Diabetologia 2006, 49, 289–297. [Google Scholar] [CrossRef]
- World Health Organization. Prevention and Control of Diabetes Mellitus. The Forty-Second World Health Assembly; World Health Organization: Geneva, Switzerland, 1989.
- WHO Study Group: Primary Prevention of Diabetes Mellitus–Techn Rep Ser 844; World Health Organization: Geneva, Switzerland, 1994.
- United Nations. Political Declaration of the High-Level Meeting of the General Assembly on the Prevention and Control of Non-Communicable Diseases; Sixty-Sixth Session. Agenda Item 117; United Nations: New York, NY, USA, 2011. [Google Scholar]
- Uusitupa, M.; Khan, T.A.; Viguiliouk, E.; Kahleova, H.; Rivellese, A.A.; Hermansen, K.; Pfeiffer, A.; Thanopoulou, A.; Salas-Salvadó, J.; Schwab, U.; et al. Prevention of Type 2 Diabetes by Lifestyle Changes: A Systematic Review and Meta-Analysis. Nutrients 2019, 11, 2611. [Google Scholar] [CrossRef]
- Barry, E.; Roberts, S.; Oke, J.; Vijayaraghavan, S.; Normansell, R.; Greenhalgh, T. Efficacy and effectiveness of screen and treat policies in prevention of type 2 diabetes: Systematic review and meta-analysis of screening tests and interventions. BMJ 2017, 356, i6538. [Google Scholar] [CrossRef]
- Salas-Salvadó, J.; Bulló, M.; Estruch, R.; Ros, E.; Covas, M.I.; Ibarrola-Jurado, N.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; et al. Prevention of diabetes with Mediterranean diets: A subgroup analysis of a randomized trial. Ann. Intern. Med. 2018, 160, 1–10, Erratum in Ann. Intern. Med. 2018, 169, 271–272. [Google Scholar] [CrossRef]
- Saito, T.; Watanabe, M.; Nishida, J.; Izumi, T.; Omura, M.; Takagi, T.; Fukunaga, R.; Bandai, Y.; Tajima, N.; Nakamura, Y.; et al. Lifestyle Modification and Prevention of Type 2 Diabetes in Overweight Japanese With Impaired Fasting Glucose Levels A Randomized Controlled Trial. Arch. Int. Med. 2011, 171, 1352–1360. [Google Scholar] [CrossRef]
- Sampson, M.; Clark, A.; Bachmann, M.; Garner, N.; Irvine, L.; Howe, A.; Greaves, C.; Auckland, S.; Smith, J.; Turner, J.; et al. Effects of the Norfolk diabetes prevention lifestyle intervention (NDPS) on glycaemic control in screen-detected type 2 diabetes: A randomised controlled trial. BMC Med. 2021, 19, 183. [Google Scholar] [CrossRef]
- Wagner, R.; Heni, M.; Tabak, A.G.; Machann, J.; Schick, F.; Randrianarisoa, E.; Hrabě de Angelis, M.; Birkenfeld, A.L.; Stefan, N.; Peter, A.; et al. Pathophysiology-based subphenotyping of individuals at elevated risk for type 2 diabetes. Nat. Med. 2021, 27, 49–57. [Google Scholar] [CrossRef]
- Haw, J.S.; Galaviz, K.I.; Straus, A.N.; Kowalski, A.J.; Magee, M.J.; Weber, M.B.; Wei, J.; Narayan, K.V.; Ali, M.K. Long-term Sustainability of Diabetes Prevention Approaches: A Systematic Review and Meta-analysis of Randomized Clinical Trials. JAMA Intern. Med. 2017, 177, 1808–1817. [Google Scholar] [CrossRef]
- Knowler, W.C.; Fowler, S.E.; Hamman, R.F.; Brenneman, A.T.; Diabetes Prevention Program Research Group. 10-Year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet 2009, 374, 1677–1686. [Google Scholar]
- Gong, Q.; Zhang, P.; Wang, J.; Ma, J.; An, Y.; Chen, Y.; Zhang, B.; Feng, X.; Li, H.; Chen, X.; et al. Morbidity and mortality after lifestyle intervention for people with impaired glucose tolerance: 30-year results of the Da Qing Diabetes Prevention Outcome Study. Lancet Diabetes Endocrinol. 2019, 7, 452–461. [Google Scholar] [CrossRef]
- Li, G.; Zhang, P.; Wang, J.; Gregg, E.W.; Yang, W.; Gong, Q.; Li, H.; Li, H.; Jiang, Y.; An, Y.; et al. The longterm effect of lifestyle interventions to prevent diabetes in the China Da Qing Diabetes Prevention Study: A 20-year follow-up study. Lancet 2008, 371, 1783–1789. [Google Scholar] [CrossRef] [PubMed]
- Lindström, J.; Ilanne-Parikka, P.; Peltonen, M.; Aunola, S.; Eriksson, J.G.; Hemiö, K.; Hämäläinen, H.; Härkönen, P.; Keinänen-Kiukaanniemi, S.; Laakso, M.; et al. Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: Follow-up of the Finnish Diabetes Prevention Study. Lancet 2006, 368, 1673–1679. [Google Scholar] [CrossRef] [PubMed]
- Lindström, J.; Peltonen, M.; Eriksson, J.G.; Ilanne-Parikka, P.; Aunola, S.; Keinänen-Kiukaanniemi, S.; Uusitupa, M.; Tuomilehto, J.; Finnish Diabetes Prevention Study (DPS). Improved lifestyle and decreased diabetes risk over 13 years: Long-term follow-up of the randomised Finnish Diabetes Prevention Study (DPS). Diabetologia 2013, 56, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Aro, A.; Kauppinen, A.; Kivinen, N.; Selander, T.; Kinnunen, K.; Tuomilehto, J.; Keinänen-Kiukaanniemi, S.; Lindström, J.; Uusitupa, M.; Kaarniranta, K.; et al. Life Style Intervention Improves Retinopathy Status-The Finnish Diabetes Prevention Study. Nutrients 2019, 11, 1691. [Google Scholar] [CrossRef] [PubMed]
- Uusitupa, M.; Peltonen, M.; Lindström, J.; Aunola, S.; Ilanne-Parikka, P.; Keinänen-Kiukaanniemi, S.; Valle, T.T.; Eriksson, J.G.; Tuomilehto, J.; Finnish Diabetes Prevention Study Group. Ten-year mortality and cardiovascular morbidity in the Finnish Diabetes Prevention Study--secondary analysis of the randomized trial. PLoS ONE 2009, 4, e5656. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.G.; Heckman-Stoddard, B.; Dabelea, D.; Gadde, K.M.; Ehrmann, D.; Ford, L.; Prorok, P.; Boyko, E.J.; Pi-Sunyer, X.; Wallia, A.; et al. Effect of Metformin and Lifestyle Interventions on Mortality in the Diabetes Prevention Program and Diabetes Prevention Program Outcomes Study. Diabetes Care 2021, 44, 2775–2782. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, R.B.; Orchard, T.J.; Crandall, J.P.; Boyko, E.J.; Budoff, M.; Dabelea, D.; Gadde, K.M.; Knowler, W.C.; Lee, C.G.; Nathan, D.M.; et al. Effects of Long-term Metformin and Lifestyle Interventions on Cardiovascular Events in the Diabetes Prevention Program and Its Outcome Study. Circulation 2022, 145, 1632–1641. [Google Scholar] [CrossRef]
- Wing, R.R. Does Lifestyle Intervention Improve Health of Adults with Overweight/Obesity and Type 2 Diabetes? Findings from the Look AHEAD Randomized Trial. Obesity 2021, 29, 1246–1258. [Google Scholar] [CrossRef]
- Lindstrom, J.; Louheranta, A.; Mannelin, M.; Rastas, M.; Salminen, V.; Eriksson, J.; Uusitupa, M.; Tuomilehto, J.; Finnish Diabetes Prevention Study Group. The Finnish Diabetes Prevention Study (DPS): Lifestyle intervention and 3-year results on diet and physical activity. Diabetes Care 2003, 26, 3230–3236. [Google Scholar] [CrossRef]
- Saaristo, T.; Peltonen, M.; Keinänen-Kiukaanniemi, S.; Vanhala, M.; Saltevo, J.; Niskanen, L.; Oksa, H.; Korpi-Hyövälti, E.; Tuomilehto, J. National type 2 diabetes prevention programme in Finland: FIN-D2D. Int. J. Circumpolar Health 2007, 66, 101–112. [Google Scholar] [CrossRef]
- Lindström, J.; Tuomilehto, J. The diabetes risk score: A practical tool to predict type 2 diabetes risk. Diabetes Care 2003, 26, 725–731. [Google Scholar] [CrossRef]
- Saaristo, T.; Moilanen, L.; Korpi-Hyövälti, E.; Vanhala, M.; Saltevo, J.; Niskanen, L.; Jokelainen, J.; Peltonen, M.; Oksa, H.; Tuomilehto, J.; et al. Lifestyle intervention for prevention of type 2 diabetes in primary health care: One-year follow-up of the Finnish National Diabetes Prevention Program (FIN-D2D). Diabetes Care 2010, 33, 2146–2151. [Google Scholar] [CrossRef]
- Rintamäki, R.; Rautio, N.; Peltonen, M.; Jokelainen, J.; Keinänen-Kiukaanniemi, S.; Oksa, H.; Saaristo, T.; Puolijoki, H.; Saltevo, J.; Tuomilehto, J.; et al. Long-term outcomes of lifestyle intervention to prevent type 2 diabetes in people at high risk in primary health care. Prim Care Diabetes 2021, 15, 444–450. [Google Scholar] [CrossRef]
- Jonas, D.E.; Crotty, K.; Yun, J.D.; Middleton, J.C.; Feltner, C.; Taylor-Phillips, S.; Barclay, C.; Dotson, A.; Baker, C.; Balio, C.P.; et al. Screening for prediabetes and type 2 diabetes: Updatesd evidence report and systematic review for the US Preventive Services Task Force. JAMA 2021, 326, 744–760. [Google Scholar] [CrossRef] [PubMed]
- Galaviz, K.I.; Weber, M.B.; Straus, A.; Haw, J.S.; Narayan, K.M.V.; Ali, M.K. Global Diabetes Prevention Interventions: A Systematic Review and Network Meta-analysis of the Real-World Impact on Incidence, Weight, and Glucose. Diabetes Care 2018, 41, 1526–1534. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.K.; Echouffo-Tcheugui, J.; Williamson, D.F. How effective were lifestyle interventions in real-world settings that were modeled on the Diabetes Prevention Program? Health Aff. 2012, 31, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Mudaliar, U.; Zabetian, A.; Goodman, M.; Echouffo-Tcheugui, J.B.; Albright, A.L.; Gregg, E.; Ali, M. Cardiometabolic Risk Factor Changes Observed in Diabetes Prevention Programs in US Settings: A Systematic Review and Meta-analysis. PLoS Med. 2016, 13, e1002095. [Google Scholar] [CrossRef]
- Zhuo, X.; Zhang, P.; Kahn, H.S.; Gregg, E.W. Cost-Effectiveness of Alternative Thresholds of the Fasting Plasma Glucose Test to Identify the Target Population for Type 2 Diabetes Prevention in Adults Aged >= 45 Years. Diabetes Care 2013, 36, 3992–3998. [Google Scholar] [CrossRef]
- Li, R.; Qu, M.S.; Zhang, P.; Chattopadhyay, S.; Gregg, E.W.; Albright, A.; Hopkins, D.; Pronk, N.P. Economic Evaluation of Combined Diet and Physical Activity Promotion Programs to Prevent Type 2 Diabetes Among Persons at Increased Risk: A Systematic Review for the Community Preventive Services Task Force. Ann. Int. Med. 2015, 163, 452–460. [Google Scholar] [CrossRef]
- Albright, A.L.; Gregg, E.W. Preventing type 2 diabetes in communities across the U.S.: The National Diabetes Prevention Program. Am. J. Prev. Med. 2013, 44 (Suppl. S4), S346–S351. [Google Scholar] [CrossRef]
- Ely, E.K.; Gruss, S.M.; Luman, E.T.; Gregg, E.W.; Ali, M.K.; Nhim, K.; Rolka, D.B.; Albright, A.L. A National Effort to Prevent Type 2 Diabetes: Participant-Level Evaluation of CDC’s National Diabetes Prevention Program. Diabetes Care 2017, 40, 1331–1341. [Google Scholar] [CrossRef]
- Cannon, M.J.; Ng, B.P.; Lloyd, K.; Reynolds, J.; Ely, E.K. Delivering the National Diabetes Prevention Program: Assessment of Enrollment in In-Person and Virtual Organizations. J. Diabetes Res. 2022, 2022, 2942918. [Google Scholar] [CrossRef]
- Chakkalakal, R.J.; Connor, L.R.; Rolando, L.A.; Huang, Y.; Byrne, D.W.; Awalt, B.M.; McGown, P.W.; Aliyu, M.; Yarbrough, M.I. Putting the National Diabetes Prevention Program to Work: Predictors of Achieving Weight-Loss Goals in an Employee Population. Prev. Chronic. Dis. 2019, 16, E125. [Google Scholar] [CrossRef]
- US Preventive Services Task Force. Screening for prediabetes and type 2 diabetes: US Preventive Services Task Force recommendation statement. JAMA 2021, 326, 736–743. [Google Scholar] [CrossRef] [PubMed]
- Gregg, E.W.; Moin, T. New USPSTF Recommendations for Screening for Prediabetes and Type 2 Diabetes: An Opportunity to Create National Momentum. JAMA 2021, 326, 701–703. [Google Scholar] [CrossRef] [PubMed]
- Penn, L.; White, M.; Lindström, J.; den Boer, A.T.; Blaak, E.; Eriksson, J.G.; Feskens, E.; Ilanne-Parikka, P.; Keinänen-Kiukaanniemi, S.M.; Walker, M.; et al. Importance of weight loss maintenance and risk prediction in the prevention of type 2 diabetes: Analysis of European Diabetes Prevention Study RCT. PLoS ONE 2013, 8, e57143. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, P.E.; Lindström, J.; Kissimova-Scarbeck, K.; Szybinski, Z.; Barengo, N.C.; Peltonen, M.; Tuomilehto, J. The European perspective of type 2 diabetes prevention: Diabetes in Europe--prevention using lifestyle, physical activity and nutritional intervention (DE-PLAN) project. Exp. Clin. Endocrinol. Diabetes 2008, 116, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Karamanakos, G.; Costa-Pinel, B.; Gilis-Januszewska, A.; Velickiene, D.; Barrio-Torrell, F.; Cos-Claramunt, X.; Mestre-Miravet, S.; Piwońska-Solska, B.; Hubalewska-Dydejczyk, A.; Tuomilehto, J.; et al. The effectiveness of a community-based, type 2 diabetes prevention programme on health-related quality of life. The DE-PLAN study. PLoS ONE 2019, 14, e0221467. [Google Scholar] [CrossRef] [PubMed]
- Paulweber, B.; Valensi, P.; Lindström, J.; Lalic, N.M.; Greaves, C.J.; McKee, M.; Kissimova-Skarbek, K.; Liatis, S.; Cosson, E.; Szendroedi, J.; et al. A European evidence-based guideline for the prevention of type 2 diabetes. Horm. Metab. Res. 2010, 42 (Suppl. S1), S3–S36. [Google Scholar] [CrossRef]
- Lindström, J.; Neumann, A.; Sheppard, K.E.; Gilis-Januszewska, A.; Greaves, C.J.; Handke, U.; Pajunen, P.; Puhl, S.; Pölönen, A.; Rissanen, A.; et al. Take action to prevent diabetes: A toolkit for the prevention of type 2 diabetes in Europe. Horm. Metab. Res. 2010, 42 (Suppl. S1), S37–S55. [Google Scholar] [CrossRef]
- Pajunen, P.; Landgraf, R.; Muylle, F.; Neumann, A.; Lindström, J.; Schwarz, P.; Peltonen, M.; Acosta, T.; Adler, M.; AlKerwi, A.; et al. Quality indicators for the prevention of type 2 diabetes in Europe-IMAGE. Horm. Metab. Res. 2010, 42 (Suppl. S1), S56–S63. [Google Scholar] [CrossRef]
- Valabhji, J.; Barron, E.; Bradley, D.; Bakhai, C.; Fagg, J.; O’Neill, S.; Young, B.; Wareham, N.; Khunti, K.; Jebb, S.; et al. Early Outcomes From the English National Health Service Diabetes Prevention Programme. Diabetes Care 2020, 43, 152–160. [Google Scholar] [CrossRef]
- Whelan, M.; Bell, L. The English national health service diabetes prevention programme (NHS DPP): A scoping review of existing evidence. Diabet Med. 2022, 39, e14855. [Google Scholar] [CrossRef]
- Zhang, X. Effect of Lifestyle Interventions on Glucose Regulation among Adults without Impaired Glucose Tolerance or Diabetes: A Systematic Review and Meta-Analysis Diabetes Research and Clinical Practice. Diabetes Res. Clin. Pract. 2017, 123, 149–164. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.B.; Ranjani, H.; Staimez, L.R.; Anjana, R.M.; Ali, M.K.; Narayan, K.V.; Mohan, V. The stepwise approach to diabetes prevention: Results from the D-CLIP randomized controlled trial. Diabetes Care 2016, 39, 1760–1767. [Google Scholar] [CrossRef] [PubMed]
- Thankappan, K.R.; Sathish, T.; Tapp, R.; Shaw, J.E.; Lotfaliany, M.; Wolfe, R.; Absetz, P.; Mathews, E.; Aziz, Z.; Williams, E.; et al. A peer-support lifestyle intervention for preventing type 2 diabetes in India: A cluster-randomized controlled trial of the Kerala Diabetes Prevention Program. PLoS Med. 2018, 15, e1002575. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Siegel, K.R.; Ng, B.P.; Jawanda, S.; Proia, K.K.; Zhang, X.; Albright, A.L.; Zhang, P. Cost-effectiveness of diabetes prevention intervention targeting high-risk individuals and whole populations: A systematic review. Diabetes Care 2020, 43, 1593–1616. [Google Scholar] [CrossRef]
- NCD Risk Factor Collaboration. Worldwide trends in diabetes since 1980: A pooled analysis of 751 population-based studies with 4.4 million participants. Lancet 2016, 387, 1513–1530. [Google Scholar] [CrossRef]
- Chan, J.C.N.; Lim, L.-L.; Wareham, N.J.; E Shaw, J.; Orchard, T.J.; Zhang, P.; Lau, E.S.H.; Eliasson, B.; Kong, A.P.S.; Ezzati, M.; et al. The Lancet Commission on diabetes: Using data to transform diabetes care and patient lives. Lancet 2021, 396, 2019–2082. [Google Scholar] [CrossRef]
- World Health Organization. Global Action Plan for the Prevention and Control of Noncommunicable Diseases 2013–2020; World Health Organization: Geneva, Switzerland, 2013.
- Besançon, S.; Sidibé, A.; Sow, D.S.; Sy, O.; Ambard, J.; Yudkin, J.S.; Beran, D. The role of non-governmental organizations in strengthening healthcare systems in low- and middle-income countries: Lessons from Santé Diabète in Mali. Glob. Health Action 2022, 15, 2061239. [Google Scholar] [CrossRef]
- Sagastume, D.; Siero, I.; Mertens, E.; Cottam, J.; Colizzi, C.; Peñalvo, J.L. The effectiveness of lifestyle interventions on type 2 diabetes and gestational diabetes incidence and cardiometabolic outcomes: A systematic review and meta-analysis of evidence from low- and middle-income countries. EClinicalMedicine 2022, 53, 101650. [Google Scholar] [CrossRef]
- Hawthorne, K.; Robles, Y.; Cannings-John, R.; Edwards, A.G.K. Culturally appropriate health education for type 2 diabetes in ethnic minority groups: A systematic and narrative review of randomized controlled trials. Diabet. Med. 2010, 27, 613–623. [Google Scholar] [CrossRef]
- Renzaho, A.M.N.; Mellor, D.; Boulton, K. Effectiveness of prevention programmes for obesity and chronic diseases among immigrants to developed countries–a systematic review. Public Health Nutr. 2010, 13, 438–450. [Google Scholar] [CrossRef]
- Latina, J.; Fernandez-Jimenez, R.; Bansilal, S.; Sartori, S.; Vedanthan, R.; Lewis, M.; Kofler, C.; Hunn, M.; Martin, F.; Bagiella, E.; et al. Grenada heart project-community health action to encourage healthy behaviors (GHP-CHANGE): A randomized control peer group-based lifestyle intervention. Am. Heart J. 2020, 220, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Catley, D.; Puoane, T.; Tsolekile, L.; Resnicow, K.; Fleming, K.K.; Hurley, E.A.; Smyth, J.M.; Materia, F.T.; Lambert, E.V.; Vitolins, M.Z.; et al. Evaluation of an adapted version of the Diabetes Program for low- and middle-income countries: A cluster randomized trial to evaluate “Lifestyle Africa” in South Africa. PLoS Med. 2022, 19, e1003964. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Laborde, C.; Hirmas-Adauy, M.; Matute, I.; Jasmen, A.; Urrejola, O.; Molina, X.; Awad, C.; Frey-Moreno, C.; Pumarino-Lira, S.; Descalzi-Rojas, F.; et al. Barriers and Facilitators in Access to Diabetes, Hypertension, and Dyslipidemia Medicines: A Scoping Review. Public Health Rev. 2022, 43, 1604796. [Google Scholar] [CrossRef] [PubMed]
- Magge, S.N.; Silverstein, J.; Elder, D.; Nadeau, K.; Hannon, T.S. Evaluation and treatment of prediabetes in youth. J. Pediatr. 2020, 219, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Savoye, M.; Caprio, S.; Dziura, J.; Camp, A.; Germain, G.; Summers, C.; Li, F.; Shaw, M.; Nowicka, P.; Kursawe, R.; et al. Reversal of early abnormalities in glucose metabolism in obese youth: Results of an intensive lifestyle randomized controlled trial. Diabetes Care 2014, 37, 317–324. [Google Scholar] [CrossRef]
- Peña, A.; Olson, M.L.; Hooker, E.; Ayers, S.L.; Castro, F.G.; Patrick, D.L.; Corral, L.; Lish, E.; Knowler, W.C.; Shaibi, G.Q. Effects of a Diabetes Prevention Program on Type 2 Diabetes Risk Factors and Quality of Life Among Latino Youths With Prediabetes: A Randomized Clinical Trial. JAMA Netw. Open 2022, 5, e2231196. [Google Scholar] [CrossRef]
- Lakka, T.A.; Lintu, N.; Väistö, J.; Viitasalo, A.; Sallinen, T.; Haapala, E.A.; Tompuri, T.T.; Soininen, S.; Karjalainen, P.; Schnurr, T.M.; et al. A 2-year physical activity and dietary intervention attenuates the increase in insulin resistance in a general population of children: The PANIC study. Diabetologia 2020, 63, 2270–2281. [Google Scholar] [CrossRef]
- Aziz, Z.; Absetz, P.; Oldroyd, J.; Pronk, N.P.; Oldenburg, B. A systematic review of real-world diabetes prevention programs: Learnings from the last 15 years. Implement. Sci. 2015, 10, 172. [Google Scholar] [CrossRef]
- MacPherson, M.M.; Merry, K.J.; Locke, S.R.; Jung, M.E. mHealth prompts within diabetes prevention programs: A scoping review. Mhealth 2022, 8, 20. [Google Scholar] [CrossRef]
- Mahajan, A.; Taliun, D.; Thurner, M.; Robertson, N.R.; Torres, J.M.; Rayner, N.W.; Payne, A.J.; Steinthorsdottir, V.; Scott, R.A.; Grarup, N.; et al. Fine-mapping type 2 diabetes loci to single-variant resolution using high-density imputation and islet-specific epigenome maps. Nat. Genet. 2018, 50, 1505–1513. [Google Scholar] [CrossRef]
- Nolan, J.J.; Kahkoska, A.R.; Semnani-Azad, Z.; Hivert, M.-F.; Ji, L.; Mohan, V.; Eckel, R.H.; Philipson, L.H.; Rich, S.S.; Gruber, C.; et al. ADA/EASD Precision Medicine in Diabetes Initiative: An International Perspective and Future Vision for Precision Medicine in Diabetes. Diabetes Care 2022, 45, 261–266. [Google Scholar] [CrossRef]
- Wang, D.D.; Hu, F.B. Precision nutrition for prevention and management of type 2 diabetes. Lancet Diabetes Endocrinol. 2018, 6, 416–426. [Google Scholar] [CrossRef]
- Wareham, N.J. Personalised prevention of type 2 diabetes. Diabetologia 2022, 65, 1796–1803. [Google Scholar] [CrossRef]
- Uusitupa, M.I.; Stančáková, A.; Peltonen, M.; Eriksson, J.G.; Lindström, J.; Aunola, S.; Ilanne-Parikka, P.; Keinänen-Kiukaanniemi, S.; Tuomilehto, J.; Laakso, M. Impact of positive family history and genetic risk variants on the incidence of diabetes–the Finnish Diabetes Prevention Study. Diabetes Care 2011, 34, 418–423. [Google Scholar] [CrossRef]
- Florez, J.C.; Jablonski, K.A.; McAteer, J.B.; Franks, P.; Mason, C.C.; Mather, K.; Horton, E.; Goldberg, R.; Dabelea, D.; Kahn, S.E.; et al. Effects of genetic variants previously associated with fasting glucose and insulin in the Diabetes Prevention Program. PLoS ONE 2012, 7, e44424. [Google Scholar] [CrossRef]
- Schwab, U.; Lankinen, M.; Laakso, M. Effect of lifestyle intervention on the risk of incident diabetes in individuals with impaired fasting glucose and low or high genetic risk for the development of type 2 diabetes in men: A T2D-GENE trial. Food Nutr. Res. 2021, 65, 7721. [Google Scholar] [CrossRef]
- Canfora, E.E.; Meex, R.C.; Venema, K.; Blaak, E.E. Gut microbial metabolites in obesity, NAFLD and T2DM. Nat. Rev. Endocrinol. 2019, 15, 261–273. [Google Scholar] [CrossRef]
- O’Keefe, S.J. The association between dietary fibre deficiency and high-income lifestyle-associated diseases: Burkitt’s hypothesis revisited. Lancet Gastroenterol. Hepatol. 2019, 4, 984–996. [Google Scholar] [CrossRef]
- De Mello, V.D.; Paananen, J.; Lindström, J.; Lankinen, M.A.; Shi, L.; Kuusisto, J.; Pihlajamäki, J.; Auriola, S.; Lehtonen, M.; Rolandsson, O.; et al. Indolepropionic acid and novel lipid metabolites are associated with a lower risk of type 2 diabetes in the Finnish Diabetes Prevention Study. Sci. Rep. 2017, 7, 46337. [Google Scholar] [CrossRef]
- Thow, A.M.; Rippin, H.L.; Mulcahy, G.; Duffey, K.; Wickramasinghe, K. Sugar-sweetened beverage taxes in Europe: Learning for the future. Eur. J. Public Health 2022, 32, 273–280. [Google Scholar] [CrossRef]
- Brownell, K.D.; Farley, T.; Willett, W.C.; Popkin, B.M.; Chaloupka, F.J.; Thompson, J.W.; Ludwig, D.S. The public health and economic benefits of taxing sugar-sweetened beverages. N. Engl. J. Med. 2009, 361, 1599–1605. [Google Scholar] [CrossRef]
- Basto-Abreu, A.; Barrientos-Gutiérrez, T.; Vidaña-Pérez, D.; Colchero, M.A.; Hernández-F, M.; Hernández-Ávila, M.; Ward, Z.J.; Long, M.W.; Gortmaker, S.L. Cost-Effectiveness Of The Sugar-Sweetened Beverage Excise Tax In Mexico. Health Aff. 2019, 38, 1824–1831. [Google Scholar] [CrossRef]
- Ottelin, A.M.; Lindstrom, J.; Peltonen, M.; Martikainen, J.; Uusitupa, M.; Gylling, H.; Poutanen, K.; Louheranta, A.; Mannelin, M.; Paturi, M.; et al. Costs of a self-selected, health-promoting diet among the participants of the Finnish Diabetes Prevention Study. Diabetes Care 2007, 30, 1275–1277. [Google Scholar] [CrossRef]
- Martikainen, J.; Jalkanen, K.; Heiskanen, J.; Lavikainen, P.; Peltonen, M.; Laatikainen, T.; Lindström, J. Type 2 Diabetes-Related Health Economic Impact Associated with Increased Whole Grains Consumption among Adults in Finland. J. Nutr. 2021, 13, 3583. [Google Scholar] [CrossRef]
- Lopez-Zetina, J.; Lee, H.; Friis, R. The link between obesity and the built environment. Evidence from an ecological analysis of obesity and vehicle miles of travel in California. Health Place 2006, 12, 656–664. [Google Scholar] [CrossRef]
- Hoehner, C.M.; Barlow, C.E.; Allen, P.; Schootman, M. Commuting distance, cardiorespiratory fitness, and metabolic risk. Am. J. Prev. Med. 2012, 42, 571–578. [Google Scholar] [CrossRef]
- Hu, G.; Qiao, Q.; Silventoinen, K.; Eriksson, J.G.; Jousilahti, P.; Lindström, J.; Valle, T.T.; Nissinen, A.; Tuomilehto, J. Occupational, commuting, and leisure-time physical activity in relation to risk for Type 2 diabetes in middle-aged Finnish men and women. Diabetologia 2003, 46, 322–329. [Google Scholar] [CrossRef]
- De la Fuente, F.; Saldías, M.; Cubillos, C.; Mery, G.; Carvajal, D.; Bowen, M.; Bertoglia, M. Green Space Exposure Association with Type 2 Diabetes Mellitus, Physical Activity, and Obesity: A Systematic Review. Int. J. Environ. Res. Public Health 2020, 18, 97. [Google Scholar] [CrossRef]
- Evans, J.M.; Newton, R.W.; Ruta, D.A.; MacDonald, T.M.; Morris, A.D. Socio-economic status, obesity and prevalence of Type 1 and Type 2 diabetes mellitus. Diabet. Med. 2000, 17, 478–480. [Google Scholar] [CrossRef]
- O’Brien, M.J.; Whitaker, R.C.; Yu, D.; Ackermann, R.T. The comparative efficacy of lifestyle intervention and metformin by educational attainment in the Diabetes Prevention Program. Prev. Med. 2015, 77, 125–130. [Google Scholar] [CrossRef]
- Wikström, K.; Peltonen, M.; Eriksson, J.G.; Aunola, S.; Ilanne-Parikka, P.; Keinänen-Kiukaanniemi, S.; Uusitupa, M.; Tuomilehto, J.; Lindström, J. Educational attainment and effectiveness of lifestyle intervention in the Finnish Diabetes Prevention Study. Diabetes Res. Clin. Pract. 2009, 86, e1–e5. [Google Scholar] [CrossRef] [PubMed]
- Blas, E.; Sommerfeld, J.; Kurup, A.S. (Eds.) Social Determinants Approaches to Public Health: From Concept to Practice [Monograph on the Internet]; World Health Organization: Geneva, Switzerland, 2012. Available online: www.who.int/sdhconference/resources/SDapproachestopublichealth_eng.pdf (accessed on 12 February 2023).
| Country; Trial Duration, | Number of Participants | Relative Risk Reduction of Diabetes | Dietary Goals, Weight Change * | Changes in Diet When Available | Physical Activity, Goals/Change | Long-Term Follow-Up | |
|---|---|---|---|---|---|---|---|
| Da Qing IGT and Diabetes Study [6] | China; 6 years | 577, all IGT; 33 health clinics | Diet 33%; exercise 47%; diet + exercise 38% | Weight reduction in overweight people; energy restriction; 6-year fall in BMI 1 kg/m2 in obese | CHO 58–60 E%; protein 11 E%; fat 25–27 E%; energy decrease 100–240 kcal; BMI goal 23 kg/m2 | Increase in leisure-time physical activities | Yes |
| DPS [7] | Finland; 3.2 years | 522, all IGT; five centers | 58% | Weight reduction >5%; reduce total and SFA; increase dietary fiber; Weight loss 3.5 kg | 3-year results: energy reduction 204 kcal; CHO increase 3 E%; fat reduction 5 E%; SFA reduction 3 E%; fiber increase 2 g/1000 kcal | ≥4 h/wk; at year 3 sedentary people: 17% in the intervention vs. 29% control group | Yes |
| DPP [8] | USA; 2.8 years | 3234, all IGT + IFG; 27 centers | Lifestyle 58%; Metformin 31%; | Weight loss goal >7%; 1-year weight loss 5.5 kg | Energy reduction 450 vs. 249 kcal and fat intake reduction 6.6 vs. 0.8 E% for intervention and control, respectively. | 150 min/wk; 74% reached at 24 months | Yes |
| IGT trial, Japan [9] | Japan; 4 years | 102 in intervention, 356 in control, all IGT | 67% | BMI goal 22 kg/m2; increase in vegetables; reduce food intake by 10%; fat < 50 g/d; alcohol restriction; Weight loss −1.8 kg | Not reported | 30–40 min walking/day | No |
| IDPP-1 [10] | India; 2.5 years | 531, all IGT | Lifestyle 29%; Metformin 26%; lifestyle + Metformin 28% | Reduce total calories, refined CHO, fat and sugar; increase high fiber-rich foods; No change in body weight | Dietary adherence increased in the intervention groups | Walking >30 min a day | No |
| 1. A very significant and consistent relative risk reduction among the RTCs. |
| 2. The preventive effect of lifestyle intervention was rapid. |
| 3. The benefit was similar in men and women. |
| 4. Lifestyle intervention was effective in all ethnic groups studied. |
| 5. The benefit did not depend on the initial body weight alone, the change in incidence of T2D between the intervention and control groups was parallel regardless baseline BMI that varied among the RCT.s |
| 6. It is not possible to tell which component of the multifactorial intervention contributed most to the preventive effect, but people who managed to reach multiple lifestyle targets benefitted most. Weight reduction is essential in overweight and obese people at high risk of T2D. |
| 7. Lifestyle intervention postponed the onset of T2D by at least for 5 years. |
| 8. A residual risk of T2D existed in the intervention group, primarily due to the lack of success in people reaching lifestyle targets satisfactorily. |
| 9. People with a high genetic risk for T2D benefitted significantly from lifestyle intervention. |
| Category | Intervention | CE Outcome |
|---|---|---|
| Fiscal policy | ||
| Sugar sweetened beverage tax | 20%, penny-per-ounce, 10%, or $0.5/L tax on Sugar sweetened beverage | Cost saving |
| Sugar tax | $0.99/100 mL ice cream; $0.9/100 g other products | Cost saving |
| Subsidy | 30% or 0.15/100 g subsidy for fruit/veg consumption | Cost saving to worse health |
| Combination tax and subsidy | Tax SSB, sat fat., sodium, sugar; subsidy fruit/veg | Cost saving |
| Environmental change | ||
| Fresh food in low-income area | Open supermarket | Cost saving |
| Workplace healthy food | Provide healthy food in cafeteria | Cost saving |
| Enhanced physical activity access | Increase facilities for physical activities | $36 k/QALY |
| Health promotion | ||
| Campaign | Community-wide, mass media, or internet campaign to promote physical activity | $87 k/QALY to cost saving |
| Healthy eating education in low-income community | Diet education and cooking classes | More QALY but no change in cost |
| Social support PA promotion | Use organized groups to promote physical activity | $35–50 k/QALY |
| Physical activity promotion for targeted population | Encourage walking and reduce car use using tailored educational information | $17,658/QALY– cost saving |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tuomilehto, J.; Uusitupa, M.; Gregg, E.W.; Lindström, J. Type 2 Diabetes Prevention Programs—From Proof-of-Concept Trials to National Intervention and Beyond. J. Clin. Med. 2023, 12, 1876. https://doi.org/10.3390/jcm12051876
Tuomilehto J, Uusitupa M, Gregg EW, Lindström J. Type 2 Diabetes Prevention Programs—From Proof-of-Concept Trials to National Intervention and Beyond. Journal of Clinical Medicine. 2023; 12(5):1876. https://doi.org/10.3390/jcm12051876
Chicago/Turabian StyleTuomilehto, Jaakko, Matti Uusitupa, Edward W. Gregg, and Jaana Lindström. 2023. "Type 2 Diabetes Prevention Programs—From Proof-of-Concept Trials to National Intervention and Beyond" Journal of Clinical Medicine 12, no. 5: 1876. https://doi.org/10.3390/jcm12051876
APA StyleTuomilehto, J., Uusitupa, M., Gregg, E. W., & Lindström, J. (2023). Type 2 Diabetes Prevention Programs—From Proof-of-Concept Trials to National Intervention and Beyond. Journal of Clinical Medicine, 12(5), 1876. https://doi.org/10.3390/jcm12051876








