The Effects of Novel Pulsed Electromagnetic Field Therapy Device on Acute Distal Radius Fractures: A Prospective, Double-Blind, Sham-Controlled, Randomized Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
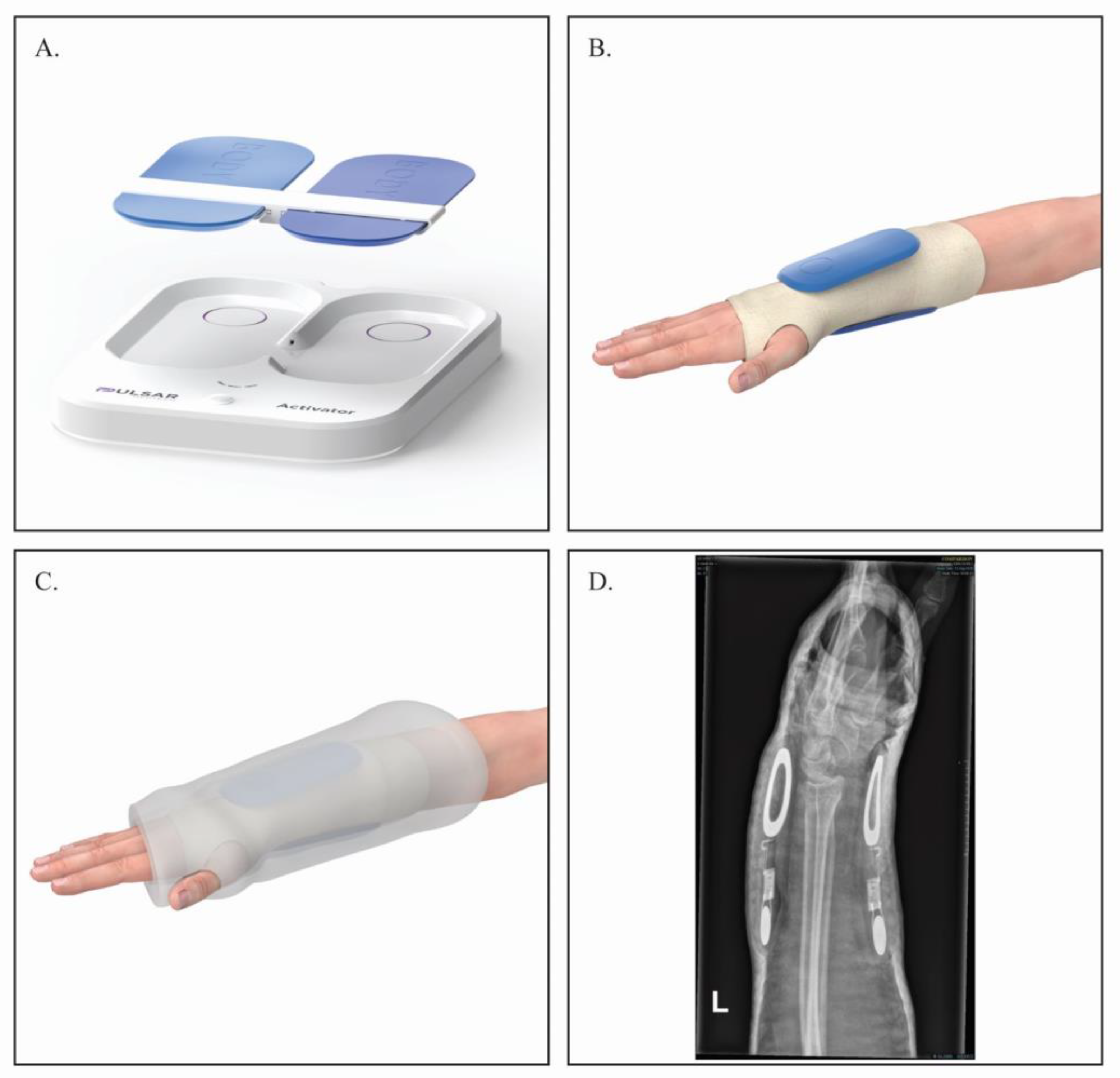
2.2. FHP Device
2.3. Outcome Measures
2.4. Radiologic Assessment
2.5. Functional Outcomes and Quality of Life Assessment
2.6. Functional Assessments
2.7. Safety Outcomes and Rehabilitation
2.8. Statistical Analysis
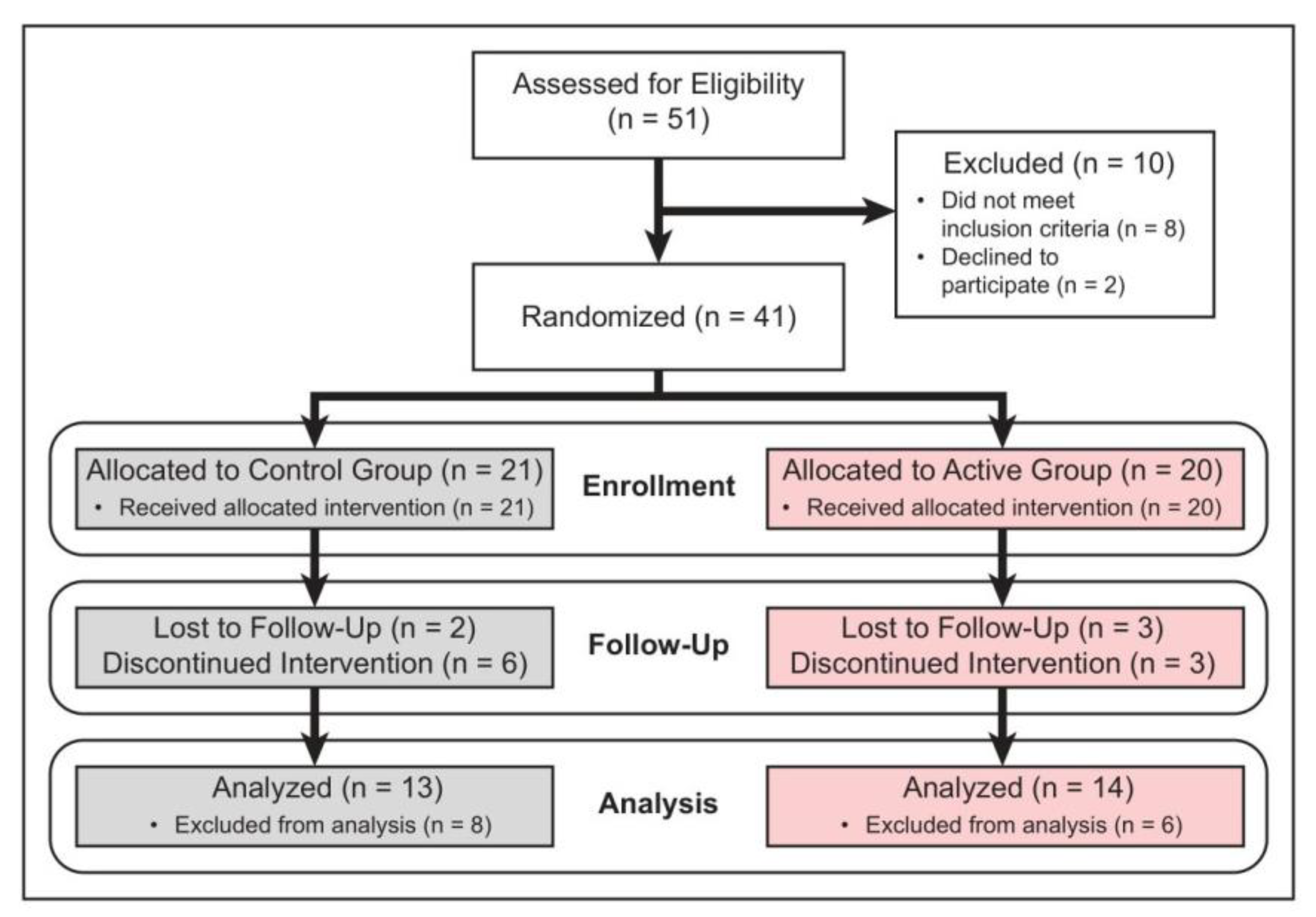
3. Results
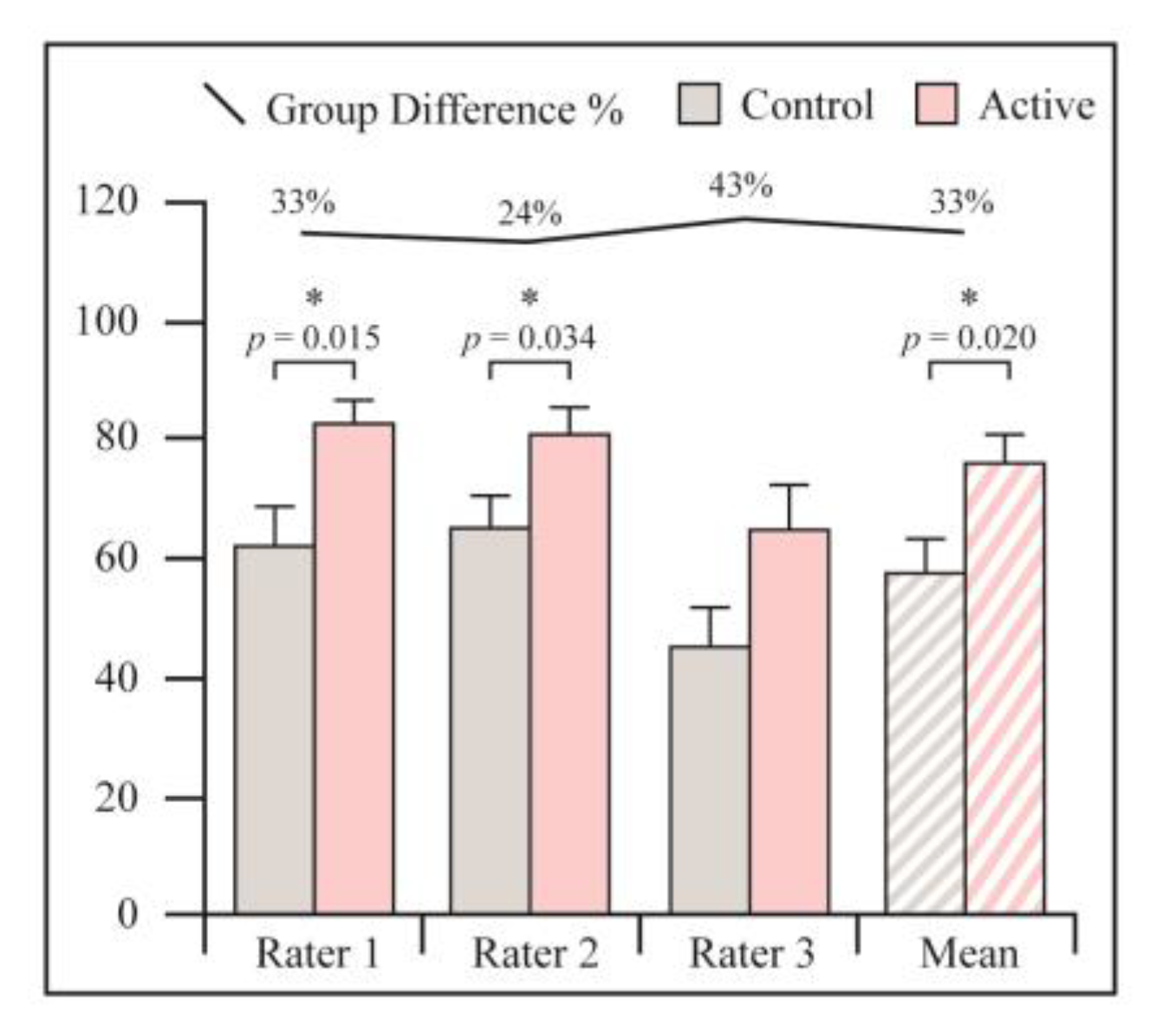
3.1. Radiological Assessment
3.2. Functional Assessment
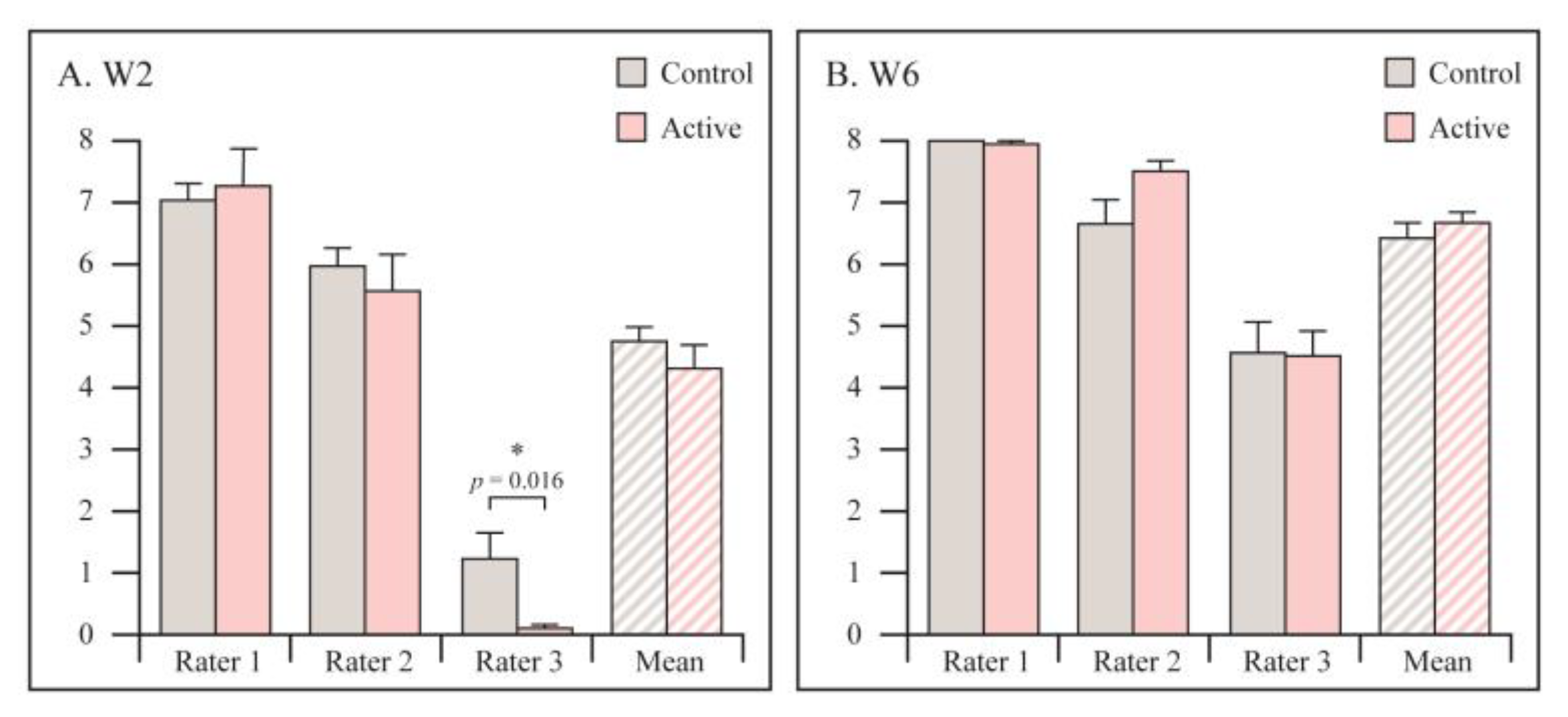
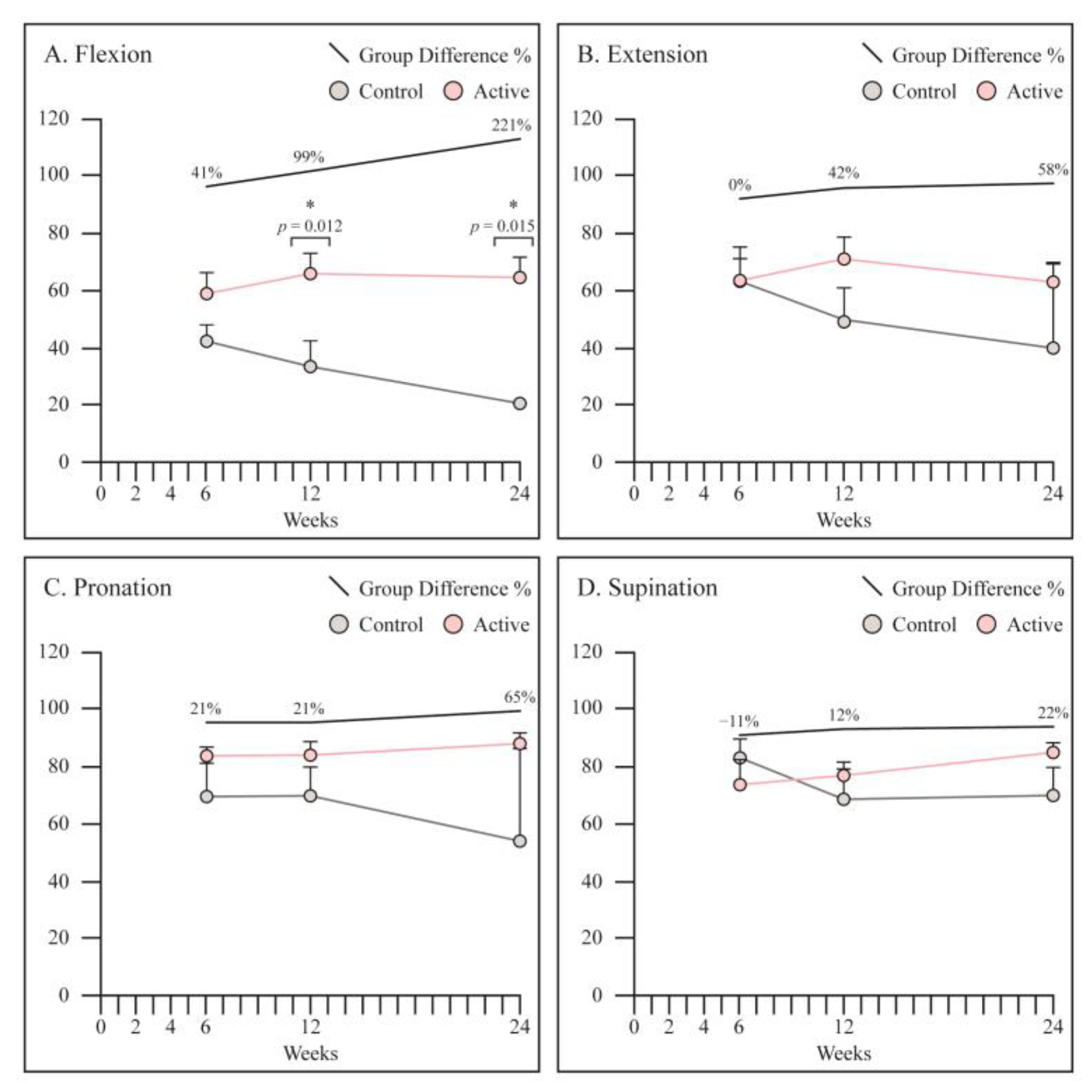
3.3. Range of Motion
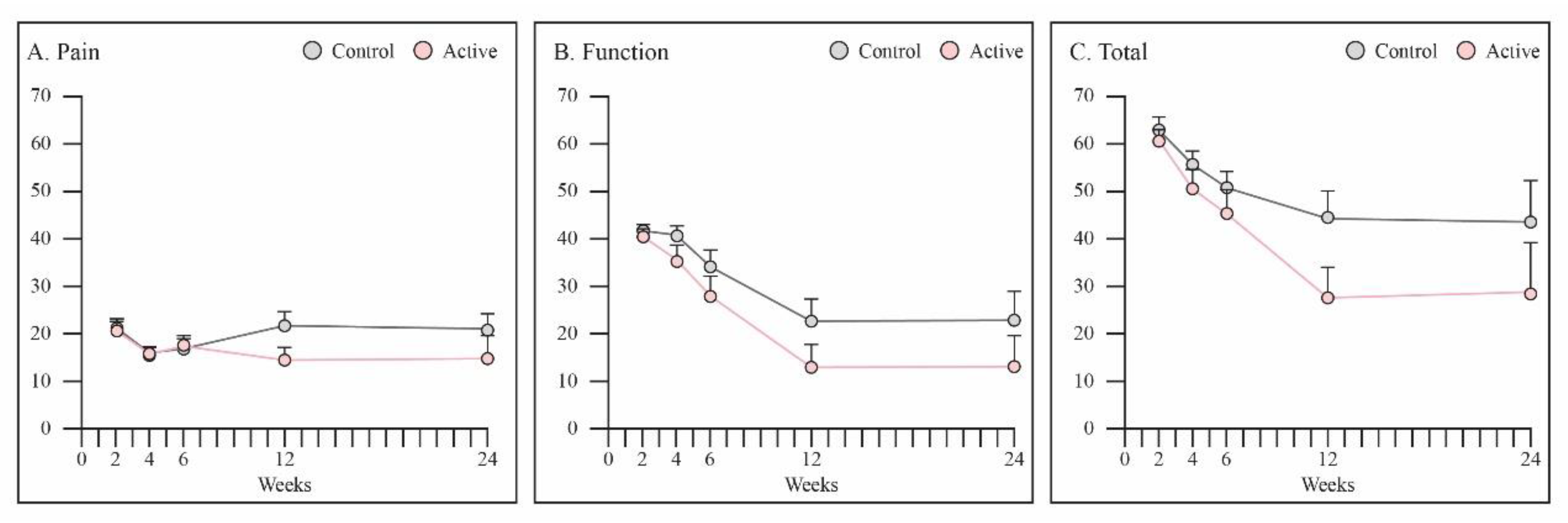
3.4. PRWE
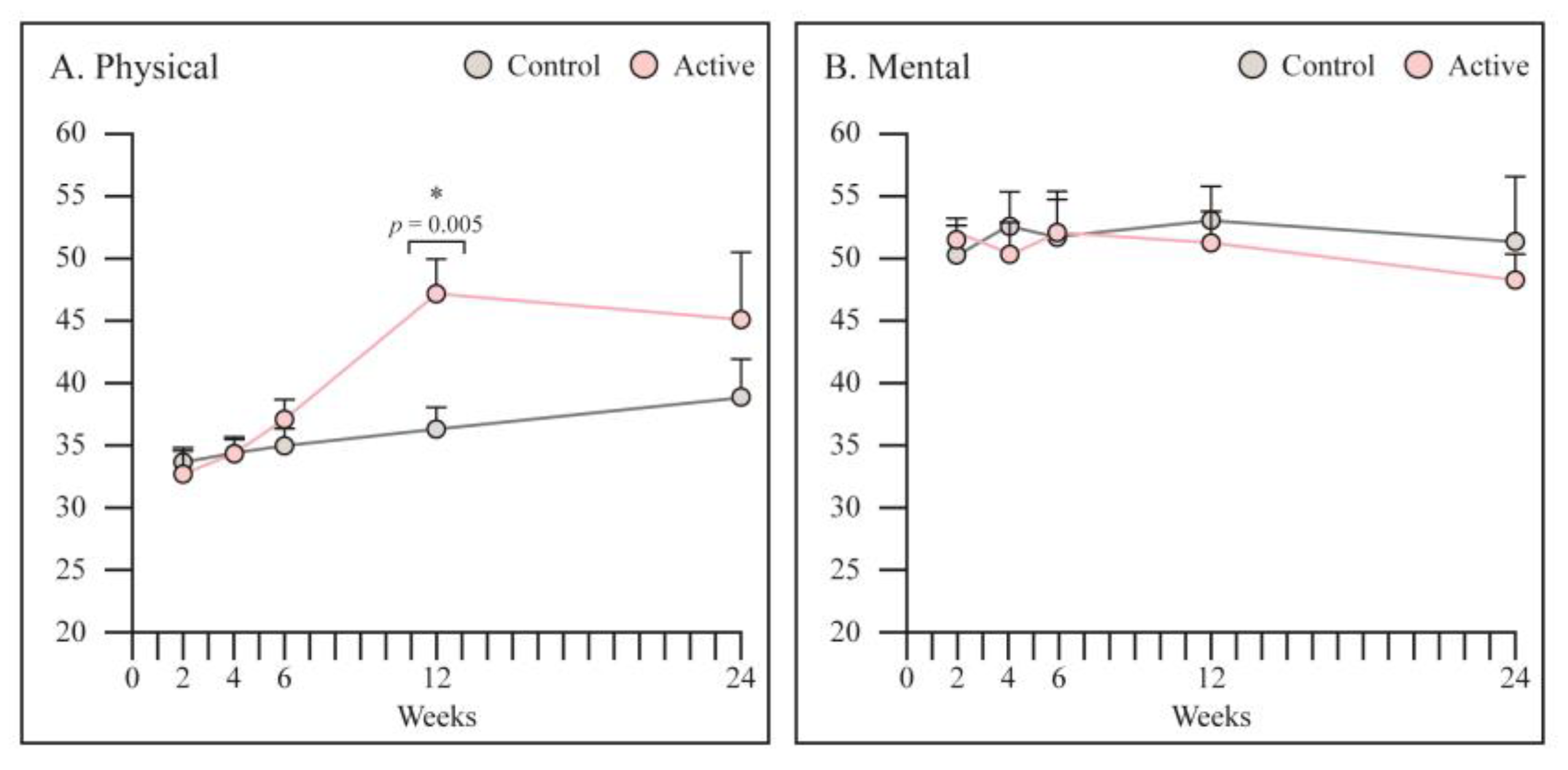
3.5. SF 12
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ponkilainen, V.; Kuitunen, I.; Liukkonen, R.; Vaajala, M.; Reito, A.; Uimonen, M. The incidence of musculoskeletal injuries: A systematic review and meta-analysis. Bone Jt. Res. 2022, 11, 814–825. [Google Scholar] [CrossRef]
- Nellans, K.W.; Kowalski, E.; Chung, K.C. The Epidemiology of Distal Radius Fractures. Hand Clin. 2012, 28, 113–125. [Google Scholar] [CrossRef]
- Court-Brown, C.M.; Caesar, B. Epidemiology of adult fractures: A review. Injury 2006, 37, 691–697. [Google Scholar] [CrossRef]
- del Piñal, F.; Jupiter, J.B.; Rozental, T.D.; Arora, R.; Nakamura, T.; Bain, G.I. Distal radius fractures. J. Hand Surg. Eur. Vol. 2022, 47, 12–23. [Google Scholar] [CrossRef]
- Egol, K.; Walsh, M.; Romo-Cardoso, S.; Dorsky, S.; Paksima, N. Distal Radial Fractures in the Elderly: Operative Compared with Nonoperative Treatment. J. Bone Jt. Surg. Am. 2010, 92, 1851–1857. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, X.; Li, Z.; Yan, H.; Zhou, F.; Gao, W. Safety and Efficacy of Operative Versus Nonsurgical Management of Distal Radius Fractures in Elderly Patients: A Systematic Review and Meta-analysis. J. Hand Surg. Am. 2016, 41, 404–413. [Google Scholar] [CrossRef]
- Stephens, A.R.; Presson, A.P.; McFarland, M.M.; Zhang, C.; Sirniö, K.; Mulders, M.A.; Schep, N.W.; Tyser, A.R.; Kazmers, N.H. Volar Locked Plating Versus Closed Reduction and Casting for Acute, Displaced Distal Radial Fractures in the Elderly: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Bone Jt. Surg. 2020, 102, 1280–1288. [Google Scholar] [CrossRef]
- Aaron, R.K.; McK Ciombor, D.; Simon, B.J. Treatment of Nonunions With Electric and Electromagnetic Fields. Clin. Orthop. Relat. Res. 2004, 419, 21–29. [Google Scholar] [CrossRef]
- Shi, H.-F.; Xiong, J.; Chen, Y.-X.; Wang, J.-F.; Qiu, X.-S.; Wang, Y.-H.; Qiu, Y. Early application of pulsed electromagnetic field in the treatment of postoperative delayed union of long-bone fractures: A prospective randomized controlled study. BMC Musculoskelet. Disord. 2013, 14, 35. [Google Scholar] [CrossRef]
- Peng, L.; Fu, C.; Xiong, F.; Zhang, Q.; Liang, Z.; Chen, L.; He, C.; Wei, Q. Effectiveness of Pulsed Electromagnetic Fields on Bone Healing: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Bioelectromagnetics 2020, 41, 323–337. [Google Scholar] [CrossRef]
- Ferroni, L.; Gardin, C.; Dolkart, O.; Salai, M.; Barak, S.; Piattelli, A.; Amir-Barak, H.; Zavan, B. Pulsed electromagnetic fields increase osteogenetic commitment of MSCs via the mTOR pathway in TNF-α mediated inflammatory conditions: An in-vitro study. Sci. Rep. 2018, 8, 5108. [Google Scholar] [CrossRef]
- Chalidis, B.; Sachinis, N.; Assiotis, A.; Maccauro, G.; Graziani, F. Stimulation of Bone Formation and Fracture Healing with Pulsed Electromagnetic Fields: Biologic Responses and Clinical Implications. Int. J. Immunopathol. Pharmacol. 2011, 24, 17–20. [Google Scholar] [CrossRef]
- Patel, S.P.; Anthony, S.G.; Zurakowski, D.; Didolkar, M.M.; Kim, P.S.; Wu, J.S.; Kung, J.W.; Dolan, M.; Rozental, T.D. Radiographic Scoring System to Evaluate Union of Distal Radius Fractures. J. Hand Surg. Am. 2014, 39, 1471–1479. [Google Scholar] [CrossRef]
- Matzon, J.L.; Lutsky, K.F.; Tulipan, J.E.; Beredjiklian, P.K. Reliability of Radiographs and Computed Tomography in Diagnosing Scaphoid Union After Internal Fixation. J. Hand Surg. Am. 2021, 46, 539–543. [Google Scholar] [CrossRef]
- Singh, H.P.; Forward, D.; Davis, T.R.C.; Dawson, J.S.; Oni, J.A.; Downing, N.D. Partial Union of Acute Scaphoid Fractures. J. Hand Surg. Br. 2005, 30, 440–445. [Google Scholar] [CrossRef]
- Udugampolage, N.; Caruso, R.; Panetta, M.; Callus, E.; Dellafiore, F.; Magon, A.; Marelli, S.; Pini, A. Is SF-12 a valid and reliable measurement of health-related quality of life among adults with Marfan syndrome? A confirmatory study. PLoS ONE 2021, 16, e0252864. [Google Scholar] [CrossRef]
- MacDermid, J.C. The PRWE/PRWHE update. J. Hand Ther. 2019, 32, 292–294. [Google Scholar] [CrossRef]
- Trampisch, U.S.; Franke, J.; Jedamzik, N.; Hinrichs, T.; Platen, P. Optimal Jamar Dynamometer Handle Position to Assess Maximal Isometric Hand Grip Strength in Epidemiological Studies. J. Hand Surg. Am. 2012, 37, 2368–2373. [Google Scholar] [CrossRef]
- Massari, L.; Benazzo, F.; Falez, F.; Perugia, D.; Pietrogrande, L.; Setti, S.; Osti, R.; Vaienti, E.; Ruosi, C.; Cadossi, R. Biophysical stimulation of bone and cartilage: State of the art and future perspectives. Int. Orthop. 2019, 43, 539–551. [Google Scholar] [CrossRef]
- Murray, H.B.; Pethica, B. A follow-up study of the in-practice results of pulsed electromagnetic field therapy in the management of nonunion fractures. Orthop. Res. Rev. 2016, 8, 67–72. [Google Scholar] [CrossRef]
- Borsalino, G.; Bagnacani, M.; Bettati, E.; Fornaciari, F.; Rocchi, R.; Uluhogian, S.; Ceccherelli, G.; Cadossi, R.; Traina, G.C. Electrical stimulation of human femoral intertrochanteric osteotomies. Double-blind study. Clin. Orthop. Relat. Res. 1988, 237, 256–263. [Google Scholar] [CrossRef]
- Mammi, G.; Rocchi, R.; Cadossi, R.; Massari, L.; Traina, G.C. The electrical stimulation of tibial osteotomies. Double-blind study. Clin. Orthop. Relat. Res. 1993, 288, 246–253. [Google Scholar] [CrossRef]
- Fontanesi, G.; Traina, G.C.; Giancecchi, F.; Tartaglia, I.; Rotini, R.; Virgili, B.; Cadossi, R.; Ceccherelli, G.; Marino, A. Slow healing fractures: Can they be prevented? (Results of electrical stimulation in fibular osteotomies in rats and in diaphyseal fractures of the tibia in humans). Ital. J. Orthop. Traumatol. 1986, 12, 371–385. [Google Scholar]
- Faldini, C.; Cadossi, M.; Luciani, D.; Betti, E.; Chiarello, E.; Giannini, S. Electromagnetic bone growth stimulation in patients with femoral neck fractures treated with screws: Prospective randomized double-blind study. Curr. Orthop. Pract. 2010, 21, 282–287. [Google Scholar] [CrossRef]
- Kristiansen, T.K.; Ryaby, J.P.; McCABE, J.; Frey, J.J.; Roe, L.R. Accelerated Healing of Distal Radial Fractures with the Use of Specific, Low-Intensity Ultrasound. A Multicenter, Prospective, Randomized, Double-Blind, Placebo-Controlled Study. J. Bone Jt. Surg. Am. 1997, 79, 961–973. [Google Scholar] [CrossRef]
- Saebø, H.; Naterstad, I.F.; Bjordal, J.M.; Stausholm, M.B.; Joensen, J. Treatment of Distal Radius Fracture during Immobilization with an Orthopedic Cast: A Double-Blinded Randomized Controlled Trial of Photobiomodulation Therapy. Photobiomodul. Photomed. Laser Surg. 2021, 39, 280–288. [Google Scholar] [CrossRef]
- Lazovic, M.; Kocic, M.; Dimitrijevic, L.; Stankovic, I.; Spalevic, M.; Ciric, T. Pulsed electromagnetic field during cast immobilization in postmenopausal women with Colles’ fracture. Srp. Arh. Celok. Lek. 2012, 140, 619–624. [Google Scholar] [CrossRef]
- Krzyżańska, L.; Straburzyńska-Lupa, A.; Rąglewska, P.; Romanowski, L. Beneficial Effects of Pulsed Electromagnetic Field during Cast Immobilization in Patients with Distal Radius Fracture. BioMed Res. Int. 2020, 2020, 6849352. [Google Scholar] [CrossRef]
- Cadossi, R.; Massari, L.; Racine-Avila, J.; Aaron, R.K. Pulsed Electromagnetic Field Stimulation of Bone Healing and Joint Preservation: Cellular Mechanisms of Skeletal Response. J. Am. Acad. Orthop. Surg. Glob. Res. Rev. 2020, 4, e19.00155. [Google Scholar] [CrossRef]
- Gupta, S.; Halai, M.; Al-Maiyah, M.; Muller, S. Which measure should be used to assess the patient’s functional outcome after distal radius fracture? Acta Orthop. Belg. 2014, 80, 116–118. [Google Scholar]
- Walenkamp, M.M.J.; Keizer, R.-J.D.M.; Goslings, J.C.; Vos, L.M.; Rosenwasser, M.P.; Schep, N.W.L. The Minimum Clinically Important Difference of the Patient-rated Wrist Evaluation Score for Patients with Distal Radius Fractures. Clin. Orthop. Relat. Res. 2015, 473, 3235–3241. [Google Scholar] [CrossRef]
| Variable | Control Group n = 21 | Active Group n = 20 |
|---|---|---|
| Age (years) | 59 (range 21–88) | 58 (range 26–79) |
| Male (n, %) | 7 (33) | 5 (25) |
| Fracture in dominant hand (n, %) | 8 (38) | 11 (55) |
| Fracture type (n, %) | ||
| Frykman Type (I) | 5 (24) | 6 (30) |
| Frykman Type (II) | 2 (9) | 3 (15) |
| Frykman Type (III) | 1 (5) | 4 (20) |
| Frykman Type (IV) | 3 (14) | 1 (5) |
| Frykman Type (V) | 0 (0) | 1 (5) |
| Frykman Type (VI) | 1 (5) | 0 (0) |
| Frykman Type (VII) | 1 (5) | 0 (0) |
| Frykman Type (VIII) | 1 (5) | 0 (0) |
| Smoking (n, %) | 4 (19) | 3 (15) |
| Osteoporosis (n, %) | 3 (14) | 4 (20) |
| Corticosteroids (n, %) | 0 (0) | 1 (5) |
| Group | No Union (0–24%) | Partial Union (25–74%) | Union (75–100%) |
|---|---|---|---|
| Control | 1 | 9 | 3 |
| Active | 0 | 5 | 9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Factor, S.; Druckmann, I.; Atlan, F.; Rosenblatt, Y.; Tordjman, D.; Krespi, R.; Kazum, E.; Pritsch, T.; Eisenberg, G. The Effects of Novel Pulsed Electromagnetic Field Therapy Device on Acute Distal Radius Fractures: A Prospective, Double-Blind, Sham-Controlled, Randomized Pilot Study. J. Clin. Med. 2023, 12, 1866. https://doi.org/10.3390/jcm12051866
Factor S, Druckmann I, Atlan F, Rosenblatt Y, Tordjman D, Krespi R, Kazum E, Pritsch T, Eisenberg G. The Effects of Novel Pulsed Electromagnetic Field Therapy Device on Acute Distal Radius Fractures: A Prospective, Double-Blind, Sham-Controlled, Randomized Pilot Study. Journal of Clinical Medicine. 2023; 12(5):1866. https://doi.org/10.3390/jcm12051866
Chicago/Turabian StyleFactor, Shai, Ido Druckmann, Franck Atlan, Yishai Rosenblatt, Daniel Tordjman, Raphael Krespi, Efi Kazum, Tamir Pritsch, and Gilad Eisenberg. 2023. "The Effects of Novel Pulsed Electromagnetic Field Therapy Device on Acute Distal Radius Fractures: A Prospective, Double-Blind, Sham-Controlled, Randomized Pilot Study" Journal of Clinical Medicine 12, no. 5: 1866. https://doi.org/10.3390/jcm12051866
APA StyleFactor, S., Druckmann, I., Atlan, F., Rosenblatt, Y., Tordjman, D., Krespi, R., Kazum, E., Pritsch, T., & Eisenberg, G. (2023). The Effects of Novel Pulsed Electromagnetic Field Therapy Device on Acute Distal Radius Fractures: A Prospective, Double-Blind, Sham-Controlled, Randomized Pilot Study. Journal of Clinical Medicine, 12(5), 1866. https://doi.org/10.3390/jcm12051866






