Effects of Dementia on Outcomes after Cervical Spine Injuries in Elderly Patients: Evaluation of 1512 Cases in a Nationwide Multicenter Study in Japan
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Variables, Classifications, and Outcomes
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics Including Injury and Treatment Status
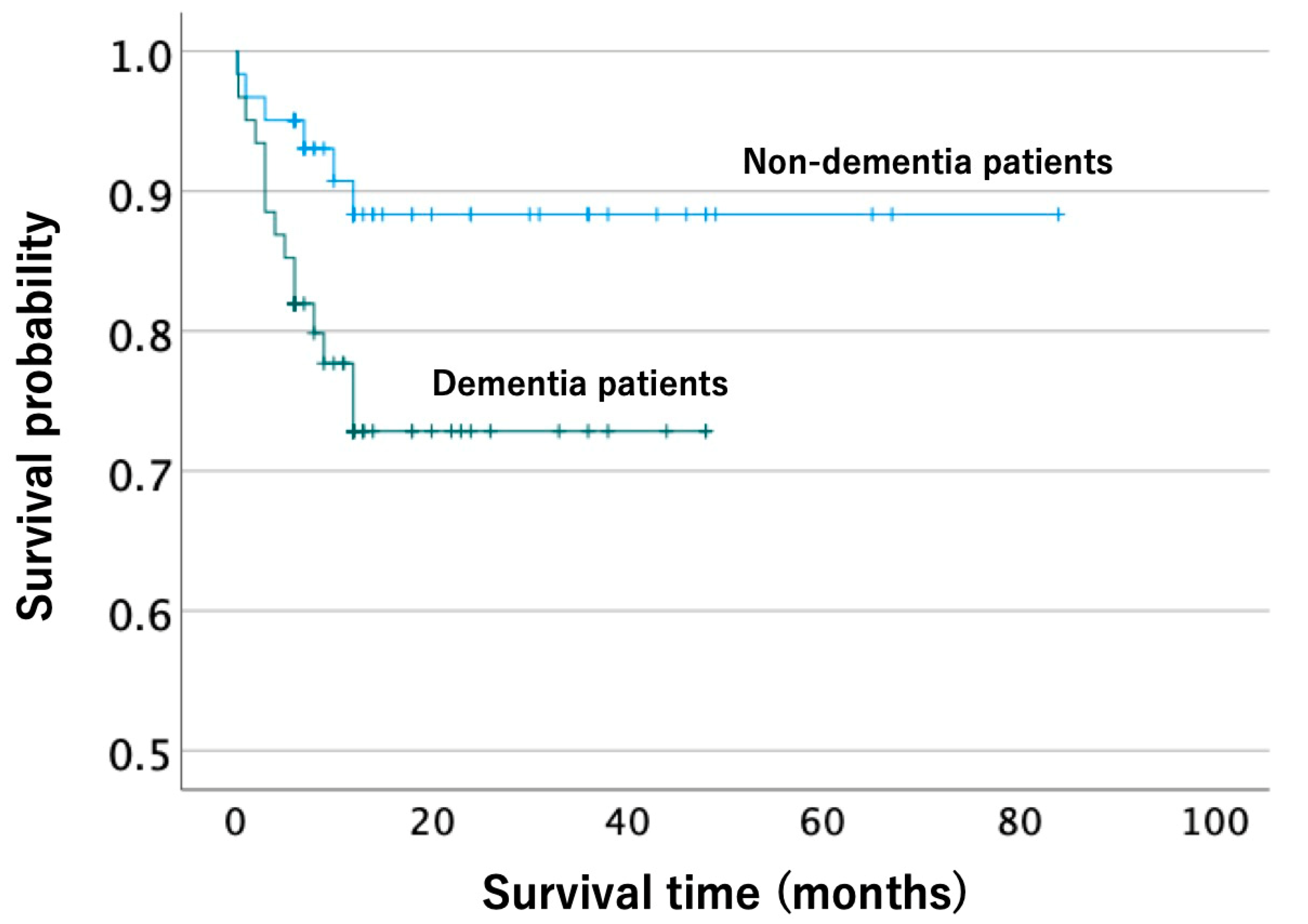
3.2. Propensity Score Matching Analysis of Complications, ADLs, and Survival after Injury
4. Discussion
4.1. Characteristics of Cervical Spine Injury in Elderly Patients with Dementia
4.2. Effects of Dementia on Clinical Outcomes Following a Cervical Spine Injury
4.3. Fall Trauma Risk in Elderly Patients with Dementia
4.4. Prevention of and Enhanced Care for Cervical Spine Injury in Elderly Patients with Dementia
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Algahtany, M.; McFaull, S.; Chen, L.; Zhang, S.; Saarela, O.; Alqahtani, F.; Cusimano, M.D. The Changing Etiology and Epidemiology of Traumatic Spinal Injury: A Population-Based Study. World Neurosurg. 2021, 149, e116–e127. [Google Scholar] [CrossRef] [PubMed]
- Kannus, P.; Palvanen, M.; Niemi, S.; Parkkari, J. Alarming Rise in the Number and Incidence of Fall-Induced Cervical Spine Injuries Among Older Adults. J. Gerontol. A Biol. Sci. Med. Sci. 2007, 62, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Bank, M.; Gibbs, K.; Sison, C.; Kutub, N.; Papatheodorou, A.; Lee, S.; Stein, A.; Bloom, O. Age and Other Risk Factors Influencing Long-Term Mortality in Patients with Traumatic Cervical Spine Fracture. Geriatr. Orthop. Surg. Rehabil. 2018, 9, 2151459318770882. [Google Scholar] [CrossRef]
- Liu, P.; Yao, Y.; Liu, M.; Fan, W.; Chao, R.; Wang, Z.; Liu, Y.; Zhou, J.; Zhao, J. Spinal trauma in mainland China from 2001 to 2007: An epidemiological study based on a nationwide database. Spine 2012, 37, 1310–1315. [Google Scholar] [CrossRef] [PubMed]
- Jawa, R.S.; Singer, A.J.; Rutigliano, D.N.; McCormack, J.E.; Huang, E.C.; Shapiro, M.J.; Fields, S.D.; Morelli, B.N.; Vosswinkel, J.A. Spinal Fractures in Older Adult Patients Admitted After Low-Level Falls: 10-Year Incidence and Outcomes. J. Am. Geriatr. Soc. 2017, 65, 909–915. [Google Scholar] [CrossRef]
- Passias, P.G.; Poorman, G.W.; Segreto, F.A.; Jalai, C.M.; Horn, S.R.; Bortz, C.A.; Vasquez-Montes, D.; Diebo, B.G.; Vira, S.; Bono, O.J.; et al. Traumatic Fractures of the Cervical Spine: Analysis of Changes in Incidence, Cause, Concurrent Injuries, and Complications Among 488,262 Patients from 2005 to 2013. World Neurosurg. 2018, 110, e427–e437. [Google Scholar] [CrossRef]
- Krueger, H.; Noonan, V.; Trenaman, L.; Joshi, P.; Rivers, C. The Economic Burden of Traumatic Spinal Cord Injury in Canada. Chronic Dis. Inj. Can. 2013, 33, 113–122. [Google Scholar] [CrossRef]
- Munce, S.E.P.; Wodchis, W.P.; Guilcher, S.J.; Couris, C.M.; Verrier, M.; Fung, K.; Craven, B.C.; Jaglal, S.B. Direct Costs of Adult Traumatic Spinal Cord Injury in Ontario. Spinal Cord 2013, 51, 64–69. [Google Scholar] [CrossRef]
- Prince, M.; Bryce, R.; Albanese, E.; Wimo, A.; Ribeiro, W.; Ferri, C.P. The Global Prevalence of Dementia: A Systematic Review and Metaanalysis. Alzheimer’s Dement. 2013, 9, 63–75.e2. [Google Scholar] [CrossRef]
- Ferri, C.P.; Prince, M.; Brayne, C.; Brodaty, H.; Fratiglioni, L.; Ganguli, M.; Hall, K.; Hasegawa, K.; Hendrie, H.; Huang, Y.; et al. Global Prevalence of Dementia: A Delphi Consensus Study. Lancet 2006, 366, 2112–2117. [Google Scholar] [CrossRef]
- Vun, J.S.H.; Ahmadi, M.; Panteli, M.; Pountos, I.; Giannoudis, P.V. Dementia and Fragility Fractures: Issues and Solutions. Injury 2017, 48, S10–S16. [Google Scholar] [CrossRef] [PubMed]
- Young, Y.; Xiong, K.; Pruzek, R.M. Longitudinal Functional Recovery After Postacute Rehabilitation in Older Hip Fracture Patients: The Role of Cognitive Impairment and Implications for Long-Term Care. J. Am. Med. Dir. Assoc. 2011, 12, 431–438. [Google Scholar] [CrossRef] [PubMed]
- van Schoor, N.M.; Smit, J.H.; Pluijm, S.M.F.; Jonker, C.; Lips, P. Different Cognitive Functions in Relation to Falls among Older Persons. Immediate Memory as an Independent Risk Factor for Falls. J. Clin. Epidemiol. 2002, 55, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.L.N.; Vogelaere, P.; Baptista, F. Role of Physical Activity in the Prevention of Falls and Their Consequences in the Elderly. Eur. Rev. Aging Phys. Act. 2008, 5, 51–58. [Google Scholar] [CrossRef]
- Bai, J.; Zhang, P.; Liang, X.; Wu, Z.; Wang, J.; Liang, Y. Association between Dementia and Mortality in the Elderly Patients Undergoing Hip Fracture Surgery: A Meta-Analysis. J. Orthop. Surg. Res. 2018, 13, 298. [Google Scholar] [CrossRef] [PubMed]
- Tolppanen, A.M.; Taipale, H.; Tanskanen, A.; Tiihonen, J.; Hartikainen, S. Comparison of Predictors of Hip Fracture and Mortality after Hip Fracture in Community-Dwellers with and without Alzheimer’s Disease—Exposure-Matched Cohort Study. BMC Geriatr. 2016, 16, 204. [Google Scholar] [CrossRef] [PubMed]
- Ha, Y.C.; Cha, Y.; Yoo, J.I.; Lee, J.; Lee, Y.K.; Koo, K.H. Effect of Dementia on Postoperative Mortality in Elderly Patients with Hip Fracture. J. Korean Med. Sci. 2021, 36, e238. [Google Scholar] [CrossRef]
- Jorissen, R.N.; Inacio, M.C.; Cations, M.; Lang, C.; Caughey, G.E.; Crotty, M. Effect of Dementia on Outcomes After Surgically Treated Hip Fracture in Older Adults. J. Arthroplast. 2021, 36, 3181–3186.e4. [Google Scholar] [CrossRef]
- Yokogawa, N.; Kato, S.; Sasagawa, T.; Hayashi, H.; Tsuchiya, H.; Ando, K.; Nakashima, H.; Segi, N.; Funayama, T.; Eto, F.; et al. Differences in Clinical Characteristics of Cervical Spine Injuries in Older Adults by External Causes: A Multicenter Study of 1512 Cases. Sci. Rep. 2022, 12, 15867. [Google Scholar] [CrossRef]
- Subramaniam, S.; Aalberg, J.J.; Soriano, R.P.; Divino, C.M. New 5-Factor Modified Frailty Index Using American College of Surgeons NSQIP Data. J. Am. Coll. Surg. 2018, 226, 173–181.e8. [Google Scholar] [CrossRef]
- Khalafallah, A.M.; Huq, S.; Jimenez, A.E.; Brem, H.; Mukherjee, D. The 5-factor modified frailty index: An effective predictor of mortality in brain tumor patients. J. Neurosurg. 2020, 135, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Kirshblum, S.C.; Burns, S.P.; Biering-Sorensen, F.; Donovan, W.; Graves, D.E.; Jha, A.; Johansen, M.; Jones, L.; Krassioukov, A.; Mulcahey, M.J.; et al. International Standards for Neurological Classification of Spinal Cord Injury (Revised 2011). J. Spinal Cord Med. 2011, 34, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, P.R.; Rubin, D.B. The Central Role of the Propensity Score in Observational Studies for Causal Effects. Biometrika 1983, 70, 41–55. [Google Scholar] [CrossRef]
- Lane, C.A.; Hardy, J.; Schott, J.M. Alzheimer’s Disease. Eur. J. Neurol. 2018, 25, 59–70. [Google Scholar] [CrossRef]
- Garre-Olmo, J. Epidemiology of Alzheimer’s Disease and Other Dementias. Rev. Neurol. 2018, 66, 377–386. [Google Scholar]
- Doraiswamy, P.M.; Leon, J.; Cummings, J.L.; Marin, D.; Neumann, P.J. Prevalence and Impact of Medical Comorbidity in Alzheimer’s Disease. J. Gerontol. A Biol. Sci. Med. Sci. 2002, 57, M173–M177. [Google Scholar] [CrossRef]
- Schubert, C.C.; Boustani, M.; Callahan, C.M.; Perkins, A.J.; Carney, C.P.; Fox, C.; Unverzagt, F.; Hui, S.; Hendrie, H.C. Comorbidity Profile of Dementia Patients in Primary Care: Are They Sicker? J. Am. Geriatr. Soc. 2006, 54, 104–109. [Google Scholar] [CrossRef]
- Kuo, T.C.; Zhao, Y.; Weir, S.; Kramer, M.S.; Ash, A.S. Implications of Comorbidity on Costs for Patients with Alzheimer Disease. Med. Care 2008, 46, 839–846. [Google Scholar] [CrossRef]
- Leon, J.; Cheng, C.K.; Neumann, P.J. Alzheimer’s Disease Care: Costs and Potential Savings. Health Aff. 2017, 17, 206–216. [Google Scholar] [CrossRef]
- Bunn, F.; Burn, A.M.; Goodman, C.; Rait, G.; Norton, S.; Robinson, L.; Schoeman, J.; Brayne, C. Comorbidity and Dementia: A Scoping Review of the Literature. BMC Med. 2014, 12, 192. [Google Scholar] [CrossRef]
- Richards, M.; Brayne, C. What Do We Mean by Alzheimer’s Disease? BMJ. 2010, 341, c4670. [Google Scholar] [CrossRef] [PubMed]
- Skoog, I. Vascular aspects in Alzheimer’s disease. J. Neural Transm. Suppl. 2000, 59, 37–43. [Google Scholar] [PubMed]
- Thuné-Boyle, I.C.V.; Iliffe, S.; Cerga-Pashoja, A.; Lowery, D.; Warner, J. The Effect of Exercise on Behavioral and Psychological Symptoms of Dementia: Towards a Research Agenda. Int. Psychogeriatr. 2012, 24, 1046–1057. [Google Scholar] [CrossRef] [PubMed]
- Eggermont, L.H.P.; Scherder, E.J.A. Physical Activity and Behaviour in Dementia: A Review of the Literature and Implications for Psychosocial Intervention in Primary Care. Dementia 2006, 5, 411–428. [Google Scholar] [CrossRef]
- Scherder, E.J.A.; Bogen, T.; Eggermont, L.H.P.; Hamers, J.P.H.; Swaab, D.F. The More Physical Inactivity, the More Agitation in Dementia. Int. Psychogeriatr. 2010, 22, 1203–1208. [Google Scholar] [CrossRef]
- Royall, D.R.; Lauterbach, E.C.; Kaufer, D.; Malloy, P.; Coburn, K.L.; Black, K.J.; Association, C. on R. of the A.N. The Cognitive Correlates of Functional Status: A Review from the Committee on Research of the American Neuropsychiatric Association. J. Neuropsychiatry Clin. Neurosci. 2007, 19, 249–265. [Google Scholar] [CrossRef]
- Kuan, Y.C.; Huang, L.K.; Wang, Y.H.; Hu, C.J.; Tseng, I.J.; Chen, H.C.; Lin, L.F. Balance and Gait Performance in Older Adults with Early-Stage Cognitive Impairment. Eur. J. Phys. Rehabil. Med. 2021, 57, 560–567. [Google Scholar] [CrossRef]
- Doi, T.; Shimada, H.; Park, H.; Makizako, H.; Tsutsumimoto, K.; Uemura, K.; Nakakubo, S.; Hotta, R.; Suzuki, T. Cognitive Function and Falling among Older Adults with Mild Cognitive Impairment and Slow Gait: MCI, Slow Gait and Fall. Geriatr. Gerontol. Int. 2014, 15, 1073–1078. [Google Scholar] [CrossRef]
- Cohen, J.A.; Verghese, J. Gait and Dementia. Handb. Clin. Neurol. 2019, 167, 419–427. [Google Scholar]
- Kostev, K.; Hadji, P.; Jacob, L. Impact of Osteoporosis on the Risk of Dementia in Almost 60,000 Patients Followed in General Practices in Germany. J. Alzheimer’s Dis. 2018, 65, 401–407. [Google Scholar] [CrossRef]
- Mughal, N.; Inderjeeth, A.J.; Inderjeeth, C.A. Osteoporosis in Patients with Dementia Is Associated with High Morbidity and Mortality: Findings from a Single Orthogeriatric Unit. Aust. J. Gen. Pract. 2019, 48, 53–58. [Google Scholar] [CrossRef]
- Hayashi, T.; Fujiwara, Y.; Ariji, Y.; Sakai, H.; Kubota, K.; Kawano, O.; Masuda, M.; Morishita, Y.; Maeda, T. Mechanism of Dysphagia after Acute Traumatic Cervical Spinal Cord Injury. J. Neurotrauma 2020, 37, 2315–2319. [Google Scholar] [CrossRef] [PubMed]
- Alagiakrishnan, K.; Bhanji, R.A.; Kurian, M. Evaluation and Management of Oropharyngeal Dysphagia in Different Types of Dementia: A Systematic Review. Arch. Gerontol. Geriatr. 2013, 56, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Easterling, C.S.E.; Robbins, E. Dementia and Dysphagia. Geriatr. Nurs. 2008, 29, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Chebib, N.; Cuvelier, C.; Malézieux-Picard, A.; Parent, T.; Roux, X.; Fassier, T.; Müller, F.; Prendki, V. Pneumonia Prevention in the Elderly Patients: The Other Sides. Aging Clin. Exp. Res. 2021, 33, 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- Marik, P.E.; Kaplan, D. Aspiration Pneumonia and Dysphagia in the Elderly. Chest 2003, 124, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Sura, L.; Madhavan, A.; Carnaby, G.; Crary, M.A. Dysphagia in the Elderly: Management and Nutritional Considerations. Clin. Interv. Aging 2012, 7, 287–298. [Google Scholar]
- Smith, T.O.; Gilbert, A.W.; Sreekanta, A.; Sahota, O.; Griffin, X.L.; Cross, J.L.; Fox, C.; Lamb, S.E. Enhanced Rehabilitation and Care Models for Adults with Dementia Following Hip Fracture Surgery. Cochrane Database Syst. Rev. 2020, 2, CD010569. [Google Scholar] [CrossRef]
- Smith, S.; Purzner, T.; Fehlings, M. The Epidemiology of Geriatric Spinal Cord Injury. Top. Spinal Cord Inj. Rehabil. 2010, 15, 54–64. [Google Scholar] [CrossRef]
- Taudorf, L.; Nørgaard, A.; Waldemar, G.; Laursen, T.M. Mortality in Dementia from 1996 to 2015: A National Registry-Based Cohort Study. J. Alzheimer’s Dis. 2020, 79, 289–300. [Google Scholar] [CrossRef]
- Dewey, M.E.; Saz, P. Dementia, Cognitive Impairment and Mortality in Persons Aged 65 and over Living in the Community: A Systematic Review of the Literature. Int. J. Geriatr. Psychiatry 2001, 16, 751–761. [Google Scholar] [CrossRef] [PubMed]
- Guehne, U.; Riedel-Heller, S.; Angermeyer, M.C. Mortality in Dementia. Neuroepidemiology 2005, 25, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Lundström, M.; Edlund, A.; Bucht, G.; Karlsson, S.; Gustafson, Y. Dementia after Delirium in Patients with Femoral Neck Fractures. J. Am. Geriatr. Soc. 2003, 51, 1002–1006. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cao, T.; Ritzel, R.M.; He, J.; Faden, A.I.; Wu, J. Dementia, Depression, and Associated Brain Inflammatory Mechanisms after Spinal Cord Injury. Cells 2020, 9, 1420. [Google Scholar] [CrossRef]
- Beauchet, O.; Sekhon, H.; Schott, A.M.; Rolland, Y.; Muir-Hunter, S.; Markle-Reid, M.; Gagne, H.; Allali, G. Motoric Cognitive Risk Syndrome and Risk for Falls, Their Recurrence, and Postfall Fractures: Results from a Prospective Observational Population-Based Cohort Study. J. Am. Med. Dir. Assoc. 2019, 20, 1268–1273. [Google Scholar] [CrossRef]
- Shaw, F.E.; Bond, J.; Richardson, D.A.; Dawson, P.; Steen, I.N.; McKeith, I.G.; Kenny, R.A. Multifactorial intervention after a fall in older people with cognitive impairment and dementia presenting to the accident and emergency department: Randomised controlled trial. BMJ 2003, 326, 73. [Google Scholar] [CrossRef]
- Hart, L.A.; Marcum, Z.A.; Gray, S.L.; Walker, R.L.; Crane, P.K.; Larson, E.B. The Association Between Central Nervous System-Active Medication Use and Fall-Related Injury in Community-Dwelling Older Adults with Dementia. Pharmacotherapy 2019, 39, 530–543. [Google Scholar] [CrossRef]
- Bokhari, A.R.; Sivakumar, B.; Sefton, A.; Lin, J.L.; Smith, M.M.; Gray, R.; Hartin, N. Morbidity and mortality in cervical spine injuries in the elderly. ANZ J. Surg. 2019, 89, 412–417. [Google Scholar] [CrossRef]
- Golob, J.F., Jr.; Claridge, J.A.; Yowler, C.J.; Como, J.J.; Peerless, J.R. Isolated cervical spine fractures in the elderly: A deadly injury. J. Trauma 2008, 64, 311–315. [Google Scholar] [CrossRef]
| Dementia (n = 95) | Non-Dementia (n = 1417) | p-Value | |
|---|---|---|---|
| Age at injury (years), mean ± SD | 82.1 ± 6.8 | 75.4 ± 6.7 | <0.001 * |
| Sex: Female, n (%) | 45 (47.4) | 460 (32.5) | 0.003 * |
| Body mass index (kg/m2), mean ± SD | 20.2 ± 3.8 | 22.2 ± 3.6 | <0.001 * |
| Residence status; living at home, n (%) | 82 (87.2) | 1357 (97.7) | <0.001 * |
| Pre-injury ADLs; walking w/ or w/o a cane, n (%) | 76 (81.7) | 1354 (96.4) | <0.001 * |
| Presence of a pre-injury comorbidity | |||
| Cerebrovascular disease, n (%) | 19 (20.0) | 126 (9.2) | <0.001 * |
| Parkinson’s disease, n (%) | 3 (3.2) | 19 (1.4) | 0.162 |
| Hypertension, n (%) | 58 (61.1) | 673 (48.8) | 0.021 * |
| Diabetes mellitus, n (%) | 29 (30.5) | 301 (22.1) | 0.057 |
| Cardiovascular disease, n (%) | 22 (23.2) | 205 (15.1) | 0.035 * |
| Respiratory disease, n (%) | 11 (11.7) | 70 (5.1) | 0.007 * |
| Renal disease, n (%) | 9 (9.5) | 64 (4.7) | 0.040 * |
| Musculoskeletal disorders, n (%) | 21 (22.3) | 169 (12.4) | 0.006 * |
| Number of medications, mean ± SD | 5.9 ± 4.6 | 3.8 ± 3.7 | <0.001 * |
| Modified 5-item frailty index, mean ± SD | 1.18 ± 0.86 | 0.77 ± 0.74 | <0.001 * |
| Blood test data at admission | |||
| Serum total protein value (g/dL), mean ± SD | 6.6 ± 0.64 | 6.6 ± 0.71 | 0.762 |
| Serum hemoglobin value (g/dL), mean ± SD | 11.7 ± 1.9 | 12.8 ± 1.9 | <0.001 * |
| Dementia (n = 95) | Non-Dementia (n = 1417) | p-Value | |
|---|---|---|---|
| Injury status | |||
| Ground-level fall, n (%) | 54 (56.8) | 525(37.1) | <0.001 * |
| Cervical spine fracture, n (%) | 67 (70.5) | 767 (54.1) | 0.002 * |
| Cervical dislocation, n (%) | 9 (9.5) | 219 (15.5) | 0.115 |
| Neurological impairment, n (%) | 0.056 | ||
| AIS A or B, n (%) | 10 (10.5) | 193 (13.6) | |
| AIS C or D, n (%) | 46 (48.4) | 807 (57.0) | |
| No spinal cord injury, n (%) | 39 (41.1) | 417 (29.4) | |
| Associated injuries, n (%) | 30 (31.6) | 381 (26.9) | 0.320 |
| Head injury, n (%) | 19 (20.0) | 198 (14.0) | 0.107 |
| Thoracic injury, n (%) | 5 (5.3) | 83 (5.9) | 0.819 |
| Abdominal injury, n (%) | 1 (1.1) | 21 (1.5) | 0.592 |
| Upper limb injury, n (%) | 4 (4.2) | 73 (5.2) | 0.459 |
| Lower limb injury, n (%) | 6 (6.3) | 49 (3.5) | 0.127 |
| Pelvic fracture, n (%) | 1 (1.1) | 26 (1.8) | 0.484 |
| Thoracolumbar vertebral fracture, n (%) | 7 (7.4) | 84 (5.9) | 0.571 |
| Treatment status | |||
| Steroid administration, n (%) | 9 (9.5) | 198 (14.0) | 0.216 |
| Surgical treatment, n (%) | 47 (49.5) | 856 (60.4) | 0.035 * |
| Dementia (n = 61) | Non-Dementia (n = 61) | p-Value | |
|---|---|---|---|
| Age at injury (years), mean ± SD | 79.7 ± 6.4 | 79.4 ± 6.5 | 0.790 |
| Sex: Female, n (%) | 23 (37.7) | 22 (36.1) | 0.851 |
| Pre-injuryADLs: walking w/ or w/o a cane, n (%) | 58 (95.1) | 59 (96.7) | 0.500 |
| Neurological impairment | 0.405 | ||
| AIS A or B, n (%) | 6 (9.8) | 8 (13.1) | |
| AIS C or D, n (%) | 30 (49.2) | 35 (57.4) | |
| No spinal cord injury, n (%) | 25 (41.0) | 18 (29.5) | |
| Surgical treatment | 36 (59.0) | 34 (55.7) | 0.714 |
| Dementia (n = 61) | Non-Dementia (n = 61) | p-Value | |
|---|---|---|---|
| Complications, n (%) | 38 (62.3) | 29 (47.5) | 0.102 |
| Respiratory impairment, n (%) | 5 (8.2) | 3 (4.9) | 0.359 |
| Dysphagia, n (%) | 8 (13.1) | 2 (3.3) | 0.048 * |
| Pneumonia, n (%) | 12 (19.7) | 5 (8.2) | 0.067 |
| Urinary tract infection, n (%) | 8 (13.1) | 4 (6.6) | 0.224 |
| Deep venous thrombosis, n (%) | 1 (1.6) | 1 (1.6) | 0.752 |
| Pulmonary embolism, n (%) | 2 (3.3) | 0 (0) | 0.248 |
| Cerebral infarction, n (%) | 0 (0) | 2 (3.3) | 0.248 |
| Myocardial infarction, n (%) | 0 (0) | 0 (0) | N/A |
| Delirium, n (%) | 16 (26.2) | 9 (14.8) | 0.116 |
| ADLsat 6 months: walking w/ or w/o a cane, n (%) | 28 (46.2) | 42 (69) | 0.016 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamada, Y.; Yokogawa, N.; Kato, S.; Sasagawa, T.; Tsuchiya, H.; Nakashima, H.; Segi, N.; Ito, S.; Funayama, T.; Eto, F.; et al. Effects of Dementia on Outcomes after Cervical Spine Injuries in Elderly Patients: Evaluation of 1512 Cases in a Nationwide Multicenter Study in Japan. J. Clin. Med. 2023, 12, 1867. https://doi.org/10.3390/jcm12051867
Yamada Y, Yokogawa N, Kato S, Sasagawa T, Tsuchiya H, Nakashima H, Segi N, Ito S, Funayama T, Eto F, et al. Effects of Dementia on Outcomes after Cervical Spine Injuries in Elderly Patients: Evaluation of 1512 Cases in a Nationwide Multicenter Study in Japan. Journal of Clinical Medicine. 2023; 12(5):1867. https://doi.org/10.3390/jcm12051867
Chicago/Turabian StyleYamada, Yohei, Noriaki Yokogawa, Satoshi Kato, Takeshi Sasagawa, Hiroyuki Tsuchiya, Hiroaki Nakashima, Naoki Segi, Sadayuki Ito, Toru Funayama, Fumihiko Eto, and et al. 2023. "Effects of Dementia on Outcomes after Cervical Spine Injuries in Elderly Patients: Evaluation of 1512 Cases in a Nationwide Multicenter Study in Japan" Journal of Clinical Medicine 12, no. 5: 1867. https://doi.org/10.3390/jcm12051867
APA StyleYamada, Y., Yokogawa, N., Kato, S., Sasagawa, T., Tsuchiya, H., Nakashima, H., Segi, N., Ito, S., Funayama, T., Eto, F., Yamaji, A., Yamane, J., Nori, S., Furuya, T., Yunde, A., Nakajima, H., Yamada, T., Hasegawa, T., Terashima, Y., ... Watanabe, K. (2023). Effects of Dementia on Outcomes after Cervical Spine Injuries in Elderly Patients: Evaluation of 1512 Cases in a Nationwide Multicenter Study in Japan. Journal of Clinical Medicine, 12(5), 1867. https://doi.org/10.3390/jcm12051867










