Vitamin D, a Regulator of Androgen Levels, Is Not Correlated to PSA Serum Levels in a Cohort of the Middle Italy Region Participating to a Prostate Cancer Screening Campaign
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sample
2.2. Study Design
2.3. Sample Collection
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Di Zazzo, E.; Galasso, G.; Giovannelli, P.; Di Donato, M.; Di Santi, A.; Cernera, G.; Rossi, V.; Abbondanza, C.; Moncharmont, B.; Sinisi, A.A.; et al. Prostate cancer stem cells: The role of androgen and estrogen receptors. Oncotarget 2016, 7, 193–208. [Google Scholar] [CrossRef] [PubMed]
- Rossi, V.; Di Zazzo, E.; Galasso, G.; De Rosa, C.; Abbondanza, C.; Sinisi, A.A.; Altucci, L.; Migliaccio, A.; Castoria, G. Estrogens Modulate Somatostatin Receptors Expression and Synergize With the Somatostatin Analog Pasireotide in Prostate Cells. Front. Pharmacol. 2019, 10, 28. [Google Scholar] [CrossRef] [PubMed]
- Mottet, N.; van den Bergh, R.C.N.; Briers, E.; Van den Broeck, T.; Cumberbatch, M.G.; De Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S.; et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer-2020 Update. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2021, 79, 243–262. [Google Scholar] [CrossRef] [PubMed]
- Pernar, C.H.; Ebot, E.M.; Wilson, K.M.; Mucci, L.A. The Epidemiology of Prostate Cancer. Cold Spring Harb. Perspect. Med. 2018, 8, a030361. [Google Scholar] [CrossRef] [PubMed]
- Gandaglia, G.; Leni, R.; Bray, F.; Fleshner, N.; Freedland, S.J.; Kibel, A.; Stattin, P.; Van Poppel, H.; La Vecchia, C. Epidemiology and Prevention of Prostate Cancer. Eur. Urol. Oncol. 2021, 4, 877–892. [Google Scholar] [CrossRef] [PubMed]
- Mazzone, E.; Preisser, F.; Nazzani, S.; Tian, Z.; Bandini, M.; Gandaglia, G.; Fossati, N.; Montorsi, F.; Graefen, M.; Shariat, S.F.; et al. The Effect of Lymph Node Dissection in Metastatic Prostate Cancer Patients Treated with Radical Prostatectomy: A Contemporary Analysis of Survival and Early Postoperative Outcomes. Eur. Urol. Oncol. 2019, 2, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.C.; Valenzuela, L.A.; Murphy, G.P.; Chu, T.M. Purification of a human prostate specific antigen. Invest. Urol. 1979, 7, i59–i163. [Google Scholar]
- Ellis, W.J.; Brawer, M.K. The role of tumor markers in the diagnosis and treatment of prostate cancer. In Prostate Diseases; Lepor, H., Lawson, R.K., Eds.; WB Saunders: Philadelphia, PA, USA, 1993; pp. 276–292. [Google Scholar]
- Stamey, T.A.; Yang, N.; Hay, A.R.; McNeal, J.E.; Freiha, F.S.; Redwine, E. Prostate-specific antigen as a serum marker for adenocarcinoma of the prostate. N. Engl. J. Med. 1987, 317, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Semjonow, A.; Brandt, B.; Oberpenning, F.; Roth, S.; Hertle, L. Discordance of assay methods creates pitfalls for the interpretation of prostate-specific antigen values. Prostate. Suppl. 1996, 7, 3–16. [Google Scholar] [CrossRef]
- Beyond PSA: The Role of Prostate Health Index (phi) PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/32053990/ (accessed on 12 October 2022).
- Catalona, W.J.; Richie, J.P.; Ahmann, F.R.; Hudson, M.A.; Scardino, P.T.; Flanigan, R.C.; DeKernion, J.B.; Ratliff, T.L.; Kavoussi, L.R.; Dalkin, B.L.; et al. Comparison of digital rectal examination and serum prostate specific antigen in the early detection of prostate cancer: Results of a multicenter clinical trial of 6630 men. J. Urol. 1994, 151, 1283–1290. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Metter, E.J.; Landis, P.; Carter, H.B. PSA velocity for assessing prostate cancer risk in men with PSA levels between 2.0 and 4.0 ng/ml. Urology 2002, 59, 889–894. [Google Scholar] [CrossRef] [PubMed]
- Ferro, M.; Crocetto, F.; Bruzzese, D.; Imbriaco, M.; Fusco, F.; Longo, N.; Napolitano, L.; La Civita, E.; Cennamo, M.; Liotti, A. Prostate Health Index and Multiparametric MRI: Partners in Crime Fighting Overdiagnosis and Overtreatment in Prostate Cancer. Cancers 2021, 13, 4723. [Google Scholar] [CrossRef] [PubMed]
- Crocetto, F.; Russo, G.; Di Zazzo, E.; Pisapia, P.; Mirto, B.F.; Palmieri, A.; Pepe, F.; Bellevicine, C.; Russo, A.; La Civita, E.; et al. Liquid Biopsy in Prostate Cancer Management-Current Challenges and Future Perspectives. Cancers 2022, 14, 3272. [Google Scholar] [CrossRef] [PubMed]
- Chandler, P.D.; Giovannucci, E.L.; Scott, J.B.; Bennett, G.G.; Ng, K.; Chan, A.T.; Hollis, B.W.; Emmons, K.M.; Fuchs, C.S.; Drake, B.F. Null association between vitamin D and PSA levels among black men in a vitamin D supplementation trial. Cancer Epidemiol. Biomark. Prev. 2014, 23, 1944–1947. [Google Scholar] [CrossRef] [PubMed]
- Nelson, S.M.; Batai, K.; Ahaghotu, C.; Agurs-Collins, T.; Kittles, R.A. Association between Serum 25-Hydroxy-Vitamin D and Aggressive Prostate Cancer in African American Men. Nutrients 2016, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, G.G.; Hulka, B.S. Is vitamin D deficiency a risk factor for prostate cancer? (Hypothesis). Anticancer. Res. 1990, 10, 1307–1311. [Google Scholar]
- Helgstrand, J.T.; Røder, M.A.; Klemann, N.; Toft, B.G.; Lichtensztajn, D.Y.; Brooks, J.D.; Brassom, K.; Vainer, B.; Iversen, P. Trends in incidence and 5-year mortality in men with newly diagnosed, metastatic prostate cancer-A population-based analysis of 2 national cohorts. Cancer 2018, 124, 2931–2938. [Google Scholar] [CrossRef]
- John, E.M.; Schwartz, G.G.; Koo, J.; Van Den Berg, D.; Ingles, S.A. Sun exposure, vitamin D receptor gene polymorphisms, and risk of advanced prostate cancer. Cancer Res. 2005, 65, 5470–5479. [Google Scholar] [CrossRef]
- Giovannucci, E. The epidemiology of vitamin D and cancer incidence and mortality: A review (United States). Cancer Causes Control 2005, 16, 83–95. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D: Importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. Am. J. Clin. Nutr. 2004, 79, 362–371. [Google Scholar] [CrossRef]
- Krill, D.; Stoner, J.; Konety, B.R.; Becich, M.J.; Getzenberg, R.H. Differential effects of vitamin D on normal human prostate epithelial and stromal cells in primary culture. Urology 1999, 54, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Corder, E.H.; Guess, H.A.; Hulka, B.S.; Friedman, G.D.; Sadler, M.; Vollmer, R.T.; Lobaugh, B.; Drezner, M.K.; Vogelman, J.H.; Orentreich, N. Vitamin D and prostate cancer: A prediagnostic study with stored sera. Cancer Epidemiol. Biomark. Prev. 1993, 2, 467–472. [Google Scholar]
- Kristal, A.R.; Till, C.; Song, X.; Goodman, P.J.; Neuhauser, M.L.; Schenk, J.M.; Thompson, I.M.; Meyskens, F.L., Jr.; Goodman, G.E.; Minasian, L.M.; et al. Plasma vitamin D and prostate cancer risk: Results from the Selenium and Vitamin E Cancer Prevention Trial. Cancer Epidemiol. Biomark. Prev. 2014, 23, 1494–1504. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Peters, U.; Albanes, D.; Purdue, M.P.; Abnet, C.C.; Chatterjee, N.; Horst, R.L.; Hollis, B.W.; Huang, W.Y.; Shikany, J.M.; et al. Serum vitamin D concentration and prostate cancer risk: A nested case-control study. J. Natl. Cancer Inst. 2008, 100, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.Y.; Yao, Q.; Zhuo, Z.; Ma, Z.; Chen, G. Circulating vitamin D level and mortality in prostate cancer patients: A dose-response meta-analysis. Endocr. Connect. 2018, 7, R294–R303. [Google Scholar] [CrossRef] [PubMed]
- Barnett, C.M.; Beer, T.M. Prostate cancer and vitamin D: What does the evidence really suggest? Urol. Clin. N. Am. 2011, 38, 333–342. [Google Scholar] [CrossRef]
- Krishnan, A.V.; Peehl, D.M.; Feldman, D. Inhibition of prostate cancer growth by vitamin D: Regulation of target gene expression. J. Cell. Biochem. 2003, 88, 363–371. [Google Scholar] [CrossRef]
- Shiota, M.; Fujimoto, N.; Kashiwagi, E.; Eto, M. The Role of Nuclear Receptors in Prostate Cancer. Cells 2019, 8, 602. [Google Scholar] [CrossRef]
- Smith, K.W.; Thompson, P.D.; Rodriguez, E.P.; Mackay, L.; Cobice, D.F. Effects of vitamin D as a regulator of androgen intracrinology in LNCAP prostate cancer cells. Biochem. Biophys. Res. Commun. 2019, 519, 579–584. [Google Scholar] [CrossRef]
- Xu, Y.; Shao, X.; Yao, Y.; Xu, L.; Chang, L.; Jiang, Z.; Lin, Z. Positive association between circulating 25-hydroxyvitamin D levels and prostate cancer risk: New findings from an updated meta-analysis. J. Cancer Res. Clin. Oncol. 2014, 140, 1465–1477. [Google Scholar] [CrossRef]
- Nair-Shalliker, V.; Smith, D.P.; Clements, M.; Naganathan, V.; Litchfield, M.; Waite, L.; Handelsman, D.; Seibel, M.J.; Cumming, R.; Armstrong, B.K. The relationship between solar UV exposure, serum vitamin D levels and serum prostate-specific antigen levels, in men from New South Wales, Australia: The CHAMP study. World J. Urol. 2014, 32, 1251–1257. [Google Scholar] [CrossRef] [PubMed]
- Marshall, D.T.; Savage, S.J.; Garrett-Mayer, E.; Keane, T.E.; Hollis, B.W.; Horst, R.L.; Ambrose, L.H.; Kindy, M.S.; Gattoni-Celli, S. Vitamin D3 supplementation at 4000 international units per day for one year results in a decrease of positive cores at repeat biopsy in subjects with low-risk prostate cancer under active surveillance. J. Clin. Endocrinol. Metab. 2012, 97, 2315–2324. [Google Scholar] [CrossRef] [PubMed]
- Olumi, A.F. Commentary on “randomized clinical trial of vitamin D3 doses on prostatic vitamin D metabolite levels and Ki67 labeling in prostate cancer patients.” Wagner, D.; Trudel, D.; Van der Kwast, T.; Nonn, L.; Giangreco, A.A.; Li, D.; Dias, A.; Cardoza, M.; Laszlo, S.; Hersey, K.; Klotz, L.; Finelli, A.; Fleshner, N.; Vieth, R. Department of Nutritional Sciences, University of Toronto, Ontario, Canada. J. Clin. Endocrinol. Metab. 2013, 98, 1498–1507 [Epub 2013 Mar 5]. Urol. Oncol. 2014, 32, 210. [Google Scholar] [CrossRef]
- Lepor, H.; Wang, B.; Shapiro, E. Relationship between prostatic epithelial volume and serum prostate-specific antigen levels. Urology 1994, 44, 199–205. [Google Scholar] [CrossRef]
- Gao, J.; Wei, W.; Wang, G.; Zhou, H.; Fu, Y.; Liu, N. Circulating vitamin D concentration and risk of prostate cancer: A dose-response meta-analysis of prospective studies. Ther. Clin. Risk Manag. 2018, 14, 95–104. [Google Scholar] [CrossRef]
- Hansen, L.; Tjønneland, A.; Køster, B.; Brot, C.; Andersen, R.; Cohen, A.S.; Frederiksen, K.; Olsen, A. Vitamin D Status and Seasonal Variation among Danish Children and Adults: A Descriptive Study. Nutrients 2018, 10, 1801. [Google Scholar] [CrossRef]
- Wong, M.C.S.; Goggins, W.B.; Wang, H.H.X.; Fung, F.D.; Leung, C.; Wong, S.Y.; Ng, C.F.; Sung, J.J. Global Incidence and Mortality for Prostate Cancer: Analysis of Temporal Patterns and Trends in 36 Countries. Eur. Urol. 2016, 70, 862–874. [Google Scholar] [CrossRef]
- Stroomberg, H.V.; Vojdeman, F.J.; Madsen, C.M.; Helgstrand, J.T.; Schwarz, P.; Heegaard, A.M.; Olsen, A.; Tjønneland, A.; Struer Lind, B.; Brasso, K.; et al. Vitamin D levels and the risk of prostate cancer and prostate cancer mortality. Acta Oncol. Stockh. Swed. 2021, 60, 316–322. [Google Scholar] [CrossRef]
- Shahvazi, S.; Soltani, S.; Ahmadi, S.M.; de Souza, R.J.; Salehi-Abargouei, A. The Effect of Vitamin D Supplementation on Prostate Cancer: A Systematic Review and Meta-Analysis of Clinical Trials. Horm. Metab. Res. 2019, 51, 11–21. [Google Scholar] [CrossRef]
- Ramakrishnan, S.; Steck, S.E.; Arab, L.; Zhang, H.; Bensen, J.T.; Fontham, E.T.H.; Johnson, C.S.; Mohler, J.L.; Smith, G.J.; Su, L.J.; et al. Association among plasma 1,25(OH)2 D, ratio of 1,25(OH)2 D to 25(OH)D, and prostate cancer aggressiveness. Prostate 2019, 79, 1117–1124. [Google Scholar] [CrossRef]
- Tice, J.A.; Halalau, A.; Burke, H. Vitamin D Does Not Prevent Cancer or Cardiovascular Disease: The VITAL Trial: Manson, J.E.; Cook, N.R.; Lee, I.M.; Christen, W.; Bassuk, S.S.; Mora, S.; Gibson, H.; Gordon, D.; Copeland, T.; D’Agostino, D.; Friedenberg, G.; Ridge, C.; Bubes, V.; Giovannucci, E.L.; Willett, W.C.; Buring, J.E. Vitamin D Supplements and Prevention of Cancer and Cardiovascular Disease. N. Engl. J. Med. 2019, 380, 33–44. [Google Scholar] [CrossRef]
- Grant, W.B. Review of Recent Advances in Understanding the Role of Vitamin D in Reducing Cancer Risk: Breast, Colorectal, Prostate, and Overall Cancer. Anticancer Res. 2020, 40, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Hollis, B.W.; Marshall, D.T.; Savage, S.J.; Garrett-Mayer, E.; Kindy, M.S.; Gattoni-Celli, S. Vitamin D3 supplementation, low-risk prostate cancer, and health disparities. J. Steroid. Biochem. Mol. Biol. 2013, 136, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Tóth, Z.; Szalay, B.; Gyarmati, B.; Jalal, D.A.; Vásárhelyi, B.; Szabó, T. Vitamin D Deficiency has no Impact on PSA Reference Ranges in a General University Hospital A Retrospective Analysis. EJIFCC 2020, 31, 225–230. [Google Scholar] [PubMed]
- Kasiappan, R.; Shen, Z.; Tse, A.K.W.; Jinwal, U.; Tang, J.; Lungchukiet, P.; Sun, Y.; Kruk, P.; Nicosia, S.V.; Zhang, X.; et al. 1,25-Dihydroxyvitamin D3 suppresses telomerase expression and human cancer growth through microRNA-498. J. Biol. Chem. 2012, 287, 41297–41309. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, R.; Martin, R.M.; Beynon, R.; Harris, R.; Savovic, J.; Zuccolo, L.; Bekkering, G.E.; Fraser, W.D.; Sterne, J.A.; Metcalfem, C. Associations of circulating and dietary vitamin D with prostate cancer risk: A systematic review and dose-response meta-analysis. Cancer Causes Control 2011, 22, 319–340. [Google Scholar] [CrossRef] [PubMed]
- Rabahn, D.M.; Farhat, K.; Al-Atawi, M.A.; Arafa, M.A. Age-Specific Reference Ranges of Prostate-Specific Antigen among Saudi Men as a Representation of the Arab Population. Med. Princ. Pract. 2019, 28, 242–246. [Google Scholar] [CrossRef]
- Reza, H.S.; Ali, Z.; Tara, H.; Ali, B. Age-specific reference ranges of prostate-specific antigen in the elderly of Amirkola: A population-based study. Asian J. Urol. 2021, 8, 183–188. [Google Scholar] [CrossRef]
- Thompson, I.M.; Pauler, D.K.; Goodman, P.J.; Tangen, C.M.; Lucia, M.S.; Parnes, H.L.; Minasian, L.M.; Ford, L.G.; Lippman, S.M.; Crawford, E.D.; et al. Prevalence of prostate cancer among men with a prostate-specific antigen level < or =4.0 ng per milliliter. N. Engl. J. Med. 2004, 350, 2239–2246, Erratum in: N. Engl. J. Med. 2004, 351, 1470. [Google Scholar] [CrossRef]
- Gentile, F.; La Civita, E.; Della Ventura, B.; Ferro, M.; Cennamo, M.; Bruzzese, D.; Crocetto, F.; Velotta, R.; Terracciano, D. A Combinatorial Neural Network Analysis Reveals a Synergistic Behaviour of Multiparametric Magnetic Resonance and Prostate Health Index in the Identification of Clinically Significant Prostate Cancer. Clin. Genitourin. Cancer 2022, 20, e406–e410. [Google Scholar] [CrossRef]
- Massanova, M.; Robertson, S.; Barone, B.; Dutto, L.; Caputo, V.F.; Bhatt, J.R.; Ahmad, I.; Bada, M.; Obeidallah, A.; Crocetto, F. The Comparison of Imaging and Clinical Methods to Estimate Prostate Volume: A Single-Centre Retrospective Study. Urol. Int. 2021, 105, 804–810. [Google Scholar] [CrossRef] [PubMed]

| Study Participants’ Characteristics | Mean | Standard Deviation | Median | Mode |
|---|---|---|---|---|
| Age | 61.14 | 5.66 | 61 | 53 |
| Height (cm) | 174 | 5.26 | 173 | 178 |
| Weight (kg) | 84.92 | 13.15 | 84 | 90 |
| Body Mass Index | 27.97 | 3.53 | 28.03 | 24.22 |
| PSA (ng/mL) | 5.24 | 14.99 | 2.14 | 0.35 |
| Vitamin D (ng/mL) | 20.69 | 11.12 | 18.45 | 8.29 |
| Count | Percentage | |||
| Taking drugs | 29 | 23 | ||
| Refer a disease | 26 | 20.6 | ||
| Hypertension | 18 | 14.3 | ||
| Diabetes | 6 | 4.8 | ||
| Familiar history for prostate cancer | 5 | 4 | ||
| Smokers | 7 | 5.6 | ||
| Alcohol | 23 | 18.3 | ||
| Sedentary lifestyle | 29 | 23 | ||
| Physical activity | 24 | 19 | ||
| Altered PSA (>4 ng/mL) | 50 | 39.7 | ||
| Vitamin D deficiency (<20.9 ng/mL) | 78 | 61.9 | ||
| Vitamin D insufficiency (>21 ng/ml but <30 ng/mL) | 31 | 24.6 | ||
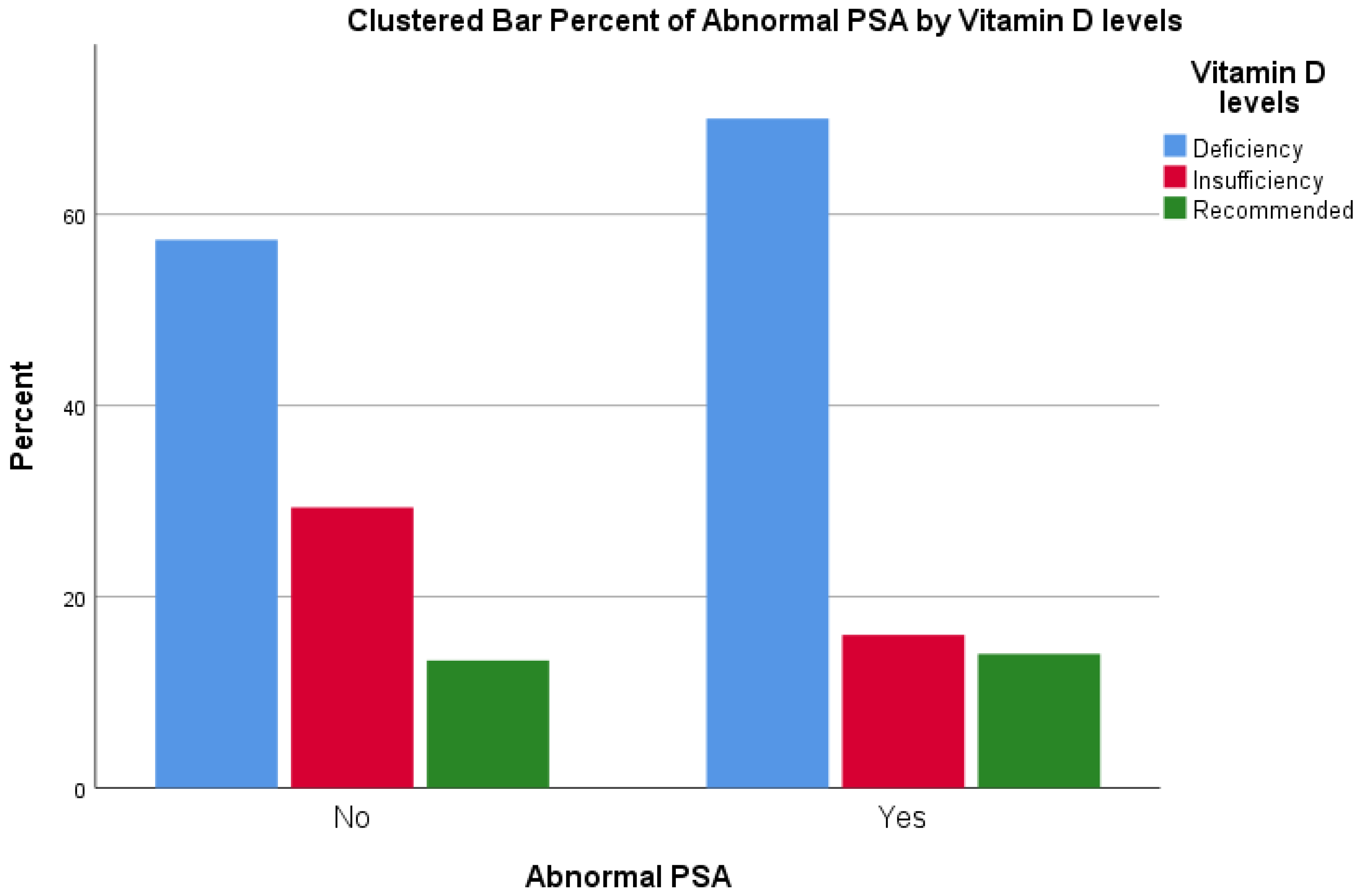
| Vitamin D Levels | Total | |||||
|---|---|---|---|---|---|---|
| Deficiency | Insufficiency | Recommended | ||||
| Abnormal PSA | No | Count | 43 | 22 | 10 | 75 |
| % within Abnormal PSA | 57.3% | 29.3% | 13.3% | 100.0% | ||
| % within Vitamin D levels | 55.1% | 73.3% | 58.8% | 60.0% | ||
| % of Total | 34.4% | 17.6% | 8.0% | 60.0% | ||
| Yes | Count | 35 | 8 | 7 | 50 | |
| % within Abnormal PSA | 70.0% | 16.0% | 14.0% | 100.0% | ||
| % within Vitamin D levels | 44.9% | 26.7% | 41.2% | 40.0% | ||
| % of Total | 28.0% | 6.4% | 5.6% | 40.0% | ||
| Total | Count | 78 | 30 | 17 | 125 | |
| % within Abnormal PSA | 62.4% | 24.0% | 13.6% | 100.0% | ||
| % within Vitamin D levels | 100.0% | 100.0% | 100.0% | 100.0% | ||
| % of Total | 62.4% | 24.0% | 13.6% | 100.0% | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crocetto, F.; Barone, B.; D’Aguanno, G.; Falcone, A.; de Vivo, R.; Rienzo, M.; Recchia, L.; Di Zazzo, E. Vitamin D, a Regulator of Androgen Levels, Is Not Correlated to PSA Serum Levels in a Cohort of the Middle Italy Region Participating to a Prostate Cancer Screening Campaign. J. Clin. Med. 2023, 12, 1831. https://doi.org/10.3390/jcm12051831
Crocetto F, Barone B, D’Aguanno G, Falcone A, de Vivo R, Rienzo M, Recchia L, Di Zazzo E. Vitamin D, a Regulator of Androgen Levels, Is Not Correlated to PSA Serum Levels in a Cohort of the Middle Italy Region Participating to a Prostate Cancer Screening Campaign. Journal of Clinical Medicine. 2023; 12(5):1831. https://doi.org/10.3390/jcm12051831
Chicago/Turabian StyleCrocetto, Felice, Biagio Barone, Giulio D’Aguanno, Alfonso Falcone, Rosamaria de Vivo, Monica Rienzo, Laura Recchia, and Erika Di Zazzo. 2023. "Vitamin D, a Regulator of Androgen Levels, Is Not Correlated to PSA Serum Levels in a Cohort of the Middle Italy Region Participating to a Prostate Cancer Screening Campaign" Journal of Clinical Medicine 12, no. 5: 1831. https://doi.org/10.3390/jcm12051831
APA StyleCrocetto, F., Barone, B., D’Aguanno, G., Falcone, A., de Vivo, R., Rienzo, M., Recchia, L., & Di Zazzo, E. (2023). Vitamin D, a Regulator of Androgen Levels, Is Not Correlated to PSA Serum Levels in a Cohort of the Middle Italy Region Participating to a Prostate Cancer Screening Campaign. Journal of Clinical Medicine, 12(5), 1831. https://doi.org/10.3390/jcm12051831










