Point-of-Care Ultrasonography in a Pulmonary Hypertension Clinic: A Randomized Pilot Study
Abstract
1. Introduction
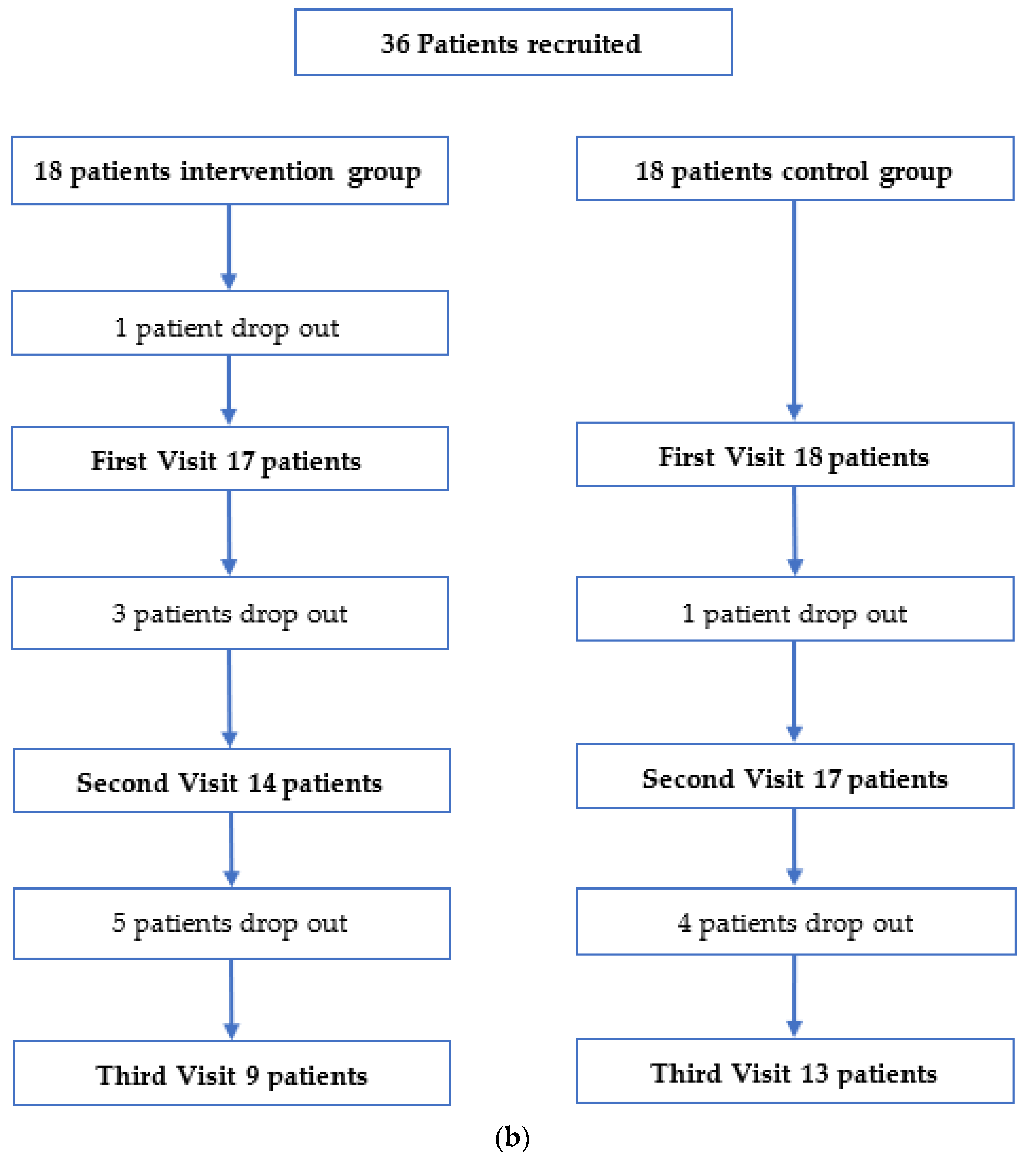
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. POCUS Examination Protocol
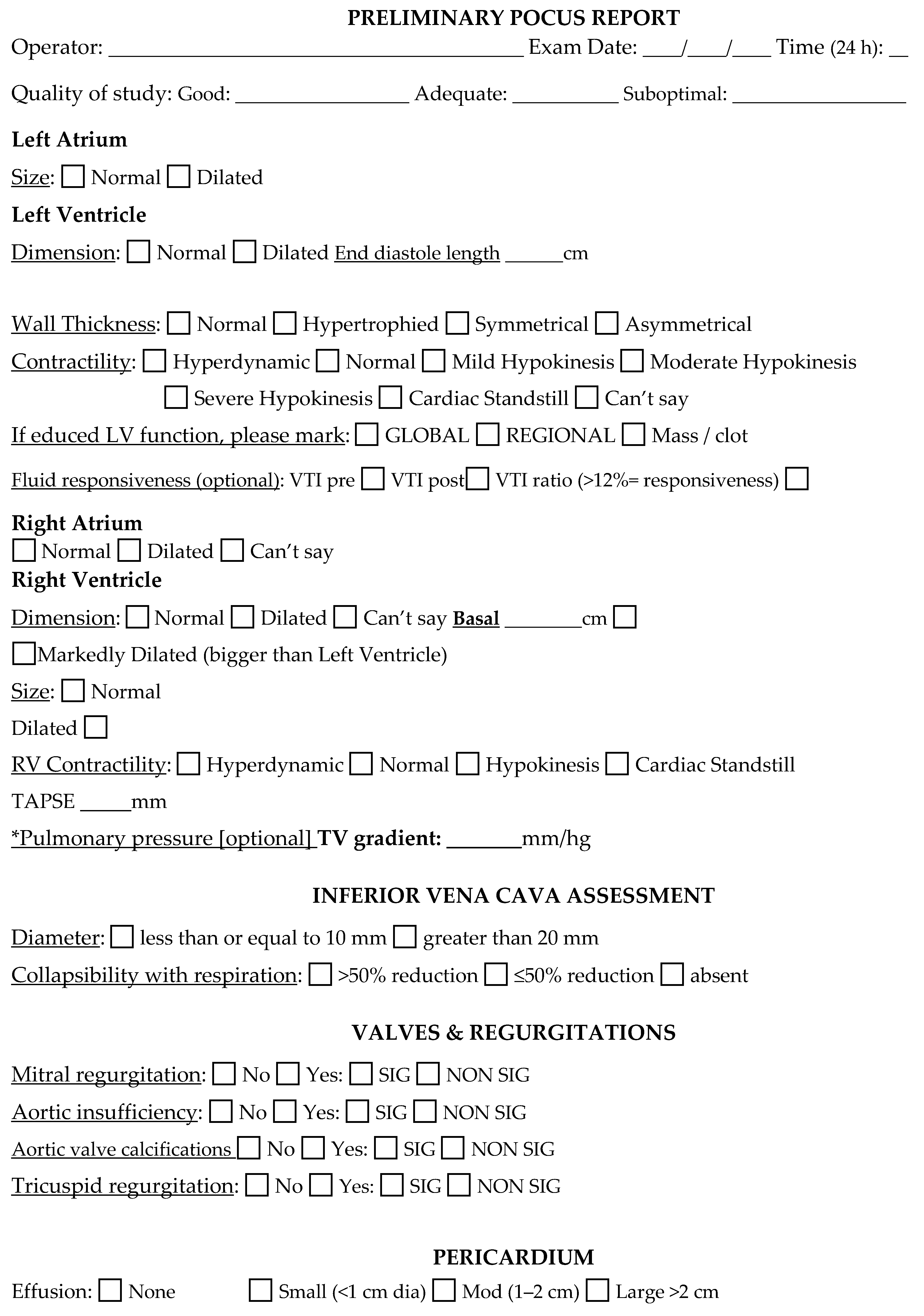

Appendix B. Case Report Form



Appendix C. Other Secondary Outcomes
| NYHA (Mean ± SD) [median] | POCUS | Control | p-Value |
| Visit 1 | 2.4 (±0.6) [2] | 2.8 (±0.4) [3] | 0.05 |
| Visit 2 | 2.1 (±0.6) [2] | 2.8 (±0.5) [3] | 0.012 |
| Visit 3 | 2.4 (±0.7) [3] | 2.9 (±0.3) [3] | 0.05 |
| 6 MWT (meter) (Mean ± SD) [median] | POCUS | Control | p-Value |
| Visit 1 | 396 (±134) [423] | 334 (±98) [333] | 0.084 |
| Visit 2 | 373 (±171) [433] | 373 (±95) [361] | 0.184 |
| Visit 3 | 334 (±165) [371] | 353 (±77) [364] | 0.8 |
| BNP (Mean ± SD) [median] | POCUS | Control | p-Value |
| Visit 1 | 757.9 (±1420) [141] | 690 (±979) [285] | 0.495 |
| Visit 2 | 734.5 (±1373) [158] | 517 (±1049) [147] | 0.817 |
| Visit 3 | 519 (±873) [257] | 730 (±1180) [273] | 0.849 |
| emPHasis-10 (Mean ± SD) [median] | POCUS | Control | p-Value |
| Visit 1 | 20.8 (±12.8) [24] | 25.5 (±11) [23] | 0.248 |
| Visit 2 | 23.9 (±13.4) [28] | 23.7 (±8.4) [24] | 0.878 |
| Visit 3 | 30.3 (±15) [34] | 24.1 (±10) [24.5] | 0.227 |
| Events of worsening | POCUS | Control | p-Value |
| Visit 1 | - | - | - |
| Visit 2 | 2 (14.3%) | 4 (23.5%) | 0.664 |
| Visit 3 | 1 (11.1%) | 2 (16.7%) | 1 |
| Prostaglandin initiation n (%) | POCUS | Control | p-Value |
| Visit 1 | 1 (5.9%) | 0 | 0.486 |
| Visit 2 | 0 | 0 | - |
| Visit 3 | 0 | 0 | - |
| Transplantation referral n (%) | POCUS | Control | p-Value |
| Visit 1 | 1 (5.9%) | 1 (5.6%) | 1 |
| Visit 2 | 0 | 1 (5.9%) | 1 |
| Visit 3 | 1 (11.1%) | 0 | 0.429 |
| Add-on PH medication n (%) | POCUS | Control | p-Value |
| Visit 1 | 5 (29.4%) | 6 (33.3%) | 1 |
| Visit 2 | 5 (35.7%) | 5 (29.4%) | 1 |
| Visit 3 | 4 (44.4%) | 3 (25%) | 0.397 |
| Medication switch n (%) | POCUS | Control | p-Value |
| Visit 1 | 2 (11.8%) | 0 | 0.229 |
| Visit 2 | 0 | 0 | - |
| Visit 3 | 0 | 1 (8.3%) | 1 |
| Management change n (%) | POCUS | Control | p-Value |
| Visit 1 | 11 (64.7%) | 5 (27.8%) | 0.044 |
| Visit 2 | 8 (57.1%) | 6 (35.3%) | 0.289 |
| Visit 3 | 9 (100%) | 5 (41.7%) | 0.003 |
| NYHA change n (%) visit 2 | POCUS, n = 14 (%) | Control, n = 17 (%) | p-Value |
| increased | 1 (7.1%) | 4 (23.5%) | 0.428 |
| decreased | 5 (35.7%) | 4 (23.5%) | |
| NYHA change n (%) visit 3 | POCUS, n = 9 (%) | Control, n = 13 (%) | p-Value |
| increased | 2 (22.2%) | 4 (36.4%) | 0.79 |
| decreased | 1 (11.1%) | 1 (9.1%) |
References
- Galie, N.; McLaughlin, V.V.; Rubin, L.J.; Simonneau, G. An overview of the 6th World Symposium on Pulmonary Hypertension. Eur. Respir. J. 2019, 53, 1802148. [Google Scholar] [CrossRef]
- McLaughlin, V.V.; Archer, S.L.; Badesch, D.B.; Barst, R.J.; Farber, H.W.; Lindner, J.R.; Mathier, M.A.; McGoon, M.D.; Park, M.H.; Rosenson, R.S.; et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension: A report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association: Developed in collaboration with the American College of Chest Physicians, American Thoracic Society, Inc., and the Pulmonary Hypertension Association. Circulation 2009, 119, 2250–2294. [Google Scholar]
- Galie, N.; Humbert, M.; Vachiery, J.L.; Gibbs, S.; Lang, I.; Torbicki, A.; Simonneau, G.; Peacock, A.; Noordegraaf, A.V.; Beghetti, M.; et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur. Heart J. 2016, 37, 67–119. [Google Scholar]
- D'Alto, M.; Romeo, E.; Argiento, P.; Di Salvo, G.; Badagliacca, R.; Cirillo, A.P.; Kaemmerer, H.; Bossone, E.; Naeije, R. Pulmonary arterial hypertension: The key role of echocardiography. Echocardiography 2015, 32 (Suppl. 1), S23–S37. [Google Scholar] [CrossRef]
- Blumberg, F.C.; Arzt, M.; Lange, T.; Schroll, S.; Pfeifer, M.; Wensel, R. Impact of right ventricular reserve on exercise capacity and survival in patients with pulmonary hypertension. Eur. J. Heart Fail. 2013, 15, 771–775. [Google Scholar] [CrossRef]
- Hoeper, M.M.; Granton, J. Intensive care unit management of patients with severe pulmonary hypertension and right heart failure. Am. J. Respir. Crit. Care Med. 2011, 184, 1114–1124. [Google Scholar] [CrossRef]
- Grignola, J.C.; Gines, F.; Bia, D.; Armentano, R. Improved right ventricular-vascular coupling during active pulmonary hypertension. Int. J. Cardiol. 2007, 115, 171–182. [Google Scholar] [CrossRef]
- Moore, C.L.; Copel, J.A. Point-of-care ultrasonography. N. Engl. J. Med. 2011, 364, 749–757. [Google Scholar] [CrossRef]
- Kobal, S.L.; Atar, S.; Siegel, R.J. Hand-carried ultrasound improves the bedside cardiovascular examination. Chest 2004, 126, 693–701. [Google Scholar] [CrossRef]
- Labovitz, A.J.; Noble, V.E.; Bierig, M.; Goldstein, S.A.; Jones, R.; Kort, S.; Porter, T.R.; Spencer, K.T.; Tayal, V.S.; Wei, K. Focused cardiac ultrasound in the emergent setting: A consensus statement of the American Society of Echocardiography and American College of Emergency Physicians. J. Am. Soc. Echocardiogr. 2010, 23, 1225–1230. [Google Scholar] [CrossRef]
- Ben-Baruch Golan, Y.; Sadeh, R.; Mizrakli, Y.; Shafat, T.; Sagy, I.; Slutsky, T.; Kobal, S.; Novack, V.; Fuchs, L. Early Point-of-Care Ultrasound Assessment for Medical Patients Reduces Time to Appropriate Treatment: A Pilot Randomized Controlled Trial. Ultrasound Med. Biol. 2020, 46, 1908–1915. [Google Scholar] [CrossRef]
- Koh, Y.; Chua, M.T.; Ho, W.H.; Lee, C.; Chan, G.W.H.; Kuan, W.S. Assessment of dyspneic patients in the emergency department using point-of-care lung and cardiac ultrasonography—A prospective observational study. J. Thorac. Dis. 2018, 10, 6221. [Google Scholar] [CrossRef]
- Braganza, M.; Shaw, J.; Solverson, K.; Vis, D.; Janovcik, J.; Varughese, R.A.; Thakrar, M.V.; Hirani, N.; Helmersen, D.; Weatherald, J. A Prospective Evaluation of the Diagnostic Accuracy of the Physical Examination for Pulmonary Hypertension. Chest 2019, 155, 982–990. [Google Scholar] [CrossRef]
- McLaughlin, V.V.; Shah, S.J.; Souza, R.; Humbert, M. Management of pulmonary arterial hypertension. J. Am. Coll. Cardiol. 2015, 65, 1976–1997. [Google Scholar] [CrossRef]
- Delcroix, M.; Howard, L. Pulmonary arterial hypertension: The burden of disease and impact on quality of life. Eur. Respir. Rev. 2015, 24, 621–629. [Google Scholar] [CrossRef]
- Simonneau, G.; Gatzoulis, M.A.; Adatia, I.; Celermajer, D.; Denton, C.; Ghofrani, A.; Sanchez, M.A.G.; Kumar, R.K.; Landzberg, M.; Machado, R.F.; et al. Updated Clinical Classification of Pulmonary Hypertension. J. Am. Coll. Cardiol. 2013, 62 (Suppl. 25), D34–D41. [Google Scholar] [CrossRef]
- Ashton-Cleary, D.T. Is thoracic ultrasound a viable alternative to conventional imaging in the critical care setting? Br. J. Anaesth. 2013, 111, 152–160. [Google Scholar] [CrossRef]
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.F.; Brida, M.; Carlsen, J.; Coats, A.J.S.; Escribano-Subias, P.; Ferrari, P.; et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: Developed by the task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS). Endorsed by the International Society for Heart and Lung Transplantation (ISHLT) and the European Reference Network on rare respiratory diseases (ERN-LUNG). Eur. Heart J. 2022, 43, 3618–3731. [Google Scholar]
- Howard, L.S.; Grapsa, J.; Dawson, D.; Bellamy, M.; Chambers, J.B.; Masani, N.D.; Nihoyannopoulos, P.; Gibbs, J.S.R. Echocardiographic assessment of pulmonary hypertension: Standard operating procedure. Eur. Respir. Rev. 2012, 21, 239–248. [Google Scholar] [CrossRef]
- Heldeweg, M.L.A.; Matta, J.E.L.; Pisani, L.M.; Slot, S.M.; Haaksma, M.E.; Smit, J.M.; Mousa, A.M.; Magnesa, G.; Massaro, F.; Touw, H.R.W.M.; et al. The Impact of Thoracic Ultrasound on Clinical Management of Critically Ill Patients (UltraMan): An International Prospective Observational Study. Crit. Care Med. 2022. [Google Scholar] [CrossRef]
- Mathai, S.C.; Puhan, M.A.; Lam, D.; Wise, R.A. The minimal important difference in the 6-minute walk test for patients with pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2012, 186, 428–433. [Google Scholar] [CrossRef]
- Mazurek, J.A.; Vaidya, A.; Mathai, S.C.; Roberts, J.D.; Forfia, P.R. Follow-up tricuspid annular plane systolic excursion predicts survival in pulmonary arterial hypertension. Pulm. Circ. 2017, 7, 361–371. [Google Scholar] [CrossRef]
- Solomon, S.D.; Saldana, F. Point-of-Care Ultrasound in Medical Education—Stop Listening and Look. N. Engl. J. Med. 2014, 370, 1083–1085. [Google Scholar] [CrossRef]
- Lait, D.; Friedman, Z.; Mizrakli, Y.; Fuchs, L. The effect of point of care ultrasonography before urgent orthopedic surgery on anesthetic management—A prospective, pilot study. J. Clin. Anesth. 2020, 66, 109902. [Google Scholar] [CrossRef]
- Lichter, Y.; Topilsky, Y.; Taieb, P.; Banai, A.; Hochstadt, A.; Merdler, I.; Oz, A.G.; Vine, J.; Goren, O.; Cohen, B.; et al. Lung ultrasound predicts clinical course and outcomes in COVID-19 patients. Intensive Care Med. 2020, 46, 1873–1883. [Google Scholar] [CrossRef]
- Gundersen, G.H.; Norekval, T.M.; Haug, H.H.; Skjetne, K.; Kleinau, J.O.; Graven, T.; Dalen, H. Adding point of care ultrasound to assess volume status in heart failure patients in a nurse-led outpatient clinic. A randomised study. Heart 2016, 102, 29–34. [Google Scholar] [CrossRef]
- Hamill, C.; Ellis, P.K.; Johnston, P.C. Point of Care Thyroid Ultrasound (POCUS) in Endocrine Outpatients: A Pilot Study. Ulster Med. J. 2020, 89, 21–24. [Google Scholar]
- Niblock, F.; Byun, H.; Jabbarpour, Y. Point-of-Care Ultrasound Use by Primary Care Physicians. J. Am. Board Fam. Med. 2021, 34, 859–860. [Google Scholar] [CrossRef]
- Samant, S.; Tran, H.V.; Uddo, R.B.; Deboisblanc, B.P.; Saketkoo, L.A.; Saito, S.; Helmcke, F.R.; Lammi, M.R. Use of Handheld Ultrasound to Estimate Right Atrial Pressure in a Pulmonary Hypertension Clinic. Ann. Am. Thorac. Soc. 2022, 19, 179–185. [Google Scholar] [CrossRef]
- Vieillard-Baron, A.; Millington, S.J.; Sanfilippo, F.; Chew, M.; Diaz-Gomez, J.; McLean, A.; Pinsky, M.R.; Pulido, J.; Mayo, P.; Fletcher, N. A decade of progress in critical care echocardiography: A narrative review. Intensiv. Care Med. 2019, 45, 770–788. [Google Scholar] [CrossRef]
- Tuvali, O.; Sadeh, R.; Kobal, S.; Yarza, S.; Golan, Y.; Fuchs, L. The long-term effect of short point of care ultrasound course on physicians’ daily practice. PLOS ONE 2020, 15, e0242084. [Google Scholar] [CrossRef]
- Clay, R.D.; Lee, E.C.; Kurtzman, M.F.; Dversdal, R.K. Teaching the internist to see: Effectiveness of a 1-day workshop in bedside ultrasound for internal medicine residents. Crit. Ultrasound J. 2016, 8, 11. [Google Scholar] [CrossRef]
- Hoppmann, R.A.; Mladenovic, J.; Melniker, L.; Badea, R.; Blaivas, M.; Montorfano, M.; Abuhamad, A.; Noble, V.; Hussain, A.; Prosen, G.; et al. International consensus conference recommendations on ultrasound education for undergraduate medical students. Ultrasound J. 2022, 14, 31. [Google Scholar] [CrossRef]
- Lopresti, C.M.; Schnobrich, D.J.; Dversdal, R.K.; Schembri, F. A road map for point-of-care ultrasound training in internal medicine residency. Ultrasound J. 2019, 11, 10. [Google Scholar] [CrossRef]
- Zanobetti, M.; Scorpiniti, M.; Gigli, C.; Nazerian, P.; Vanni, S.; Innocenti, F.; Stefanone, V.T.; Savinelli, C.; Coppa, A.; Bigiarini, S.; et al. Point-of-Care Ultrasonography for Evaluation of Acute Dyspnea in the ED. Chest 2017, 151, 1295–1301. [Google Scholar] [CrossRef]

| Variable | POCUS (n = 17) | Control (n = 18) | p-Value | |
|---|---|---|---|---|
| Sex (female) n (%) | 13 (76.5%) | 16 (88.9%) | 0.402 | |
| Age (years) mean ± SD | 65 ± 14 | 65 ± 16 | 0.934 | |
| BMI (weight) mean ± SD | 29 (±6.3) | 28.7 ± 10.9 | 0.666 | |
| Duration of disease (month) median (IQR) | 33 (8, 39) | 21 (5, 30) | 0.105 | |
| Left ventricular dysfunction n (%) | 2 (11.8%) | 0 | 0.229 | |
| Lung disease n (%) | 2 (11.8%) | 0 | 0.402 | |
| Chronic kidney disease n (%) | 1 (5.9%) | 1 (5.6%) | 1 | |
| Chronic liver disease n (%) | 1 (5.9%) | 0 | 0.486 | |
| Malignancy n (%) PAH Diagnosis: | 1 (5.9%) | 1 (5.6%) | 1 | |
| Connective tissue disease | 2 (11.8%) | 7 (38.9%) | 0.121 | |
| IPAH | 14 (82.4%) | 9 (50%) | 0.044 | |
| FPAH | 0 | 2 (11.1%) | 0.486 | |
| Drug and toxins exposure | 1 (5.9%) | 0 | 0.486 | |
| HIV | 0 | 0 | NA | |
| PH medications | No | 2 (11.8%) | 3 (16.7%) | 0.194 |
| Yes | 7 (41.2%) | 11 (61.1%) | 0.862 | |
| >1 PH drug | 8 (47.1%) | 4 (22.2%) | 0.862 | |
| Diuretics | 8 (47.1%) | 9 (50%) | 0.862 | |
| NYHA Class | 1 | 1(5.9%) | 0 | 0.05 |
| 2 | 8 (47.1%) | 4 (22.2%) | 0.05 | |
| 3 | 8 (47.1%) | 14 (77.8%) | 0.05 | |
| Symptoms on enrolment | None | 6 (35.3%) | 5 (27.8%) | 0.948 |
| Orthopnea | 1 (5.9%) | 0 | 0.948 | |
| Chest pain | 3 (17.6%) | 0 | 0.948 | |
| Palpitations | 0 | 0 | 0.948 | |
| Syncope | 0 | 0 | 0.948 | |
| Edema | 1 (5.9%) | 2 (11.1%) | 0.948 | |
| Increased abdominal girth | 0 | 0 | 0.948 | |
| Constitutional symptoms | 0 | 0 | 0.948 | |
| Dyspnea | 6 (35.3%) | 11 (61.1%) | 0.948 | |
| Right heart failure | 5 (31.3%) | 7 (38.9%) | 0.642 | |
| BNP mean ± SD | 758 ± 1420.3 | 847 ± 1064.3 | 0.369 | |
| 6 Min walk test mean ± SD | 374 ± 166 | 334 ± 98 | 0.179 | |
| EmPHasis-10 evaluation median (IQR) | 19 (8, 26) | 26 (18, 33) | 0.143 | |
| Variable | POCUS | Control | p Value |
|---|---|---|---|
| Total PH outpatient visits | 43 | 49 | 1 |
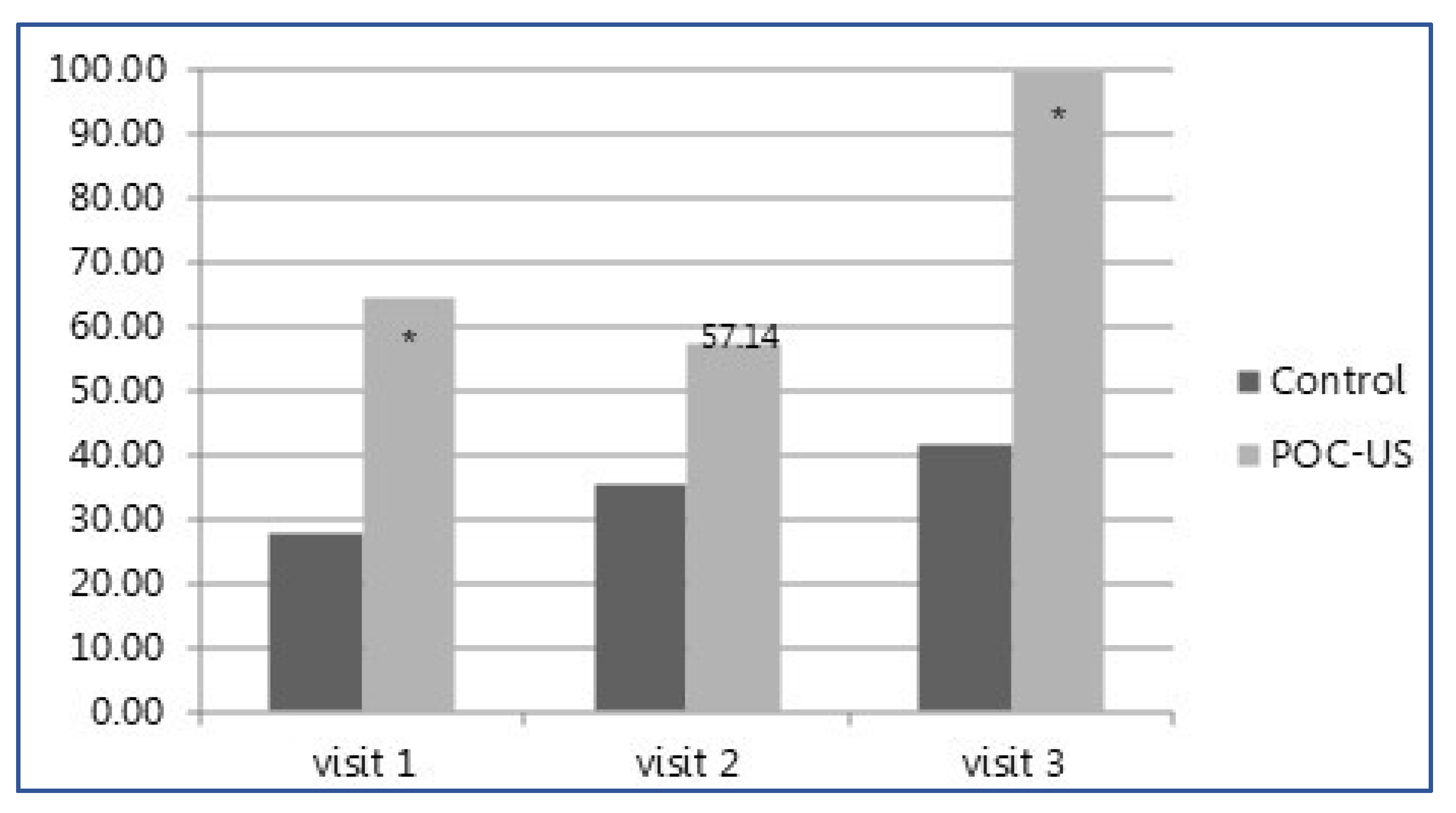
| Visits with management changes (%) | 32 (74.4%) | 17 (34.7%) | <0.001 |
| Total management changes | 48 | 18 | <0.001 |
| Average management changes per visit | 1.2 | 0.37 | <0.001 |
| Model | Odds Ratio | Confidence Interval 95% | p-Value | |
|---|---|---|---|---|
| Lower | Upper | |||
| Model 1 | ||||
| Age | 0.99 | 0.96 | 1.02 | 0.51 |
| Sex (male) | 1.7 | 0.45 | 6.3 | 0.43 |
| POCUS group | 6.02 | 2.3 | 15.9 | <0.001 |
| Model 2 | ||||
| Age | 0.99 | 0.96 | 1.02 | 0.47 |
| Sex (male) | 1.8 | 0.45 | 7.03 | 0.40 |
| Symptoms | 1.12 | 0.96 | 1.30 | 0.14 |
| BMI | 0.99 | 0.94 | 1.04 | 0.99 |
| POCUS group | 8.7 | 2.92 | 25.97 | <0.001 |
| Model 3 | ||||
| Age | 0.98 | 0.95 | 1.01 | 0.23 |
| Sex (male) | 2.34 | 0.56 | 9.89 | 0.24 |
| Symptoms | 1.17 | 0.96 | 1.30 | 0.16 |
| BMI | 0.97 | 0.92 | 1.03 | 0.42 |
| Physical examination | 4.66 | 1.34 | 16.22 | 0.01 |
| POCUS group | 11.98 | 3.59 | 40.02 | <0.001 |
| POCUS Findings | Number (%) Total n = 26 |
|---|---|
| Suspected aortic stenosis | 1 (3.8) |
| Right ventricular hypertrophy | 1 (3.8) |
| Left ventricular hypertrophy | 5 (19.3) |
| “Unexpectedly normal” RVSP | 6 (23.2) |
| Pericardial effusion | 1 (3.8) |
| Pulmonary congestion | 4 (15.4) |
| D-shaped septum | 2 (7.7) |
| Arrhythmia | 1 (3.8) |
| Elevated central venous pressure | 3 (11.5) |
| Restrictive diastolic pattern | 2 (7.7) |
| Patient Code/Visit | POCUS Findings | Management Change |
|---|---|---|
| Patient 1/Visit 2 | Calcified aortic valve Suspected aortic stenosis | A new formal TTE study was ordered |
| Patient 10/Visit 1 | Signs of LVH, small pericardial effusion B-lines suggestive of pulmonary congestion | Diuretics were added |
| Patient 12/Visit 3 | B lines suggestive of pulmonary congestion | Diuretics were added A new formal Echo study was ordered |
| Patient 21/Visit 1 | Surprisingly normal POCUS study with low CVP | Diuretics were stopped A new right catheterization was scheduled |
| Patient 22/Visit 1 | Small left ventricle Low CVP | Diuretics dosage was reduced Chest computed tomography study ordered Full pulmonary function test ordered A new right catheterization was scheduled |
| Patient 23/Visit 2 | High CVP Elevated RVSP (58 mm Hg) Restrictive diastolic pattern | A new formal TTE to assess suspected diastolic left ventricular dysfunction was ordered |
| Patient 24/visit 2 | B lines suggestive of lung congestion | Diuretic dosage increased |
| Patient 24/visit 3 | LVH B lines suggestive of lung congestion | A new right catheterization was scheduled |
| Patient 30/visit 1 | Elevated CVP Elevated RVSP (55) D-shaped Septum | A new formal TTE study was ordered |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avriel, A.; Bar Lavie Shay, A.; Hershko Klement, A.; Taylor, J.; Shamia, D.; Tsaban, G.; Abu-Shakra, M.; Granton, J.; Fuchs, L. Point-of-Care Ultrasonography in a Pulmonary Hypertension Clinic: A Randomized Pilot Study. J. Clin. Med. 2023, 12, 1752. https://doi.org/10.3390/jcm12051752
Avriel A, Bar Lavie Shay A, Hershko Klement A, Taylor J, Shamia D, Tsaban G, Abu-Shakra M, Granton J, Fuchs L. Point-of-Care Ultrasonography in a Pulmonary Hypertension Clinic: A Randomized Pilot Study. Journal of Clinical Medicine. 2023; 12(5):1752. https://doi.org/10.3390/jcm12051752
Chicago/Turabian StyleAvriel, Avital, Anat Bar Lavie Shay, Anat Hershko Klement, Jonathan Taylor, David Shamia, Gal Tsaban, Mahmoud Abu-Shakra, John Granton, and Lior Fuchs. 2023. "Point-of-Care Ultrasonography in a Pulmonary Hypertension Clinic: A Randomized Pilot Study" Journal of Clinical Medicine 12, no. 5: 1752. https://doi.org/10.3390/jcm12051752
APA StyleAvriel, A., Bar Lavie Shay, A., Hershko Klement, A., Taylor, J., Shamia, D., Tsaban, G., Abu-Shakra, M., Granton, J., & Fuchs, L. (2023). Point-of-Care Ultrasonography in a Pulmonary Hypertension Clinic: A Randomized Pilot Study. Journal of Clinical Medicine, 12(5), 1752. https://doi.org/10.3390/jcm12051752






