Perioperative Drug Treatment in Pancreatic Surgery—A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Study Selection and Data Extraction
2.3. Critical Appraisal
2.4. Statistical Analysis
3. Results
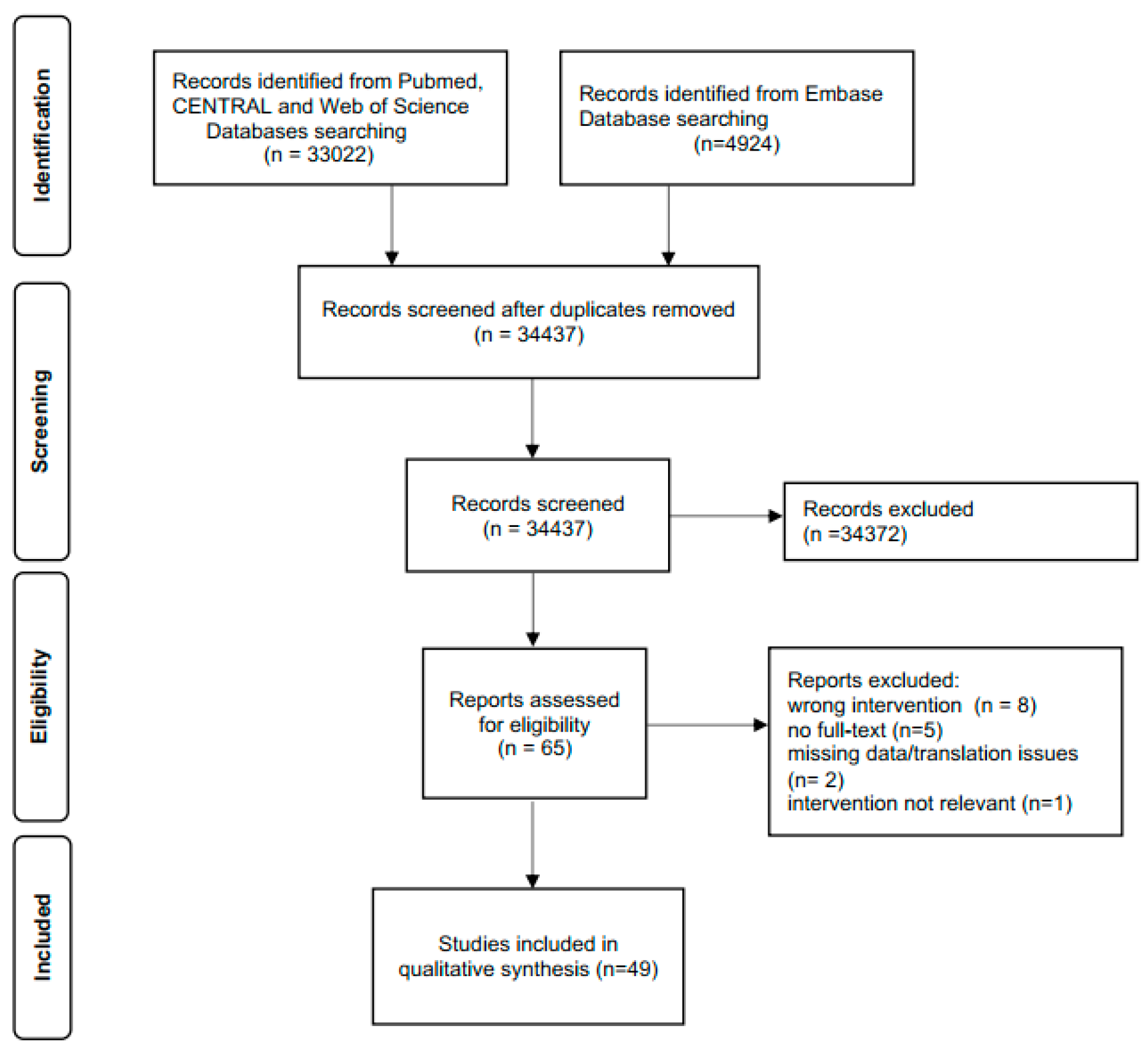
3.1. Literature Search
3.2. Somatostatin Analogues
3.3. Glucocorticoids
3.4. Prokinetics
3.5. Proton Pump Inhibitor
3.6. Antidiabetic Drugs
3.7. Pancreatic Enzyme Replacement Therapy
4. Discussion
4.1. Somatostatin Analogues
4.2. Glucocorticoids
4.3. Erythromycin
4.4. Proton Pump Inhibitor
4.5. Antidiabetic Drugs
4.6. Pancreatic Enzyme Replacement Therapy
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wei, K.; Hackert, T. Surgical Treatment of Pancreatic Ductal Adenocarcinoma. Cancers 2021, 13, 1971. [Google Scholar] [CrossRef]
- Probst, P.; Huttner, F.J.; Meydan, O.; Abu Hilal, M.; Adham, M.; Barreto, S.G.; Besselink, M.G.; Busch, O.R.; Bockhorn, M.; Del Chiaro, M.; et al. Evidence Map of Pancreatic Surgery-A living systematic review with meta-analyses by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 2021, 170, 1517–1524. [Google Scholar] [CrossRef]
- Klaiber, U.; Probst, P.; Strobel, O.; Michalski, C.W.; Dorr-Harim, C.; Diener, M.K.; Buchler, M.W.; Hackert, T. Meta-analysis of delayed gastric emptying after pylorus-preserving versus pylorus-resecting pancreatoduodenectomy. Br. J. Surg. 2018, 105, 339–349. [Google Scholar] [CrossRef]
- Liu, X.; Pausch, T.; Probst, P.; Cui, J.; Wei, J.; Hackert, T.; Miao, Y. Efficacy of Pasireotide for Prevention of Postoperative Pancreatic Fistula in Pancreatic Surgery: A Systematic Review and Meta-analysis. J. Gastrointest. Surg. 2020, 24, 1421–1429. [Google Scholar] [CrossRef]
- Raty, S.; Sand, J.; Lantto, E.; Nordback, I. Postoperative acute pancreatitis as a major determinant of postoperative delayed gastric emptying after pancreaticoduodenectomy. J. Gastrointest. Surg. 2006, 10, 1131–1139. [Google Scholar] [CrossRef]
- Yeo, C.J.; Barry, M.K.; Sauter, P.K.; Sostre, S.; Lillemoe, K.D.; Pitt, H.A.; Cameron, J.L. Erythromycin accelerates gastric emptying after pancreaticoduodenectomy. A prospective, randomized, placebo-controlled trial. Ann. Surg. 1993, 218, 229–237, discussion 237–288. [Google Scholar] [CrossRef]
- Toyota, N.; Takada, T.; Yasuda, H.; Amano, H.; Yoshida, M.; Isaka, T.; Hijikata, H.; Takada, K. The effects of omeprazole, a proton pump inhibitor, on early gastric stagnation after a pylorus-preserving pancreaticoduodenectomy: Results of a randomized study. Hepatogastroenterology 1998, 45, 1005–1010. [Google Scholar]
- Gianotti, L.; Besselink, M.G.; Sandini, M.; Hackert, T.; Conlon, K.; Gerritsen, A.; Griffin, O.; Fingerhut, A.; Probst, P.; Abu Hilal, M.; et al. Nutritional support and therapy in pancreatic surgery: A position paper of the International Study Group on Pancreatic Surgery (ISGPS). Surgery 2018, 164, 1035–1048. [Google Scholar] [CrossRef]
- Jang, J.Y.; Kim, S.W.; Han, J.K.; Park, S.J.; Park, Y.C.; Joon Ahn, Y.; Park, Y.H. Randomized prospective trial of the effect of induced hypergastrinemia on the prevention of pancreatic atrophy after pancreatoduodenectomy in humans. Ann. Surg. 2003, 237, 522–529. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Cumpston, M.; Li, T.; Page, M.J.; Chandler, J.; Welch, V.A.; Higgins, J.P.; Thomas, J. Updated guidance for trusted systematic reviews: A new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst. Rev. 2019, 10, ED000142. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.P.; Altman, D.G.; Gotzsche, P.C.; Juni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [Green Version]
- Guyatt, G.H.; Oxman, A.D.; Schunemann, H.J.; Tugwell, P.; Knottnerus, A. GRADE guidelines: A new series of articles in the Journal of Clinical Epidemiology. J. Clin. Epidemiol. 2011, 64, 380–382. [Google Scholar] [CrossRef]
- Bassi, C.; Marchegiani, G.; Dervenis, C.; Sarr, M.; Abu Hilal, M.; Adham, M.; Allen, P.; Andersson, R.; Asbun, H.J.; Besselink, M.G.; et al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 Years After. Surgery 2017, 161, 584–591. [Google Scholar] [CrossRef] [Green Version]
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005, 5, 13. [Google Scholar] [CrossRef] [Green Version]
- Kalkum, E.; Klotz, R.; Seide, S.; Huttner, F.J.; Kowalewski, K.F.; Nickel, F.; Khajeh, E.; Knebel, P.; Diener, M.K.; Probst, P. Systematic reviews in surgery-recommendations from the Study Center of the German Society of Surgery. Langenbecks Arch. Surg. 2021, 406, 1723–1731. [Google Scholar] [CrossRef]
- Allen, P.J.; Gonen, M.; Brennan, M.F.; Bucknor, A.A.; Robinson, L.M.; Pappas, M.M.; Carlucci, K.E.; D’Angelica, M.I.; DeMatteo, R.P.; Kingham, T.P.; et al. Pasireotide for postoperative pancreatic fistula. N. Engl. J. Med. 2014, 370, 2014–2022. [Google Scholar] [CrossRef] [Green Version]
- Beguiristain, A.; Espi, A.; Balen, E.; Pardo, F.; Hernandez Lizoain, J.L.; Alvarez Cienfuegos, J. [Somatostatin prophylaxis following cephalic duodenopancreatectomy]. Rev. Esp. Enferm. Dig. 1995, 87, 221–224. [Google Scholar]
- Belyaev, O.; Polle, C.; Herzog, T.; Munding, J.; Chromik, A.M.; Meurer, K.; Tannapfel, A.; Bergmann, U.; Muller, C.A.; Uhl, W. Effects of intra-arterial octreotide on pancreatic texture: A randomized controlled trial. Scand. J. Surg. 2013, 102, 164–170. [Google Scholar] [CrossRef]
- Bonora, A.; Bassi, C.; Falconi, M.; Sartori, N.; De Santis, L.; Dragonetti, C.; Innocenti, P.; Pederzoli, P. [Gabexate mesilate vs gabexate mesilate combined with octreotide in the prevention of postoperative complications of pancreatic surgery: Preliminary results]. Chir. Ital. 2001, 53, 65–72. [Google Scholar]
- Buccoliero, F.; Pansini, G.C.; Mascoli, F.; Mari, C.; Donini, A.; Navarra, G. [Somatostatin in duodenocephalopancreatectomy for neoplastic pathology]. Minerva Chir. 1992, 47, 713–716. [Google Scholar]
- Buchler, M.; Friess, H.; Klempa, I.; Hermanek, P.; Sulkowski, U.; Becker, H.; Schafmayer, A.; Baca, I.; Lorenz, D.; Meister, R.; et al. Role of octreotide in the prevention of postoperative complications following pancreatic resection. Am. J. Surg. 1992, 163, 125–130, discussion 130–121. [Google Scholar] [CrossRef]
- Cao, Z.; Qiu, J.; Guo, J.; Xiong, G.; Jiang, K.; Zheng, S.; Kuang, T.; Wang, Y.; Zhang, T.; Sun, B.; et al. A randomised, multicentre trial of somatostatin to prevent clinically relevant postoperative pancreatic fistula in intermediate-risk patients after pancreaticoduodenectomy. J. Gastroenterol. 2021, 56, 938–948. [Google Scholar] [CrossRef]
- Closset, J.; Journe, S.; Mboti, F.; El Nakadi, I.; Gelin, M. Randomized controlled trial comparing somatostatin with octreotide in the prevention of complications after pancreatectomy. Hepatogastroenterology 2008, 55, 1818–1823. [Google Scholar]
- El Nakeeb, A.; ElGawalby, A.; Ali, M.A.; Shehta, A.; Hamed, H.; El Refea, M.; Moneer, A.; Abd El Rafee, A. Efficacy of octreotide in the prevention of complications after pancreaticoduodenectomy in patients with soft pancreas and non-dilated pancreatic duct: A prospective randomized trial. Hepatobiliary Pancreat Dis. Int. 2018, 17, 59–63. [Google Scholar] [CrossRef]
- Falconi, M.; Contro, C.; Ballabio, M.; Bassi, C.; Salvia, R.; Pederzoli, P. Evaluation of lanreotide effects on human exocrine pancreatic secretion after a single dose: Preliminary study. Dig. Liver Dis. 2002, 34, 127–132. [Google Scholar] [CrossRef]
- Fernandez-Cruz, L.; Jimenez Chavarria, E.; Taura, P.; Closa, D.; Boado, M.A.; Ferrer, J. Prospective randomized trial of the effect of octreotide on pancreatic juice output after pancreaticoduodenectomy in relation to histological diagnosis, duct size and leakage. HPB 2013, 15, 392–399. [Google Scholar] [CrossRef] [Green Version]
- Friess, H.; Beger, H.G.; Sulkowski, U.; Becker, H.; Hofbauer, B.; Dennler, H.J.; Buchler, M.W. Randomized controlled multicentre study of the prevention of complications by octreotide in patients undergoing surgery for chronic pancreatitis. Br. J. Surg. 1995, 82, 1270–1273. [Google Scholar] [CrossRef]
- Gouillat, C.; Chipponi, J.; Baulieux, J.; Partensky, C.; Saric, J.; Gayet, B. Randomized controlled multicentre trial of somatostatin infusion after pancreaticoduodenectomy. Br. J. Surg. 2001, 88, 1456–1462. [Google Scholar] [CrossRef]
- Hesse, U.J.; DeDecker, C.; Houtmeyers, P.; Demetter, P.; Ceelen, W.; Pattyn, P.; Troisi, R.; deHemptinne, B. Prospectively randomized trial using perioperative low-dose octreotide to prevent organ-related and general complications after pancreatic surgery and pancreatico-jejunostomy. World J. Surg. 2005, 29, 1325–1328. [Google Scholar] [CrossRef]
- Katsourakis, A.; Oikonomou, L.; Chatzitheoklitos, E.; Noussios, G.; Pitiakoudis, M.; Polychronidis, A.; Simopoulos, K.; Sioga, A. The role of somatostatin in 67 consecutive pancreatectomies: A randomized clinical trial. Clin. Exp. Gastroenterol. 2010, 3, 179–183. [Google Scholar] [CrossRef] [Green Version]
- Klempa, I.; Baca, I.; Menzel, J.; Schuszdiarra, V. [Effect of somatostatin on basal and stimulated exocrine pancreatic secretion after partial duodenopancreatectomy. A clinical experimental study]. Chirurg 1991, 62, 293–299. [Google Scholar]
- Kollmar, O.; Moussavian, M.R.; Richter, S.; de Roi, P.; Maurer, C.A.; Schilling, M.K. Prophylactic octreotide and delayed gastric emptying after pancreaticoduodenectomy: Results of a prospective randomized double-blinded placebo-controlled trial. Eur. J. Surg. Oncol. 2008, 34, 868–875. [Google Scholar] [CrossRef]
- Kurumboor, P.; Palaniswami, K.N.; Pramil, K.; George, D.; Ponnambathayil, S.; Varma, D.; Aikot, S. Octreotide Does Not Prevent Pancreatic Fistula Following Pancreatoduodenectomy in Patients with Soft Pancreas and Non-dilated Duct: A Prospective Randomized Controlled Trial. J. Gastrointest. Surg. 2015, 19, 2038–2044. [Google Scholar] [CrossRef]
- Lange, J.R.; Steinberg, S.M.; Doherty, G.M.; Langstein, H.N.; White, D.E.; Shawker, T.H.; Eastman, R.C.; Jensen, R.T.; Norton, J.A. A randomized, prospective trial of postoperative somatostatin analogue in patients with neuroendocrine tumors of the pancreas. Surgery 1992, 112, 1033–1037, discussion 1037–1038. [Google Scholar]
- Lowy, A.M.; Lee, J.E.; Pisters, P.W.; Davidson, B.S.; Fenoglio, C.J.; Stanford, P.; Jinnah, R.; Evans, D.B. Prospective, randomized trial of octreotide to prevent pancreatic fistula after pancreaticoduodenectomy for malignant disease. Ann. Surg. 1997, 226, 632–641. [Google Scholar] [CrossRef]
- Montorsi, M.; Zago, M.; Mosca, F.; Capussotti, L.; Zotti, E.; Ribotta, G.; Fegiz, G.; Fissi, S.; Roviaro, G.; Peracchia, A.; et al. Efficacy of octreotide in the prevention of pancreatic fistula after elective pancreatic resections: A prospective, controlled, randomized clinical trial. Surgery 1995, 117, 26–31. [Google Scholar] [CrossRef]
- Pederzoli, P.; Bassi, C.; Falconi, M.; Camboni, M.G. Efficacy of octreotide in the prevention of complications of elective pancreatic surgery. Italian Study Group. Br. J. Surg. 1994, 81, 265–269. [Google Scholar] [CrossRef]
- Sarr, M.G.; Pancreatic Surgery, G. The potent somatostatin analogue vapreotide does not decrease pancreas-specific complications after elective pancreatectomy: A prospective, multicenter, double-blinded, randomized, placebo-controlled trial. J. Am. Coll. Surg. 2003, 196, 556–564, discussion 564–555; author reply 565. [Google Scholar] [CrossRef]
- Shan, Y.S.; Sy, E.D.; Lin, P.W. Role of somatostatin in the prevention of pancreatic stump-related morbidity following elective pancreaticoduodenectomy in high-risk patients and elimination of surgeon-related factors: Prospective, randomized, controlled trial. World J. Surg. 2003, 27, 709–714. [Google Scholar] [CrossRef]
- Suc, B.; Msika, S.; Piccinini, M.; Fourtanier, G.; Hay, J.M.; Flamant, Y.; Fingerhut, A.; Fagniez, P.L.; Chipponi, J.; French Associations for Surgical, R. Octreotide in the prevention of intra-abdominal complications following elective pancreatic resection: A prospective, multicenter randomized controlled trial. Arch. Surg. 2004, 139, 288–294, discussion 295. [Google Scholar] [CrossRef]
- Tulassay, Z.; Flautner, L.; Sandor, Z.; Fehervari, I. Perioperative use of somatostatin in pancreatic surgery. Acta Biomed. Ateneo Parmense 1993, 64, 205–211. [Google Scholar]
- Wang, W.; Tian, B.; Babu, S.R.; Zhang, Y.; Yang, M. Randomized, placebo-controlled study of the efficacy of preoperative somatostatin administration in the prevention of postoperative complications following pancreaticoduodenectomy. Hepatogastroenterology 2013, 60, 400–405. [Google Scholar] [CrossRef]
- Yeo, C.J.; Cameron, J.L.; Lillemoe, K.D.; Sauter, P.K.; Coleman, J.; Sohn, T.A.; Campbell, K.A.; Choti, M.A. Does prophylactic octreotide decrease the rates of pancreatic fistula and other complications after pancreaticoduodenectomy? Results of a prospective randomized placebo-controlled trial. Ann. Surg. 2000, 232, 419–429. [Google Scholar] [CrossRef]
- You, D.D.; Paik, K.Y.; Park, I.Y.; Yoo, Y.K. Randomized controlled study of the effect of octreotide on pancreatic exocrine secretion and pancreatic fistula after pancreatoduodenectomy. Asian J. Surg. 2019, 42, 458–463. [Google Scholar] [CrossRef]
- Tarvainen, T.; Siren, J.; Kokkola, A.; Sallinen, V. Effect of Hydrocortisone vs Pasireotide on Pancreatic Surgery Complications in Patients With High Risk of Pancreatic Fistula: A Randomized Clinical Trial. JAMA Surg. 2020, 155, 291–298. [Google Scholar] [CrossRef] [Green Version]
- Kriger, A.G.; Gorin, D.S.; Kaldarov, A.R.; Galkin, G.V. Prevention of pancreatic fistula after pancreatoduodenectomy. Khirurgiia 2020, 11, 61–65. [Google Scholar] [CrossRef]
- Wente, M.N.; Bassi, C.; Dervenis, C.; Fingerhut, A.; Gouma, D.J.; Izbicki, J.R.; Neoptolemos, J.P.; Padbury, R.T.; Sarr, M.G.; Traverso, L.W.; et al. Delayed gastric emptying (DGE) after pancreatic surgery: A suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 2007, 142, 761–768. [Google Scholar] [CrossRef]
- Kong, R.; Hu, J.; Li, L.; Wang, G.; Chen, H.; Bai, X.; Wang, Y.; Wu, L.; Jiang, H.; Sun, B. Impact of octreotide on pancreatic fistula after pancreaticoduodenectomy: A prospective study. Chin. J. Surg. 2016, 54, 21–24. [Google Scholar] [CrossRef]
- Ramos-De la Medina, A.; Sarr, M.G. Somatostatin analogues in the prevention of pancreas-related complications after pancreatic resection. J. Hepato-Biliary-Pancreat. Surg. 2006, 13, 190–193. [Google Scholar] [CrossRef]
- Antila, A.; Siiki, A.; Sand, J.; Laukkarinen, J. Perioperative hydrocortisone treatment reduces postoperative pancreatic fistula rate after open distal pancreatectomy. A randomized placebo-controlled trial. Pancreatology 2019, 19, 786–792. [Google Scholar] [CrossRef]
- Laaninen, M.; Sand, J.; Nordback, I.; Vasama, K.; Laukkarinen, J. Perioperative Hydrocortisone Reduces Major Complications After Pancreaticoduodenectomy: A Randomized Controlled Trial. Ann. Surg. 2016, 264, 696–702. [Google Scholar] [CrossRef]
- Ohwada, S.; Satoh, Y.; Kawate, S.; Yamada, T.; Kawamura, O.; Koyama, T.; Yoshimura, S.; Tomizawa, N.; Ogawa, T.; Morishita, Y. Low-dose erythromycin reduces delayed gastric emptying and improves gastric motility after Billroth I pylorus-preserving pancreaticoduodenectomy. Ann. Surg. 2001, 234, 668–674. [Google Scholar] [CrossRef]
- Okabayashi, T.; Nishimori, I.; Yamashita, K.; Sugimoto, T.; Maeda, H.; Yatabe, T.; Kohsaki, T.; Kobayashi, M.; Hanazaki, K. Continuous postoperative blood glucose monitoring and control by artificial pancreas in patients having pancreatic resection: A prospective randomized clinical trial. Arch. Surg. 2009, 144, 933–937. [Google Scholar] [CrossRef] [Green Version]
- van Veldhuisen, C.L.; Latenstein, A.E.J.; Blauw, H.; Vlaskamp, L.B.; Klaassen, M.; Lips, D.J.; Bonsing, B.A.; van der Harst, E.; Stommel, M.W.J.; Bruno, M.J.; et al. Bihormonal Artificial Pancreas With Closed-Loop Glucose Control vs Current Diabetes Care After Total Pancreatectomy: A Randomized Clinical Trial. JAMA Surg. 2022, 157, 950–957. [Google Scholar] [CrossRef]
- Farkas, G.; Leindler, L.; Mihalovits, G. [Dose-dependent effect of pancreatin replacement upon the pancreatic function in the period after pancreatic surgery]. Magy Seb. 2001, 54, 347–350. [Google Scholar]
- Kim, H.; Yoon, Y.S.; Han, Y.; Kwon, W.; Kim, S.W.; Han, H.S.; Yoon, D.S.; Park, J.S.; Park, S.J.; Han, S.S.; et al. Effects of Pancreatic Enzyme Replacement Therapy on Body Weight and Nutritional Assessments After Pancreatoduodenectomy in a Randomized Trial. Clin. Gastroenterol. Hepatol. 2020, 18, 926–934.e4. [Google Scholar] [CrossRef]
- Neoptolemos, J.P.; Ghaneh, P.; Andren-Sandberg, A.; Bramhall, S.; Patankar, R.; Kleibeuker, J.H.; Johnson, C.D. Treatment of pancreatic exocrine insufficiency after pancreatic resection. Results of a randomized, double-blind, placebo-controlled, crossover study of high vs standard dose pancreatin. Int. J. Pancreatol. 1999, 25, 171–180. [Google Scholar] [CrossRef]
- Satoi, S.; Sho, M.; Yanagimoto, H.; Yamamoto, T.; Akahori, T.; Kinoshita, S.; Nagai, M.; Hirooka, S.; Yamaki, S.; Nishiwada, S.; et al. Do pancrelipase delayed-release capsules have a protective role against nonalcoholic fatty liver disease after pancreatoduodenectomy in patients with pancreatic cancer? A randomized controlled trial. J. Hepatobiliary Pancreat Sci. 2016, 23, 167–173. [Google Scholar] [CrossRef]
- Seiler, C.M.; Izbicki, J.; Varga-Szabo, L.; Czako, L.; Fiok, J.; Sperti, C.; Lerch, M.M.; Pezzilli, R.; Vasileva, G.; Pap, A.; et al. Randomised clinical trial: A 1-week, double-blind, placebo-controlled study of pancreatin 25 000 Ph. Eur. minimicrospheres (Creon 25000 MMS) for pancreatic exocrine insufficiency after pancreatic surgery, with a 1-year open-label extension. Aliment. Pharmacol. Ther. 2013, 37, 691–702. [Google Scholar] [CrossRef] [Green Version]
- Van Hoozen, C.M.; Peeke, P.G.; Taubeneck, M.; Frey, C.F.; Halsted, C.H. Efficacy of enzyme supplementation after surgery for chronic pancreatitis. Pancreas 1997, 14, 174–180. [Google Scholar] [CrossRef]
- Whitcomb, D.C.; Lehman, G.A.; Vasileva, G.; Malecka-Panas, E.; Gubergrits, N.; Shen, Y.; Sander-Struckmeier, S.; Caras, S. Pancrelipase delayed-release capsules (CREON) for exocrine pancreatic insufficiency due to chronic pancreatitis or pancreatic surgery: A double-blind randomized trial. Am. J. Gastroenterol. 2010, 105, 2276–2286. [Google Scholar] [CrossRef]
- Yasukawa, K.; Shimizu, A.; Yokoyama, T.; Kubota, K.; Notake, T.; Seki, H.; Kobayashi, A.; Soejima, Y. Preventive Effect of High-Dose Digestive Enzyme Management on Development of Nonalcoholic Fatty Liver Disease after Pancreaticoduodenectomy: A Randomized Controlled Clinical Trial. J. Am. Coll. Surg. 2020, 231, 658–669. [Google Scholar] [CrossRef]
- Shulkes, A. Somatostatin: Physiology and clinical applications. Baillieres Clin. Endocrinol. Metab. 1994, 8, 215–236. [Google Scholar] [CrossRef]
- Hackert, T.; Klaiber, U.; Hinz, U.; Kehayova, T.; Probst, P.; Knebel, P.; Diener, M.K.; Schneider, L.; Strobel, O.; Michalski, C.W.; et al. Sphincter of Oddi botulinum toxin injection to prevent pancreatic fistula after distal pancreatectomy. Surgery 2017, 161, 1444–1450. [Google Scholar] [CrossRef]
- Creutzfeldt, W.; Lembcke, B.; Folsch, U.R.; Schleser, S.; Koop, I. Effect of somatostatin analogue (SMS 201-995, Sandostatin) on pancreatic secretion in humans. Am. J. Med. 1987, 82, 49–54. [Google Scholar] [CrossRef]
- Panni, R.Z.; Panni, U.Y.; Liu, J.; Williams, G.A.; Fields, R.C.; Sanford, D.E.; Hawkins, W.G.; Hammill, C.W. Re-defining a high volume center for pancreaticoduodenectomy. HPB 2021, 23, 733–738. [Google Scholar] [CrossRef]
- Schuh, F.; Mihaljevic, A.L.; Probst, P.; Trudeau, M.T.; Muller, P.C.; Marchegiani, G.; Besselink, M.G.; Uzunoglu, F.; Izbicki, J.R.; Falconi, M.; et al. A Simple Classification Of Pancreatic Duct Size and Texture Predicts Postoperative Pancreatic Fistula: A classification of the International Study Group of Pancreatic Surgery (ISGPS). Ann. Surg. 2021, 277, e597–e608. [Google Scholar] [CrossRef]
- Lamberts, S.W.J.; Hofland, L.J. ANNIVERSARY REVIEW: Octreotide, 40 years later. Eur. J. Endocrinol. 2019, 181, R173–R183. [Google Scholar] [CrossRef] [Green Version]
- Cecire, J.; Adams, K.; Pham, H.; Pang, T.; Burnett, D. Pharmacological prevention of post-operative pancreatitis: Systematic review and meta-analysis of randomized controlled trials on animal studies. ANZ J. Surg. 2022, 92, 1338–1346. [Google Scholar] [CrossRef]
- Zhang, X.P.; Chen, L.; Hu, Q.F.; Tian, H.; Xu, R.J.; Wang, Z.W.; Wang, K.Y.; Cheng, Q.H.; Yan, W.; Li, Y.; et al. Effects of large dose of dexamethasone on inflammatory mediators and pancreatic cell apoptosis of rats with severe acute pancreatitis. World J. Gastroenterol. 2007, 13, 5506–5511. [Google Scholar] [CrossRef] [Green Version]
- Laaninen, M.; Blauer, M.; Sand, J.; Nordback, I.; Laukkarinen, J. Difference in Early Activation of NF-kappaB and MCP-1 in Acinar-Cell-Rich versus Fibrotic Human Pancreas Exposed to Surgical Trauma and Hypoxia. Gastroenterol. Res. Pract. 2014, 2014, 460363. [Google Scholar] [CrossRef] [Green Version]
- Call, T.R.; Pace, N.L.; Thorup, D.B.; Maxfield, D.; Chortkoff, B.; Christensen, J.; Mulvihill, S.J. Factors associated with improved survival after resection of pancreatic adenocarcinoma: A multivariable model. Anesthesiology 2015, 122, 317–324. [Google Scholar] [CrossRef]
- McSorley, S.T.; Horgan, P.G.; McMillan, D.C. The impact of preoperative corticosteroids on the systemic inflammatory response and postoperative complications following surgery for gastrointestinal cancer: A systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2016, 101, 139–150. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.K.; Hindenburg, A.A.; Sharma, S.K.; Suk, C.H.; Gress, F.G.; Staszewski, H.; Grendell, J.H.; Reed, W.P. Is pylorospasm a cause of delayed gastric emptying after pylorus-preserving pancreaticoduodenectomy? Ann. Surg. Oncol. 2005, 12, 222–227. [Google Scholar] [CrossRef]
- Navas, C.M.; Wadas, E.D.; Zbib, N.H.; Crowell, M.D.; Lacy, B.E. Gastroparesis and Severity of Delayed Gastric Emptying: Comparison of Patient Characteristics, Treatments and Medication Adverse Events. Dig Dis. Sci. 2021, 66, 526–534. [Google Scholar] [CrossRef]
- Camilleri, M.; Parkman, H.P.; Shafi, M.A.; Abell, T.L.; Gerson, L. Clinical guideline: Management of gastroparesis. Am. J. Gastroenterol. 2013, 108, 18–37, quiz 38. [Google Scholar] [CrossRef] [Green Version]
- Shin, J.M.; Sachs, G. Pharmacology of proton pump inhibitors. Curr. Gastroenterol. Rep. 2008, 10, 528–534. [Google Scholar] [CrossRef] [Green Version]
- Butler, J.R.; Rogers, T.; Eckart, G.; Martens, G.R.; Ceppa, E.P.; House, M.G.; Nakeeb, A.; Schmidt, C.M.; Zyromski, N.J. Is antisecretory therapy after pancreatoduodenectomy necessary? Meta-analysis and contemporary practices of pancreatic surgeons. J. Gastrointest. Surg. 2015, 19, 604–612. [Google Scholar] [CrossRef]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Okita, K.; Mizuguchi, T.; Shigenori, O.; Ishii, M.; Nishidate, T.; Ueki, T.; Meguro, M.; Kimura, Y.; Tanimizu, N.; Ichinohe, N.; et al. Pancreatic regeneration: Basic research and gene regulation. Surg. Today 2016, 46, 633–640. [Google Scholar] [CrossRef]
- Xu, G.; Sumi, S.; Koike, M.; Tanigawa, K.; Nio, Y.; Tamura, K. Role of endogenous hypergastrinemia in regenerating endocrine pancreas after partial pancreatectomy. Dig Dis. Sci. 1996, 41, 2433–2439. [Google Scholar] [CrossRef]
- Lim, P.W.; Dinh, K.H.; Sullivan, M.; Wassef, W.Y.; Zivny, J.; Whalen, G.F.; LaFemina, J. Thirty-day outcomes underestimate endocrine and exocrine insufficiency after pancreatic resection. HPB 2016, 18, 360–366. [Google Scholar] [CrossRef] [Green Version]
- Muller, M.W.; Friess, H.; Kleeff, J.; Dahmen, R.; Wagner, M.; Hinz, U.; Breisch-Girbig, D.; Ceyhan, G.O.; Buchler, M.W. Is there still a role for total pancreatectomy? Ann. Surg. 2007, 246, 966–974, discussion 974–965. [Google Scholar] [CrossRef]
- Maeda, H.; Okabayashi, T.; Yatabe, T.; Yamashita, K.; Hanazaki, K. Perioperative intensive insulin therapy using artificial endocrine pancreas in patients undergoing pancreatectomy. World J. Gastroenterol. 2009, 15, 4111–4115. [Google Scholar] [CrossRef]
- Heckler, M.; Klaiber, U.; Huttner, F.J.; Haller, S.; Hank, T.; Nienhuser, H.; Knebel, P.; Diener, M.K.; Hackert, T.; Buchler, M.W.; et al. Prospective trial to evaluate the prognostic value of different nutritional assessment scores for survival in pancreatic ductal adenocarcinoma (NURIMAS Pancreas SURVIVAL). J. Cachexia Sarcopenia Muscle 2021, 12, 1940–1947. [Google Scholar] [CrossRef]


| First Author | Year | Country | Treatment | Primary Outcome | Secondary Outcome | Patient Randomized | Patients Analyzed | Pathology | Surgical Approach |
|---|---|---|---|---|---|---|---|---|---|
| Cao [23] | 2021 | China | Somatostatin vs. Placebo | POPF | Biochemical leak, morbidity, pancreatectomy-related complications | 205 | 199 | PDAC or pancreatitis n = 90 Other diseases n = 109 | Open PD n = 127 Laparoscopic PD n = 72 |
| Tarvainen [46] | 2020 | Finland | Hydrocortisone vs. Pasireotide | Comprehensive Complication Index (CCI) score within 30 days | Clavien–Dindo classification | 168 | 126 | PDAC n = 27 Cholangiocarcinoma n = 9 IPMN n = 12 MCN n = 8 PNET n = 27 Serous cystadenoma n = 4 Papilla adenoma n = 3 Dysplasia n = 11 Metastasis of another carcinoma n = 5 | DP n = 57 PD n = 60 Papillectomy n = 1 Enucleation n = 6 |
| Kriger [47] | 2020 | Russia | somatostatin analogues and glucocorticoids vs. somatostatin analogue | POPF | N/A | 78 | 78 | N/A | N/A |
| You [45] | 2019 | Korea | Octreotide vs. Placebo | Pancreatic juice output | Incidence of POPF and postoperative complications | 66 | 59 | Bile duct cancer 24 Pancreatic cancer 17 Ampullary cancer 11 Others 5 | PD n = 59 |
| El Nakeeb [25] | 2018 | Egypt | Octreotide vs. Placebo | POPF (period: 30 days after surgery) | DGE [48], length of hospital stay | 104 | 104 | Adenocarcinoma n = 89 Neuroendocrine n = 1 Cholangiocarcinoma n = 2 Solid pseudopapillary tumor n = 3 Adenoma n = 5 Pancreatitis n = 3 Benign cyst n = 1 | PD n = 104 |
| Kurumboor [34] | 2015 | India | Octreotide vs. Placebo | POPF (period: 30 days after surgery) | Postoperative complications | 109 | 109 | Soft pancreas Non-dilated ducts | PD n = 109 |
| Kong [49] | 2016 | China | Octreotide vs. Placebo | POPF | Hospitalization days, treatment cost | 306 | 306 | N/A | N/A |
| Allen [17] | 2014 | USA | Pasireotide vs. Placebo | 60-day ≥grade 3 pancreatic complication rates (fistula, leak, and abscess) | 60-day: overall complication rate, mortality, pancreatic complication rate; Amylase level; duration of drainage, daily drain volume, time of return of bowel function as defined by passage of flatus | 443 | 300 | PDAC n = 154 | PD n = 220 DP n = 80 |
| Belyaev [19] | 2013 | Germany | Octreotide (intra-arterial) | Increased pancreatic hardness | POPF, DGE | 26 | 25 | PDAC n = 14 Ampullary cancer n = 1 Distal hepatic duct cancer n = 1 Duodenal cancer n = 1 Melanoma metastasis n = 1 Benign n = 7 Chronic pancreatitis n = 3 Pseudocyst n = 1 IPMN n = 1 Cystadenoma n = 1 Duodenal adenoma n = 1 | PD n = 19 TP n = 6 |
| Fernandez-Cruz [27] | 2013 | Spain | Octreotide vs. placebo | POPF | Morbidity, hospital mortality and duration of postoperative hospital length of stay | 62 | 62 | PDAC n = 32 Ampullary carcinoma n = 10 IPMN n = 5 Cholangiocarcinoma n = 4 Neuroendocrine tumor n = 3 Metastatic tumor n = 2 Duodenal cancer n = 2 Pseudopapillary solid tumor n = 2 Serous cystadenoma n = 2 | PD n = 62 |
| Wang [43] | 2013 | China | Somatostatin vs. Placebo | POPF | Postoperative complications | 38 | 38 | Pancreatic neoplasm n = 21 Chronic pancreatitis n = 14 CBDC n = 4 Benign pancreatic cancer n = 11 Duodenal cancer 10 | PD |
| Katsourakis [31] | 2010 | Greece | Somatostatin vs. Placebo | Effect of somatostatin administration on the ultra-structure of exocrine pancreatic cells | Postoperative complications | 67 | 67 | PDAC n = 53 Pancreatitis n = 9 Neuroendocrine tumor n = 1 Metastatic adenocarcinoma n = 1 Lymphoma n = 1 Acinar cell n = 1 Cystadenoma n = 1 | PD n = 59 DP + splenectomy n = 7 DP n = 1 |
| Kollmar [33] | 2008 | Germany | Somatostatin vs. Placebo | Incidence DGE | Perioperative morbidity other than DGE | 67 | 67 | N/A | PD n = 67 |
| Closset [24] | 2008 | Belgium | Somatostatin vs. Octreotide | Pancreatic stump-related complications | - | 50 | 50 | IPMT n = 11 Ampulloma n = 7 Serous cystadenoma n = 2 GIST n = 1 Endocrine tumor n = 2 Duodenal tumor n = 1 Cholangiocarcinoma n = 1 PAN IN 3: n = 1 | PD n = 50 |
| Ramos-De la Medina [50] | 2006 | USA | Vapreotide vs. placebo | Pancreas-specific complications; mortality | Overall complications; duration of hospitalization | 381 | 275 | Benign neoplasm n = 41 PDAC n = 80 Ampullary carcinoma n = 22 Duodenal carcinoma n = 6 Bile duct carcinoma n = 8 IPMN n = 1 Neuroendocrine n = 14 Cystadenocarcinoma n = 1 Other n = 7 | DP n = 38 PD n = 98 PPPD n = 58 |
| Hesse [30] | 2005 | Belgium | Low-dose Octreotide vs. Placebo | General complications, including extended length of hospital stay | N/A | 105 | 105 | Cancer n = 80 Benign tumor n = 8 Chronic pancreatitis | PD n = 80 LPJ= 12 DP = 10 CJ = 3 |
| Suc [41] | 2004 | France | Octreotide vs. Placebo | IACs | EACs isolated or associated with IACs | 230 | 230 | N/A | N/A |
| Sarr [39] | 2003 | USA | Somatostatin analogue: vapreotide vs. Placebo | Development of pancreatic-related complications | Overall complication rate | 275 | 275 | Benign neoplasm n = 44 Malignant n = 138 IPMN n = 1 Neuroendocrine n = 14 Cystadenocarcinoma n = 1 Other n = 7 | PD n = 134 PPPD n = 80 DP n = 52 Central pancreatectomy n = 8 |
| Shan [40] | 2003 | Taiwan | Somatostatin vs. Placebo | Prevention of pancreatic stump-related complications | N/A | 60 | 54 | Pancreatic cancer n = 12 Distal CBD cancer n = 7 Ampullary cancer n = 15 Duodenal cancer n = 4 Duodenal stromal cancer n = 4 Benign lesion of periampullary area n = 6 Lymphoma n = 3 Other malignancy n = 3 | Whipple n = 31 PPPD n = 24 |
| Falconi [26] | 2002 | Italy | Lanreotide vs. Placebo | Exocrine pancreatic secretion | N/A | 8 | 7 | PDAC n = 3 Periampullary cancer n = 2 Duodenal carcinoma n = 1 Cystic carcinoma n = 1 Neuroendrocrine tumor n = 1 | PPPD |
| Gouillat [29] | 2001 | France | Somatostatin vs. Placebo | Reduction in pancreatic juice outcome | Amylase and lipase output | 75 | 75 | PDAC n = 61 Chronic pancreatitis n = 4 Other tumors n = 10 | PD = 38 PPPD n = 37 |
| Bonora [20] | 2001 | Italy | Gabexate mesilate vs Gabexate mesilate combined with Octreotide | Postoperative complications | - | 50 | 50 | PDAC n = 15 Periampullary carcinoma n = 5 Duodenal carcinoma n = 1 Endocrine neoplasm n = 6 | DPPHR PD Enucleation |
| Yeo [44] | 2000 | USA | Octreotide vs. Saline (Placebo) | POPF, total complications, death, and length of hospital stay | Cost of octreotide and the potential cost savings associated with the cessation of its use | 383 | 211 | PDAC n = 84 Bile duct carcinoma n = 37 Ampullary carcinoma n = 27 Chronic pancreatitis n = 22 Islet cell tumor n = 9 Periampullary adenoma n = 8 Duodenal adenocarcinoma n = 4 Gastrointestinal stromal tumor n = 2 | PD |
| Briceno Delgado [2] | 1998 | Spain | Octreotide | POPF | Overall postoperative complications | 34 | 34 | Ampulloma n = 17 Pancreatic cancer n = 8 Cholangiocarcinoma n = 2 Duodenal carcinoma n = 1 Chronic pancreatitis n = 5 | PD n = 34 |
| Lowy [36] | 1997 | USA | Octreotide | Development of a clinical or biochemical pancreatic anastomotic leak | Gastrointestinal function | 120 | 110 | PDAC n = 64 Periampullary adenocarcinoma n = 20 Neuroendocrine tumor n = 9 Other malignant tumor n = 12 Benign n = 5 | PD |
| Friess [28] | 1995 | Switzerland | Octreotide | Postoperative complications | - | 247 | 247 | Chronic pancreatitis | PD n = 70 DP n = 55 PPPD n = 54 PJ n = 61 Other n = 7 |
| Beguiristain [18] | 1995 | Spain | Somatostatin | POPF | Postoperative complications | 35 | 35 | Periampullary cancer n = 14 Pancreatic cancer n = 13 Chronic pancreatitis n = 3 Endocrine tumor n = 1 Gastric cancer n = 1 Cystadenoma n = 1 | PD |
| Montorsi [37] | 1995 | Italy | Octreotide vs. Placebo | POPF | Other postoperative complications | 218 | 218 | Pancreatic and periampullary cancer n = 139 Other abdominal neoplasm n = 37 Chronic pancreatitis n = 18 Endocrine tumor n = 14 Miscellaneous n = 8 | PD n = 143 LR n = 54 SP n = 12 Enucleation n = 5 Other n = 4 |
| Pederzoli [38] | 1994 | Italy | Octreotide | Postoperative complications | - | 303 | 252 | PDAC n = 61 Periampullary tumor n = 43 Endocrine tumor n = 24 Cystic tumor n = 24 Chronic pancreatitis n = 95 Other n = 5 | Whipple n = 100 DPPHR n = 5 DP n = 60 Intermediate resection n = 7 Enucleation n = 14 PJ n = 66 |
| Tulassay [42] | 1993 | Hungary | Somatostatin | Postop. increase in pancreatic enzymes | - | 33 | 33 | Cyst of pancreas n = 19 Chronic pancreatitis n = 14 | Cysto-duodenostomy n = 12 Cysto-gastrostomy n = 7 Wirsungo-gastrostomy n = 7 Wirsungoplastic n = 7 |
| Büchler [22] | 1992 | Germany | Octreotide | Pancreatic fistula. | Abscess, acute pancreatitis, pulmonary insufficiency, shock, and sepsis, which represent local and systemic sequelae of a pancreatic leak | 322 | N/A | PDAC n = 71 Periampullary cancer n = 40 Endocrine tumor n = 9 Chronic pancreatitis n = 112 Others n = 14 | DPPHR n = 48 Whipple n = 152 DP n = 31 PJ n = 8 Enucleation n = 3 Others n = 4 |
| Lange [35] | 1992 | USA | Somatostatin vs. Placebo | Reducing pancreatic drainage | Postoperative complications | 21 | 21 | Gastrinoma n = 7 Insulinoma n = 14 | Enucleation n = 10 Resection n = 11 |
| Buccoliero [21] | 1992 | Italy | Somatostatin | Volume, pancreatic, and gall bladder secretion | Amylase and Lipase secretion, concentration of bicarbonates and chlorides | 31 | 31 | N/A | PD |
| Klempa [32] | 1991 | Germany | Somatostatin | Pancreatic juice: volume, amylase, lipase, protein and bicarbonate | Pancreatic exocrine function | 30 | 30 | Pancreas carcinoma n = 19 Ampullary carcinoma n = 5 | PD |
| First Author | Year | Country | Treatment | Primary Outcome | Secondary Outcome | Patient Randomized | Patients Analyzed | Pathology | Surgical Approach |
|---|---|---|---|---|---|---|---|---|---|
| Tarvainen [46] | 2020 | Finland | Hydrocortisone vs. Pasireotide | Comprehensive Complication Index (CCI) score within 30 days | POPF, DGE, PPH, readmissions, all within 30 days after the operation, length of hospital stay | 168 | 126 | PDAC n = 27 Cholangiocarcinoma n = 9 IPMN n = 12 MCN n = 8 pNET n = 27 Serous cystadenoma n = 4 Papilla adenoma n = 3 Dysplasia n = 11 Metastasis of another carcinoma n = 5 | DP n = 57 PD n = 60 Papillectomy n = 1 Enucleation n = 6 |
| Antila [51] | 2019 | Finland | Hydrocortisone vs. Placebo | Overall complications (C-D III-V) | Clinically significant POPF | 40 | 31 | PDAC n = 5 pNET n = 4 Cystic tumor n = 17 Other n = 2 | DP |
| Laaninen [52] | 2016 | Finland | Hydrocortisone vs. Placebo | Urine trypsinogen positive days, overall complications (Clavien–Dindo III-IV). | Clinically pancreatoduodenectomy-related complications (POPF, PPH, DGE; grades B and C), mortality, and general infectious complications | 155 | 62 | PDAC n = 27 BDC n = 14 IPMN n = 5 Duodenal carcinoma n = 7 Gastrointestinal stromal tumor n = 3 Chronic pancreatitis n = 3 other = 3 |
| First Author | Year | Country | Treatment | Primary Outcome | Secondary Outcome | Patient Randomized | Patients Analyzed | Pathology | Surgical Approach |
|---|---|---|---|---|---|---|---|---|---|
| Ohwada [53] | 2001 | Japan | Erythromycin vs. Placebo | DGE | Gastric motility Nasogastric Tube Removal | 31 | 31 | PDAC n = 4 Bile duct carcinoma n = 8 Ampullary tumor n = 11 Duodenal tumor n = 2 Chronic pancreatitis n = 3 | PPPD |
| Yeo [6] | 1993 | USA | Erythromycin vs. Placebo | DGE | N/A | 118 | 118 | Pancreas cancer n = 78 Bile duct carcinoma n = 12 Ampulla n = 17 Duodenum n = 11 | TP n = 3 Partial pancreatectomy n = 115 |
| First Author | Year | Country | Treatment | Primary Outcome | Secondary Outcome | Patient Randomized | Patients Analyzed | Pathology | Surgical Approach |
|---|---|---|---|---|---|---|---|---|---|
| Jang [9] | 2003 | Korea | Lansoprazole (PPI) vs. Placebo | Hypergastrinemia, volume of the distal pancreas, nutritional status, endocrine and exocrine function, | Serum gastrin levels before surgery and 3 months after surgery. | 56 | 37 | Ampullary cancer n = 16 Bile duct cancer n = 13 PDAC n = 6 Duodenal cancer n = 2 | PD |
| Toyota [7] | 1998 | Japan | Omeprazole (PPI) vs. Placebo | Gastric stasis | Volume and acidity of the gastric juice | 42 | 42 | Bile duct cancer n = 7 Gallbladder cancer n = 1 Pancreatic head carcinoma n = 19 Papilla of Vater Cancer n = 5 Chronic pancreatitis n = 7 Papillitis n = 2 Congenital biliary dilatation n = 1 | PPPD |
| First Author | Year | Country | Treatment | Primary Outcome | Secondary Outcome | Patient Randomized | Patients Analyzed | Pathology | Surgical Approach |
|---|---|---|---|---|---|---|---|---|---|
| Van Veldhuisen [55] | 2022 | Netherland | Closed-Loop Glucose Control vs Current Diabetes Care | Median percentage of time spent in euglycemia | Safety and efficacy of the BIHAP | 12 | 10 | IPMN n = 1 Benign n = 3 Malignant n = 6 | TP |
| Okayabashi [54] | 2009 | Japan | Glucose Control by Artificial Pancreas | Incidence of severe hypoglycemia (40 mg/dL) | Total amount of insulin required for glycemic control in the first 18 h after pancreatic resection | 32 | 30 | Pancreatic disease | PD n = 15 DP n = 11 TP n = 2 |
| First Author | Year | Country | Treatment | Primary Outcome | Secondary Outcome | Patient Randomized | Patients Analyzed | Inclusion Criteria | Exclusion Criteria |
|---|---|---|---|---|---|---|---|---|---|
| Kim [57] | 2020 | Korea | PERT vs. Placebo | Change in body weight | Changes in bowel habits, nutritional parameters, and QoL | 237 | 164 | PDAC n = 103 AoVC n = 50 CBDC n = 36 IPMN. n = 14 PNET n = 10 Other n = 24 | PD n = 40 PPPD n = 197 |
| Yasukawa [63] | 2020 | Japan | PERT vs. Placebo | NAFLD within 1 year | Incidences of NAFLD at 1, 3, 6, and 12 months, the rate of improvement in NAFLD with high-dose transfer in the control group | 84 | 80 | PDAC 35 Bile duct cancer 25 Ampullar PNT 8 IPMN 5 | PD n = 80 |
| Satoi [59] | 2016 | Japan | PERT vs. Placebo | Frequency of NAFLD-development within 12 months after starting treatment | Postoperative exocrine and endocrine pancreatic insufficiency; BMI, serum albumin level, nutritional status | 57 | 57 | PDAC 53 IPMC 4 | PD |
| Seiler [60] | 2013 | Germany | PERT | Mean CFA change from baseline to end of double-blind treatment | Stool frequency, body weight and BMI | 58 | 51 | Malignancy n = 14 Chronic pancreatitis n = 44 | PD/PPPD n = 29 DPPHR n = 13 Other n = 12 |
| Farkas [56] | 2001 | Hungary | PERT vs. Placebo | Exocrine function via fecal elastase determinations, amylum tolerance test, checks on the symptoms of maldigestion | N/A | 40 | 40 | Chronic pancreatitis n = 40 | Partial and total pancreatectomy n = 14 |
| Whitcomb [62] | 2010 | USA | PERT vs. Placebo | Coefficient of fat absorption (CFA) | Coefficient of nitrogen absorption (CNA), clinical symptoms, and safety parameters | 54 | 53 | N/A | N/A |
| Neoptolemos [58] | 1999 | Great Britain | PERT vs. Placebo | Stool fat (as g/d) | Stool volume (mL/d), clinical global impression of disease symptoms, diary card recordings, and patient’s treatment preference | 39 | 36 | Chronic pancreatitis 17 Necrotizing pancreatitis | PPPD |
| Van Hoozen [61] | 1997 | Nether-lands | PERT vs. Placebo | Nutritional status and intestinal absorption | N/A | 11 | 11 | Chronic pancreatitis | LR-LPJ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rompen, I.F.; Merz, D.C.; Alhalabi, K.T.; Klotz, R.; Kalkum, E.; Pausch, T.M.; Strothmann, H.; Probst, P. Perioperative Drug Treatment in Pancreatic Surgery—A Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 12, 1750. https://doi.org/10.3390/jcm12051750
Rompen IF, Merz DC, Alhalabi KT, Klotz R, Kalkum E, Pausch TM, Strothmann H, Probst P. Perioperative Drug Treatment in Pancreatic Surgery—A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2023; 12(5):1750. https://doi.org/10.3390/jcm12051750
Chicago/Turabian StyleRompen, Ingmar F., Daniela C. Merz, Karam T. Alhalabi, Rosa Klotz, Eva Kalkum, Thomas M. Pausch, Hendrik Strothmann, and Pascal Probst. 2023. "Perioperative Drug Treatment in Pancreatic Surgery—A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 12, no. 5: 1750. https://doi.org/10.3390/jcm12051750
APA StyleRompen, I. F., Merz, D. C., Alhalabi, K. T., Klotz, R., Kalkum, E., Pausch, T. M., Strothmann, H., & Probst, P. (2023). Perioperative Drug Treatment in Pancreatic Surgery—A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 12(5), 1750. https://doi.org/10.3390/jcm12051750





