“Neuro-Fiber Mapping”: An Original Concept of Spinal Cord Neural Network Spatial Targeting Using Live Electrostimulation Mapping to (Re-)Explore the Conus Medullaris Anatomy
Abstract
1. Introduction
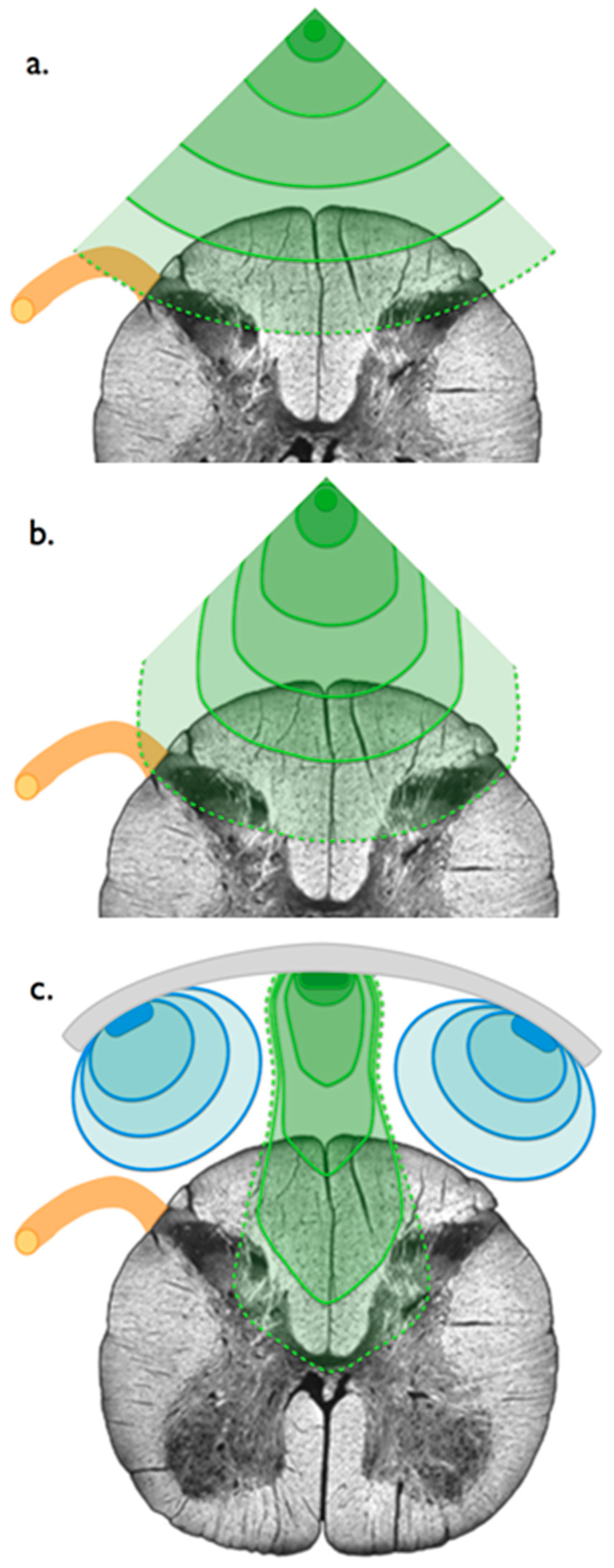
2. Materials and Methods
2.1. Phase 1: Clinical Exploration
2.1.1. Ethical Issues
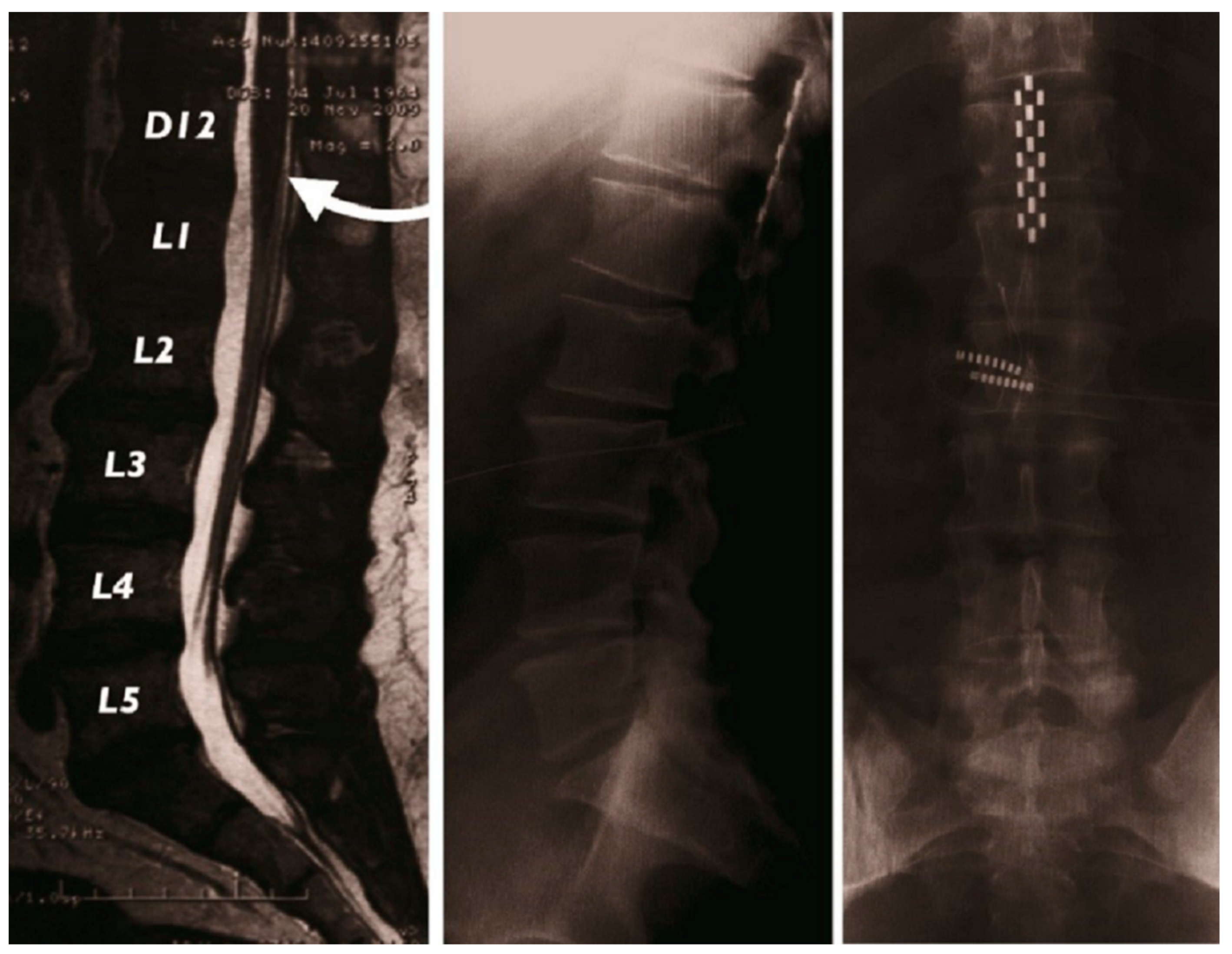
2.1.2. Surgical Implantation
2.1.3. Electrostimulation Mapping of the Conus Medullaris
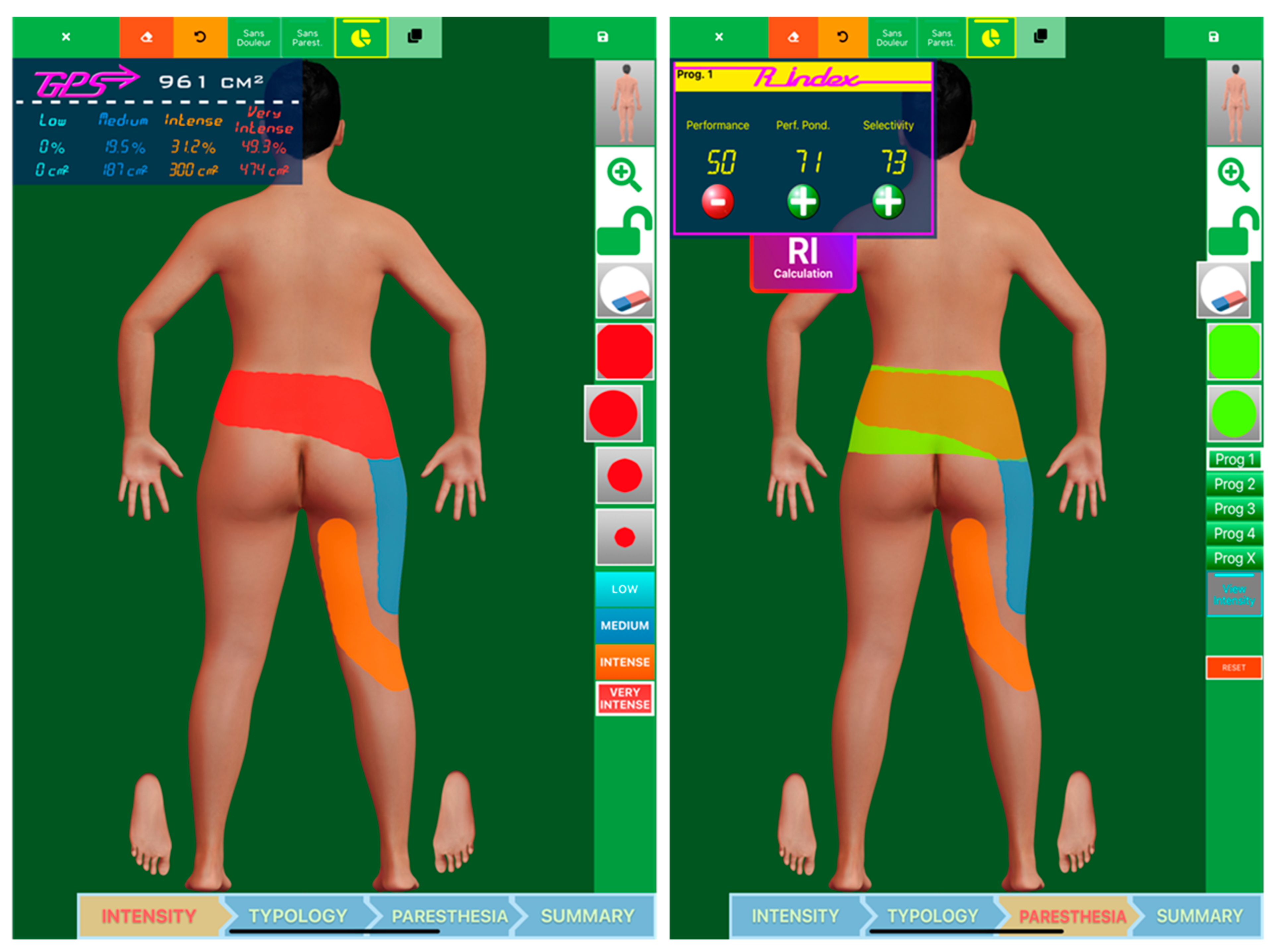
2.1.4. Data Analysis
3. Results
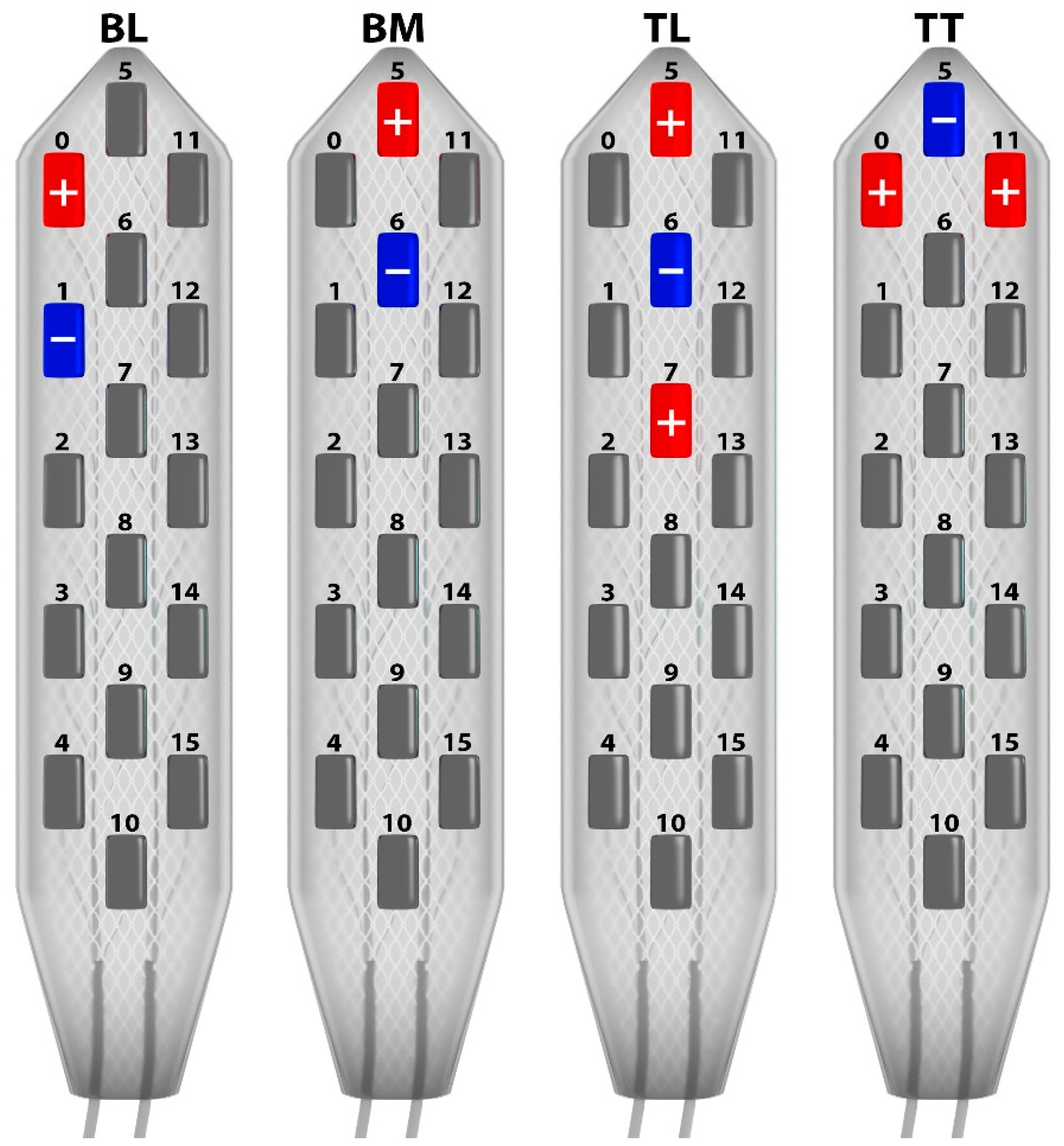
3.1. Programming Sessions
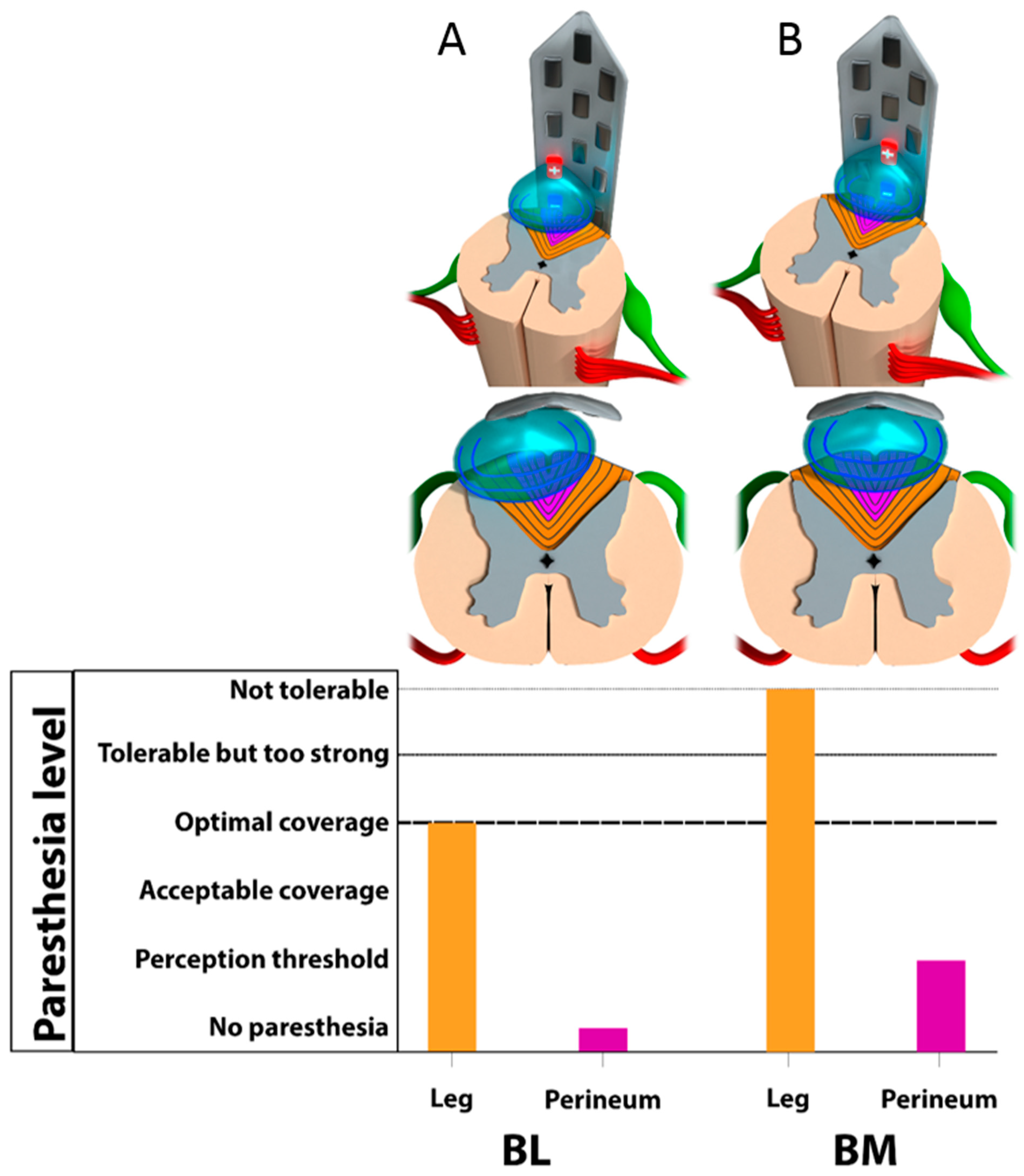
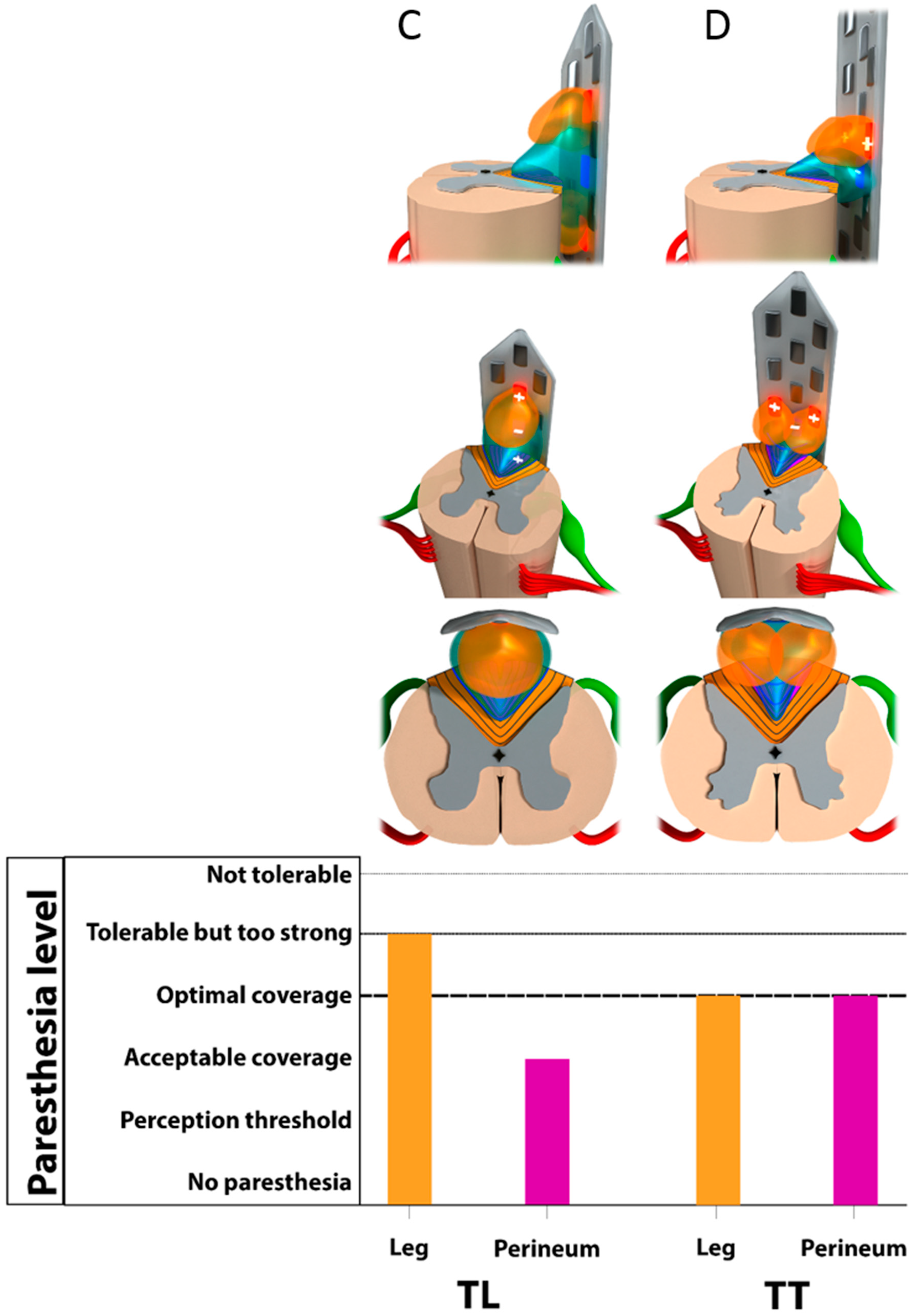
3.2. Monocolumn Combinations
3.3. Multicolumn Combinations
4. Discussion
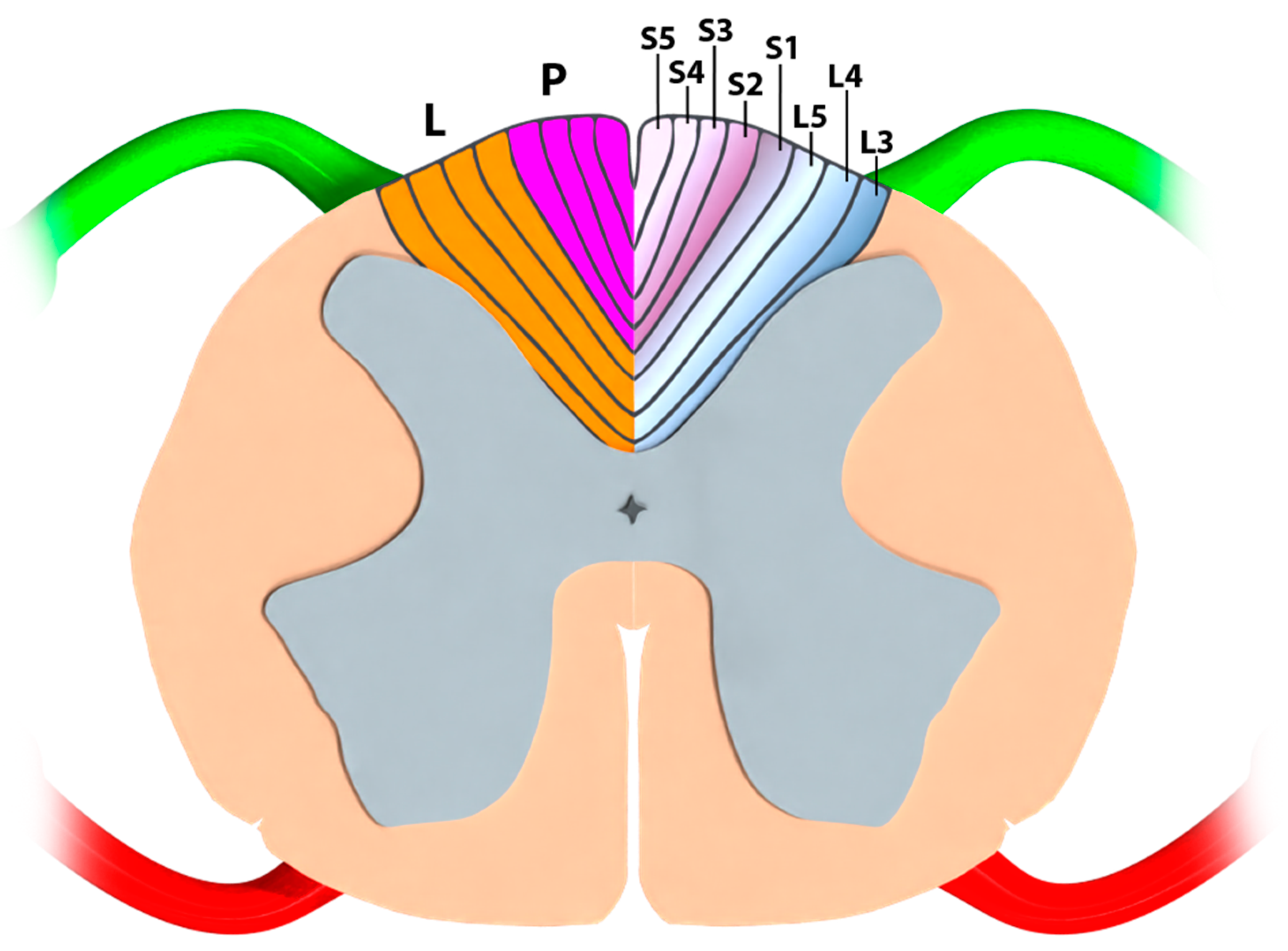
4.1. Part I—Classical Anatomy and Somatotopical Organization of the Conus Medullaris
4.1.1. Neuroanatomical Textbook Data
4.1.2. Anatomo-Electrophysiological Correlations Regarding a Clinical Blackbox
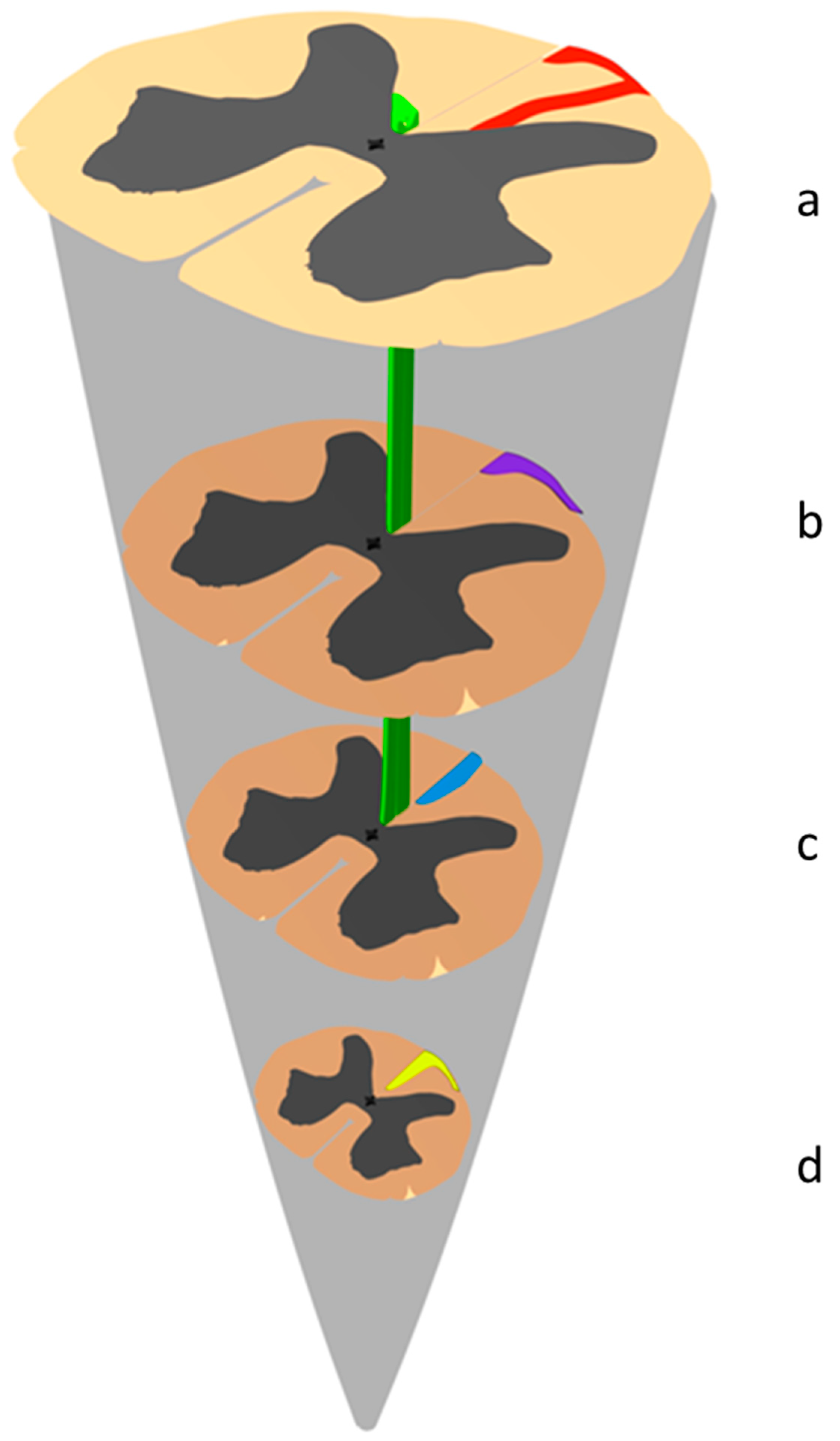
4.1.3. Historical Revisitation of the Morphological Anatomy of the Dorsal Funiculi at the Conus Medullaris
Notion of Endogenous Fibers and Intraspinal Commissural Pathways
Original Anatomical Description of the Inter-Commissural Endogenous Fiber Network by Philippe
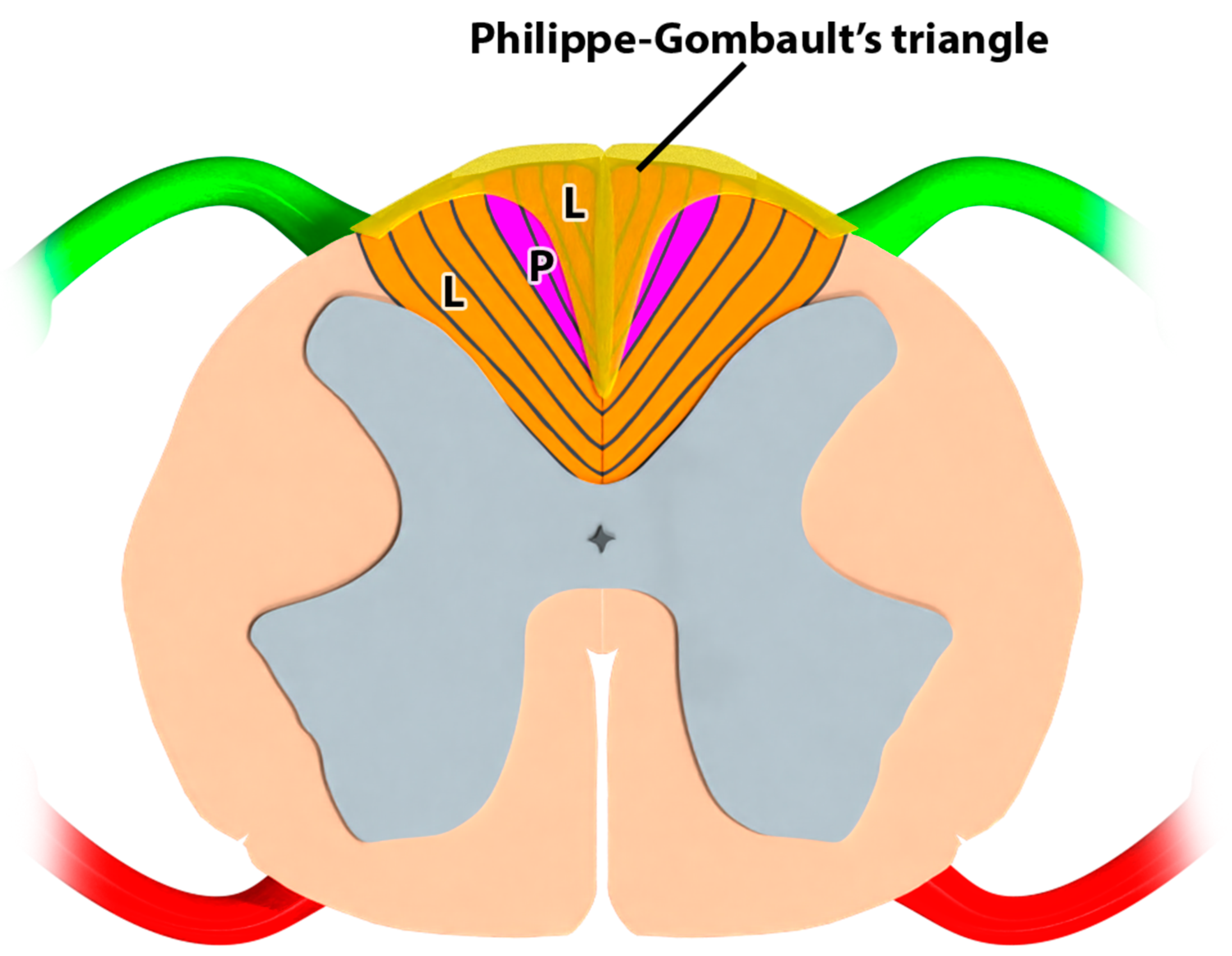
Description of the Triangle of Philippe and Gombault
4.1.4. Historical Anatomical and Clinical Correlations
4.2. Part III-Study Strengths and Limitations
4.2.1. An Alternative Approach Based on Neuron Electrical Properties and 3D-Computerized Modeling
4.2.2. Historical Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gray, H. Anatomy, Descriptive and Surgical, 5th ed.; Henry C. Lea: Philadelphia, PA, USA, 1870. [Google Scholar]
- Rouvière, H. Anatomie Humaine Descriptive et Topographique; Masson et Cie: Paris, France, 1924. [Google Scholar]
- Nieuwenhuys, R. Comparative Anatomy of the Spinal Cord. In Progress in Brain Research; Eccles, J.C., Schadé, J.P., Eds.; Organization of the Spinal Cord; Elsevier: Amsterdam, The Netherlands, 1964; Volume 11, pp. 1–57. [Google Scholar]
- Carpenter, M.B. Core Text of Neuroanatomy; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 1991. [Google Scholar]
- Fehlings, M.G.; Vaccaro, A.R.; Boakye, M. Essentials of Spinal Cord Injury: Basic Research to Clinical Practice; Thieme: New York, NY, USA, 2012; ISBN 978-1-60406-727-9. [Google Scholar]
- Rossignol, S.; Bouyer, L.; Barthélemy, D.; Langlet, C.; Leblond, H. Recovery of Locomotion in the Cat Following Spinal Cord Lesions. Brain Res. Brain Res. Rev. 2002, 40, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Rossignol, S.; Schwab, M.; Schwartz, M.; Fehlings, M.G. Spinal Cord Injury: Time to Move? J. Neurosci. 2007, 27, 11782–11792. [Google Scholar] [CrossRef]
- Bossy, J. Neuro-Anatomie; Springer: Paris, France, 1990. [Google Scholar]
- Kandel, E.R.; Schwartz, J.H.; Jessel, T.M. Principles of Neural Science, 4th ed.; McGraw-Hill: New York, NY, USA, 2000. [Google Scholar]
- Netter, F.H. Atlas D’anatomie Humaine; Maloine: Paris, France, 1997. [Google Scholar]
- Lazorthes, G. Vascularisation et Circulation Cérébral; Masson: Paris, France, 1961. [Google Scholar]
- D’Houtaud, S.; Sztermer, E.; Buffenoir, K.; Giot, J.-P.; Wager, M.; Bauche, S.; Lapierre, F.; Rigoard, P. Synapse formation and regeneration. Neurochirurgie 2009, 55 (Suppl. S1), S49–S62. [Google Scholar] [CrossRef]
- Charnay, P.; Coulpier, F.; Decker, L.; Funalot, B.; Vallat, J.-M.; Garcia-Bragado, F.; Topilko, P. Krox20 inactivation in the PNS leads to CNS/PNS boundary transgression by central glia. Bull. Acad. Natl. Med. 2010, 194, 743–744. [Google Scholar] [PubMed]
- Mertens, P.; Blond, S.; David, R.; Rigoard, P. Anatomy, Physiology and Neurobiology of the Nociception: A Focus on Low Back Pain (Part A). Neurochirurgie 2015, 61 (Suppl. S1), S22–S34. [Google Scholar] [CrossRef]
- Blond, S.; Mertens, P.; David, R.; Roulaud, M.; Rigoard, P. From “Mechanical” to “Neuropathic” Back Pain Concept in FBSS Patients. A Systematic Review Based on Factors Leading to the Chronification of Pain (Part C). Neurochirurgie 2015, 61 (Suppl. S1), S45–S56. [Google Scholar] [CrossRef] [PubMed]
- Rigoard, P.; Blond, S.; David, R.; Mertens, P. Pathophysiological Characterisation of Back Pain Generators in Failed Back Surgery Syndrome (Part B). Neurochirurgie 2015, 61 (Suppl. S1), S35–S44. [Google Scholar] [CrossRef] [PubMed]
- Courtine, G.; Sofroniew, M.V. Spinal Cord Repair: Advances in Biology and Technology. Nat. Med. 2019, 25, 898–908. [Google Scholar] [CrossRef] [PubMed]
- North, R.B.; Fischell, T.A.; Long, D.M. Chronic Dorsal Column Stimulation via Percutaneously Inserted Epidural Electrodes. Preliminary Results in 31 Patients. Appl. Neurophysiol. 1977, 40, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Krames, E.; Peckham, P.H.; Rezai, A.R. Neuromodulation: Comprehensive Textbook of Principles, Technologies, and Therapies; Elsevier Science: Amsterdam, The Netherlands, 2018; ISBN 978-0-12-809302-3. [Google Scholar]
- Shealy, C.N.; Mortimer, J.T.; Reswick, J.B. Electrical Inhibition of Pain by Stimulation of the Dorsal Columns: Preliminary Clinical Report. Anesth. Analg. 1967, 46, 489–491. [Google Scholar] [CrossRef]
- Melzack, R.; Wall, P.D. Pain Mechanisms: A New Theory. Science 1965, 150, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Goudman, L.; De Groote, S.; Linderoth, B.; De Smedt, A.; Eldabe, S.; Duarte, R.V.; Moens, M. Exploration of the Supraspinal Hypotheses about Spinal Cord Stimulation and Dorsal Root Ganglion Stimulation: A Systematic Review. J. Clin. Med. 2021, 10, 2766. [Google Scholar] [CrossRef] [PubMed]
- De Ridder, D.; Vanneste, S. Burst and Tonic Spinal Cord Stimulation: Different and Common Brain Mechanisms. Neuromodulation 2016, 19, 47–59. [Google Scholar] [CrossRef]
- Linderoth, B.; Foreman, R.D. Conventional and Novel Spinal Stimulation Algorithms: Hypothetical Mechanisms of Action and Comments on Outcomes. Neuromodulation 2017, 20, 525–533. [Google Scholar] [CrossRef]
- Guzzi, G.; Della Torre, A.; La Torre, D.; Volpentesta, G.; Stroscio, C.A.; Lavano, A.; Longhini, F. Spinal Cord Stimulation in Chronic Low Back Pain Syndrome: Mechanisms of Modulation, Technical Features and Clinical Application. Healthcare 2022, 10, 1953. [Google Scholar] [CrossRef]
- Rigoard, P.; Basu, S.; Desai, M.; Taylor, R.; Annemans, L.; Tan, Y.; Johnson, M.J.; Van den Abeele, C.; North, R. PROMISE Study Group Multicolumn Spinal Cord Stimulation for Predominant Back Pain in Failed Back Surgery Syndrome Patients: A Multicenter Randomized Controlled Trial. Pain 2019, 160, 1410–1420. [Google Scholar] [CrossRef]
- Rigoard, P.; Billot, M.; Ingrand, P.; Durand-Zaleski, I.; Roulaud, M.; Peruzzi, P.; Dam Hieu, P.; Voirin, J.; Raoul, S.; Page, P.; et al. How Should We Use Multicolumn Spinal Cord Stimulation to Optimize Back Pain Spatial Neural Targeting? A Prospective, Multicenter, Randomized, Double-Blind, Controlled Trial (ESTIMET Study). Neuromodulation 2021, 24, 86–101. [Google Scholar] [CrossRef]
- Veizi, E.; Hayek, S.M.; North, J.; Brent Chafin, T.; Yearwood, T.L.; Raso, L.; Frey, R.; Cairns, K.; Berg, A.; Brendel, J.; et al. Spinal Cord Stimulation (SCS) with Anatomically Guided (3D) Neural Targeting Shows Superior Chronic Axial Low Back Pain Relief Compared to Traditional SCS-LUMINA Study. Pain Med. 2017, 18, 1534–1548. [Google Scholar] [CrossRef]
- Rigoard, P.; Ounajim, A.; Goudman, L.; Banor, T.; Héroux, F.; Roulaud, M.; Babin, E.; Bouche, B.; Page, P.; Lorgeoux, B.; et al. The Challenge of Converting “Failed Spinal Cord Stimulation Syndrome” Back to Clinical Success, Using SCS Reprogramming as Salvage Therapy, through Neurostimulation Adapters Combined with 3D-Computerized Pain Mapping Assessment: A Real Life Retrospective Study. J. Clin. Med. 2022, 11, 272. [Google Scholar] [CrossRef]
- Labat, J.-J.; Robert, R.; Delavierre, D.; Sibert, L.; Rigaud, J. Symptomatic approach to chronic neuropathic somatic pelvic and perineal pain. Prog. Urol. 2010, 20, 973–981. [Google Scholar] [CrossRef]
- Holsheimer, J.; Struijk, J.J.; Wesselink, W.A. Analysis of Spinal Cord Stimulation and Design of Epidural Electrodes by Computer Modeling. Neuromodulation 1998, 1, 14–18. [Google Scholar] [CrossRef]
- Rigoard, P.; Delmotte, A.; Moles, A.; Hervochon, R.; Vrignaud, T.; Misbert, L.; Lafay, N.; D’houtaud, S.; Frasca, D.; Guenot, C.; et al. Successful Treatment of Pudendal Neuralgia with Tricolumn Spinal Cord Stimulation: Case Report. Neurosurgery 2012, 71, E757–E763. [Google Scholar] [CrossRef]
- Sankarasubramanian, V.; Buitenweg, J.R.; Holsheimer, J.; Veltink, P. Triple Leads Programmed to Perform as Longitudinal Guarded Cathodes in Spinal Cord Stimulation: A Modeling Study. Neuromodulation 2011, 14, 401–410; discussion 411. [Google Scholar] [CrossRef]
- Rigoard, P.; Delmotte, A.; D’Houtaud, S.; Misbert, L.; Diallo, B.; Roy-Moreau, A.; Durand, S.; Royoux, S.; Giot, J.-P.; Bataille, B. Back Pain: A Real Target for Spinal Cord Stimulation? Neurosurgery 2012, 70, 574–585. [Google Scholar] [CrossRef] [PubMed]
- Guetarni, F.; Rigoard, P. The “Neuro-Mapping Locator” Software. A Real-Time Intraoperative Objective Paraesthesia Mapping Tool to Evaluate Paraesthesia Coverage of the Painful Zone in Patients Undergoing Spinal Cord Stimulation Lead Implantation. Neurochirurgie 2015, 61 (Suppl. S1), S90–S98. [Google Scholar] [CrossRef]
- Rigoard, P.; Nivole, K.; Blouin, P.; Monlezun, O.; Roulaud, M.; Lorgeoux, B.; Bataille, B.; Guetarni, F. A Novel, Objective, Quantitative Method of Evaluation of the Back Pain Component Using Comparative Computerized Multi-Parametric Tactile Mapping before/after Spinal Cord Stimulation and Database Analysis: The “Neuro-Pain’t” Software. Neurochirurgie 2015, 61 (Suppl. S1), S99–S108. [Google Scholar] [CrossRef]
- Rigoard, P.; Ounajim, A.; Goudman, L.; Bouche, B.; Roulaud, M.; Page, P.; Lorgeoux, B.; Baron, S.; Nivole, K.; Many, M.; et al. The Added Value of Subcutaneous Peripheral Nerve Field Stimulation Combined with SCS, as Salvage Therapy, for Refractory Low Back Pain Component in Persistent Spinal Pain Syndrome Implanted Patients: A Randomized Controlled Study (CUMPNS Study) Based on 3D-Mapping Composite Pain Assessment. J. Clin. Med. 2021, 10, 5094. [Google Scholar] [CrossRef] [PubMed]
- Rigoard, P.; Ounajim, A.; Goudman, L.; Louis, P.-Y.; Slaoui, Y.; Roulaud, M.; Naiditch, N.; Bouche, B.; Page, P.; Lorgeoux, B.; et al. A Novel Multi-Dimensional Clinical Response Index Dedicated to Improving Global Assessment of Pain in Patients with Persistent Spinal Pain Syndrome after Spinal Surgery, Based on a Real-Life Prospective Multicentric Study (PREDIBACK) and Machine Learning Techniques. J. Clin. Med. 2021, 10, 4910. [Google Scholar] [CrossRef] [PubMed]
- Moens, M.; De Smedt, A.; D’Haese, J.; Droogmans, S.; Chaskis, C. Spinal Cord Stimulation as a Treatment for Refractory Neuropathic Pain in Tethered Cord Syndrome: A Case Report. J. Med. Case Rep. 2010, 4, 74. [Google Scholar] [CrossRef]
- Cliffer, K.D.; Giesler, G.J. Postsynaptic Dorsal Column Pathway of the Rat. III. Distribution of Ascending Afferent Fibers. J. Neurosci. 1989, 9, 3146–3168. [Google Scholar] [CrossRef]
- Creasey, G.H. Electrical Stimulation of Sacral Roots for Micturition after Spinal Cord Injury. Urol. Clin. N. Am. 1993, 20, 505–515. [Google Scholar] [CrossRef]
- Creasey, G.H.; Bodner, D.R. Review of Sacral Electrical Stimulation in the Management of the Neurogenic Bladder. Neurorehabilitation 1994, 4, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Bican, O.; Minagar, A.; Pruitt, A.A. The Spinal Cord: A Review of Functional Neuroanatomy. Neurol. Clin. 2013, 31, 1–18. [Google Scholar] [CrossRef]
- Grasset, J. La Dissociation Dite Syringomyélique des Sensibilités; Nouveau Montpellier Médical, IX; Leçon cliniques; Delord-Boehm et Martial: Montpellier, France, 1899. [Google Scholar]
- Gerest, J. Les Affections Nerveuses Systématiques et la Théorie des Neurones; JB Baillière et Fils: Paris, France, 1898. [Google Scholar]
- Brouardel, P.; Gilbert, A. Traité de Médecine et de Thérapeutique; JB Baillière et Fils: Paris, France, 1902. [Google Scholar]
- Debove, M.; Sallard, A. Traité Élémentaire de Clinique Médicale; Masson et Cie: Paris, France, 1905. [Google Scholar]
- Philippe, C. Contribution à L’étude Anatomique et Clinique Du Tabes Dorsalis; G. Steinheil: Paris, France, 1897; Volume 263. [Google Scholar]
- Gombault, A.; Philippe, C. Contribution à l’étude Des Lésions Systématisées Des Cordons Blancs de La Moelle Épinière. Arch. Med. Exp. Anat. Pathol. 1894, 6, 365–424. [Google Scholar]
- Gombault, A.; Philippe, C. Etat Actuel Des Connaissances Sur La Systématisation Des Cordons Postérieurs de La Moelle Épinière. Sem. Med. 1895, 15, 161–166. [Google Scholar]
- Dèjérine, J.; Spiller, W. Contributions à l’étude de La Texture Des Cordons Postérieurs de La Moelle Épinière. Du Trajet Intramédullaire Des Racines Postérieurs Sacrées et Lombaires Inférieuses. C. R. Soc. Biol. 1895, 27. [Google Scholar]
- Van Gehuchten, A. Anatomie du Système Nerveux de L’homme: Leçons Professées à L’université de Louvain; Imprimerie des Trois Rois: Mulhouse, France, 1897. [Google Scholar]
- Gracie, I. Tuberculose et Système Nerveux: Contribution à L’étude Clinique et Anatomo-Pathologique de Leurs Rapports; Hachette Book Group: New York, NY, USA, 1900. [Google Scholar]
- Rigoard, P.; Ounajim, A.; Goudman, L.; Wood, C.; Roulaud, M.; Page, P.; Lorgeoux, B.; Baron, S.; Nivole, K.; Many, M.; et al. Combining Awake Anesthesia with Minimal Invasive Surgery Optimizes Intraoperative Surgical Spinal Cord Stimulation Lead Placement. J. Clin. Med. 2022, 11, 5575. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rigoard, P.; Moens, M.; Goudman, L.; Le Tutour, T.; Rochette, M.; Dany, J.; Et Talby, M.; Roulaud, M.; Hervochon, R.; Ounajim, A.; et al. “Neuro-Fiber Mapping”: An Original Concept of Spinal Cord Neural Network Spatial Targeting Using Live Electrostimulation Mapping to (Re-)Explore the Conus Medullaris Anatomy. J. Clin. Med. 2023, 12, 1747. https://doi.org/10.3390/jcm12051747
Rigoard P, Moens M, Goudman L, Le Tutour T, Rochette M, Dany J, Et Talby M, Roulaud M, Hervochon R, Ounajim A, et al. “Neuro-Fiber Mapping”: An Original Concept of Spinal Cord Neural Network Spatial Targeting Using Live Electrostimulation Mapping to (Re-)Explore the Conus Medullaris Anatomy. Journal of Clinical Medicine. 2023; 12(5):1747. https://doi.org/10.3390/jcm12051747
Chicago/Turabian StyleRigoard, Philippe, Maarten Moens, Lisa Goudman, Tom Le Tutour, Michel Rochette, Jonathan Dany, Mohamed Et Talby, Manuel Roulaud, Rémi Hervochon, Amine Ounajim, and et al. 2023. "“Neuro-Fiber Mapping”: An Original Concept of Spinal Cord Neural Network Spatial Targeting Using Live Electrostimulation Mapping to (Re-)Explore the Conus Medullaris Anatomy" Journal of Clinical Medicine 12, no. 5: 1747. https://doi.org/10.3390/jcm12051747
APA StyleRigoard, P., Moens, M., Goudman, L., Le Tutour, T., Rochette, M., Dany, J., Et Talby, M., Roulaud, M., Hervochon, R., Ounajim, A., Nivole, K., David, R., & Billot, M. (2023). “Neuro-Fiber Mapping”: An Original Concept of Spinal Cord Neural Network Spatial Targeting Using Live Electrostimulation Mapping to (Re-)Explore the Conus Medullaris Anatomy. Journal of Clinical Medicine, 12(5), 1747. https://doi.org/10.3390/jcm12051747






