Belief Inflexibility and Cognitive Biases in Anorexia Nervosa—The Role of the Bias against Disconfirmatory Evidence and Its Clinical and Neuropsychological Correlates
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical Assessment
2.2. Neuropsychological Assessment
2.3. The BADE Task
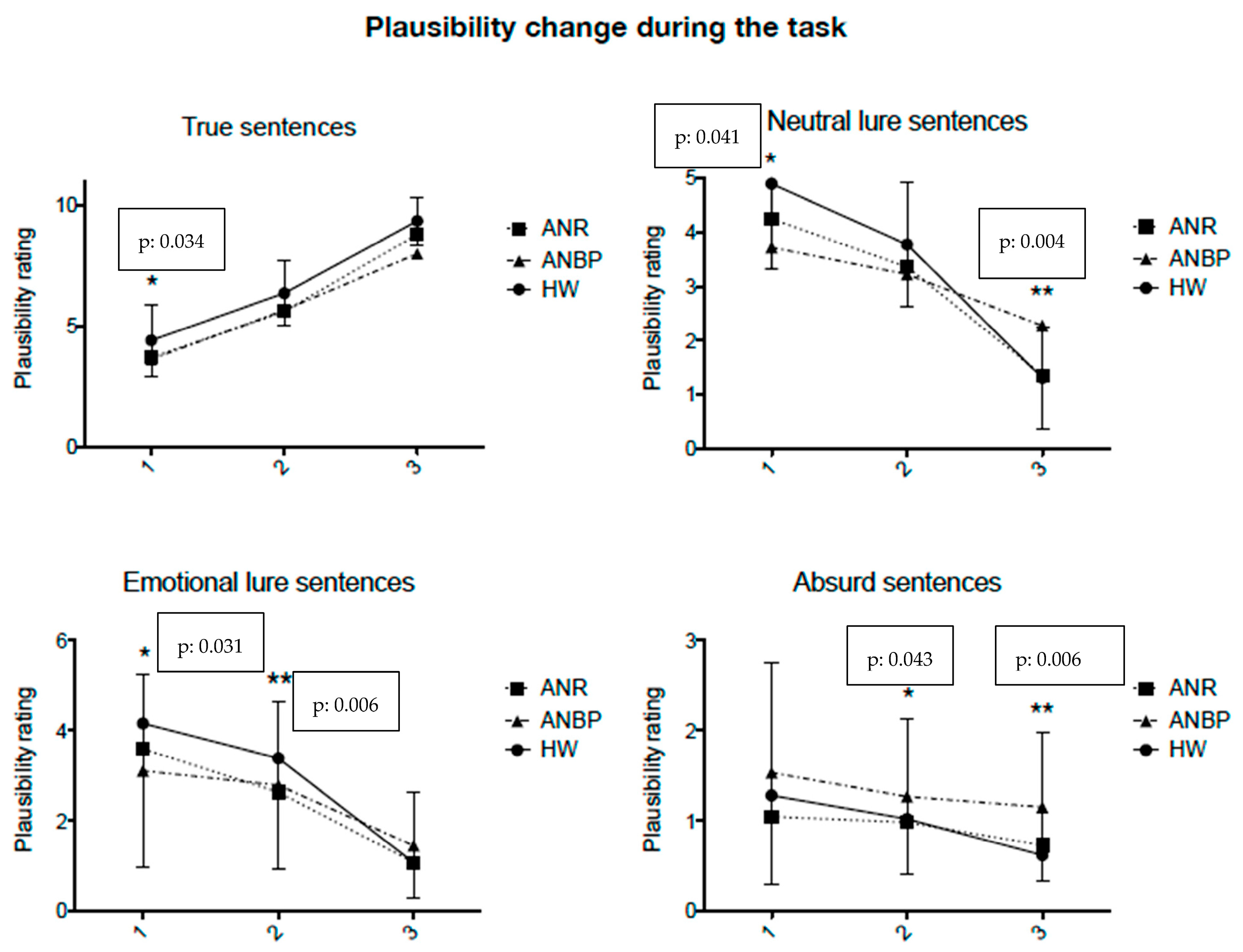
Interpretation of the BADE Task Scores
2.4. Other Main Cognitive Tasks
- −
- The Wisconsin Card Sorting Test (WCST) [43] assesses abstract thinking and the ability to both infer and adapt to external changes, shifting cognitive strategies (i.e., cognitive flexibility). It is widely considered a good task for assessing the integrity of reasoning and executive functioning. We considered as outcome variables the number of achieved categories, the total number of correct answers, the total number of errors, perseverative responses and errors, and the global score (i.e., an overall index of global cognitive efficiency) [31,44].
- −
- The Trail Making Test A and B [45] measures attentional speed, sequencing, visual search, and mental flexibility. Part A (TMT-A) assesses motor speed, part B (TMT-B) assesses complex divided attention and set-shifting, and B-A difference (delta trail) gives a measure of cognitive shifting cost and allows us to control for motor impairment.
- −
- The Rey–Osterrieth Complex Figure Test (ROCF) [46], gives a measure of visuospatial constructional abilities and visuographic memory, but cognitive planning, organizational strategies, and executive functions are also involved. The task request is to both directly copy and reproduce, after a delay of 3 min, the complex figure. The former assesses perception and visuospatial constructive abilities, and the latter implicit visuospatial memory. As described elsewhere (for a detailed description see [30,31]), this task was used as a measure of central coherence (the ability to put together different details in order to gain the “big picture”) by means of both the Order of Construction Index (the order in which the different global elements were drawn) and the Style Index (indicative of the degree of continuity in the drawing process). We considered the following outcomes of the ROCF: copy and memory accuracy scores, the time needed to complete the figure copy and the figure reproduction after a delay, and the central coherence index (CCI) of the figure copy. The CCI ranges from 0 (indicating weak central coherence) to 2 (indicating high central coherence).
- −
- All participants over the age of 20 completed the Brief Intelligence Test (TIB) [47], as a measure of premorbid intellectual ability. The task requires reading 34 irregular words which violate typical stress rules, very similar to the National Adult Reading Test. All patients and controls younger than 21 were administered the Information subtest of the Wechsler Intelligence Scale-IV edition [48] as a measure of premorbid verbal intelligence.
2.5. Statistical Analyses
3. Results
3.1. Acute AN, Weight-Recovered AN, Healthy Women in Comparison, and Disconfirmatory/Confirmatory Cognitive Bias
3.2. Acute AN Restricting (ANR) and AN Binge-Eating/Purging (ANBP) Subtypes and Healthy Women in Comparison and Disconfirmatory/Confirmatory Cognitive Bias
3.3. BADE Task Performance and Clinical and Psychopathological Variables: Correlational Analyses
3.4. BADE Task Performance and Neuropsychological Functioning: Correlational Analyses
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Publishing: Arlington, VA, USA, 2013. [Google Scholar]
- Willamson, D.A.; Muller, S.L.; Reas, D.L.; Thaw, J.M. Cognitive bias in eating disorders. Behav. Modif. 1999, 23, 556–577. [Google Scholar] [CrossRef]
- Cardi, V.; Corfield, F.; Leppanen, J.; Rhind, C.; Deriziotis, S.; Hadjimichalis, A.; Hibbs, R.; Micali, N. Emotional processing, recognition, empathy and evoked facial expression in eating disorders: An experimental study to map deficits in social cognition. PLoS ONE 2015, 10, e0133827. [Google Scholar] [CrossRef]
- Rowlands, K.; Grafton, B.; Cerea, S.; Simic, M.; Hirsch, C.; Cruwys, T.; Yellowlees, R.; Treasure, J.; Cardi, V. A multifaceted study of interpersonal functioning and cognitive biases towards social stimuli in adolescents with eating disorders and healthy controls. J. Affect. Disord. 2021, 295, 397–404. [Google Scholar] [CrossRef]
- Rienecke, R.D.; Johnson, C.; Le Grange, D.; Manwaring, J.; Mehler, P.S.; Duffy, A.; McClanahan, S.; Blalock, D.V. Adverse childhood experiences among adults with eating disorders: Comparison to a nationally representative sample and identification of trauma profiles. J. Eat. Disord. 2022, 10, 7. [Google Scholar] [CrossRef]
- Teicher, M.H.; Samson, J.A.; Anderson, C.M.; Ohashi, K. The effects of childhood maltreatment on brain structure, function and connectivity. Nat. Rev. Neurosci. 2016, 17, 652e666. [Google Scholar] [CrossRef]
- Morrison, J.; Williams, M.O.; Fox, J.R. Negative childhood events and the development of the anorexic voice: A grounded theory. Psychol. Psychother. Theory Res. Pract. 2022, 95, 1018–1035. [Google Scholar] [CrossRef]
- Veale, D. Over-valued ideas: A conceptual analyis. Behav. Res. Ther. 2002, 40, 383–400. [Google Scholar] [CrossRef]
- Shafran, R.; Teachman, B.A.; Kerry, S.; Rachman, S.A. A cognitive distortion associated with eating disorders: Thought-shape fusion. Br. J. Clin. Psychol. 1999, 38, 167–179. [Google Scholar] [CrossRef]
- Coelho, J.S.; Baeyens, C.; Purdon, C.; Shafran, R.; Roulin, J.-L.; Bouvard, M. Assessment of thought-shape fusion: Initial validation of a short version of the trait thought-shape fusion scale. Int. J. Eat. Disord. 2013, 46, 77–85. [Google Scholar] [CrossRef]
- Steinglass, J.E.; Eisen, J.L.; Attia, E.; Mayer, L.; Walsh, B.T. Is Anorexia Nervosa a Delusional Disorder? An Assessment of Eating Beliefs in Anorexia Nervosa. J. Psychiatr. Pract. 2007, 13, 65–71. [Google Scholar] [CrossRef]
- O’Connell, J.E.; Bendall, S.; Morley, E.; Huang, C.; Krug, I. Delusion-like beliefs in anorexia nervosa: An interpretative phenomeonological analysis. Clin. Psychol. 2017, 22, 317–326. [Google Scholar] [CrossRef]
- Hartmann, A.S.; Thomas, J.J.; Wilson, A.C.; Wilhelm, S. Insight impairment in body image disorders: Delusionality and overvalued ideas in anorexia nervosa versus body dysmorphic disorder. Psychiatry Res. 2013, 210, 1129–1135. [Google Scholar] [CrossRef]
- Konstantakopoulos, G.; Tchanturia, K.; Surguladze, S.; David, A. Insight in eating disorders: Clinical and cognitive correlates. Psychol. Med. 2011, 41, 1951–1961. [Google Scholar] [CrossRef]
- Mullen, R.; Linscott, R.J. A comparison of delusions and overvalued ideas. J. Nerv. Ment. Dis. 2010, 198, 35–38. [Google Scholar] [CrossRef]
- Meheler, C.; Wewetzer, C.; Schulze, U.; Warnke, A.; Theisen, F.; Dittmann, R. Olanzapine in childern and adolescents with chronic anorexia nervosa. A study of five cases. Eur. Child. Adolesc. Psychiatry 2001, 10, 151–157. [Google Scholar] [CrossRef]
- Delsedime, N.; Nicotra, B.; Giovannone, M.C.; Marech, L.; Barosio, M.; Marzola, E.; Abbate-Daga, G.; Fassino, S. Psychotic symptoms in a woman with severe anorexia nervosa. Eat. Weight Disord. 2013, 18, 95–98. [Google Scholar] [CrossRef]
- Kelly, L.; Kamali, M.; Brennan, T. Anorectic symptomatology as a prodrome of schziophrenia: Four case reports. Eur. Eat. Disord. Rev. 2004, 12, 230–233. [Google Scholar] [CrossRef]
- Keef, K.M.; Warman, D.M. Reasoning, delusion proneness and stress: An experimental investigation. Clin. Psychol. Psychother. 2011, 18, 138–147. [Google Scholar] [CrossRef]
- Garety, P.A.; Freeman, D.; Jolley, S.; Dunn, G.; Bebbington, P.E.; Fowler, D.G.; Kuipers, E.; Dudley, R. Reasoning emotions, and delusional conviction in psychosis. J. Abnorm. Psychol. 2005, 114, 373–384. [Google Scholar] [CrossRef]
- Woodward, T.S.; Moritz, S.; Cuttler, C.; Whitman, J.C. The contribution of a cognitive bias against disconfirmatory evidence (BADE) to delusions in schizophrenia. J. Clin. Exp. Neuropsychol. 2006, 28, 605–617. [Google Scholar] [CrossRef]
- Garety, P.A.; Freeman, D. Cognitive approaches to delusions: A critical review of the evidence. Br. J. Clin. Psychol. 1999, 38, 113–154. [Google Scholar] [CrossRef]
- Moritz, S.; Woodward, T.S. Plausibility judgment in schizophrenic patients: Evidence for a liberal acceptance bias. Ger. J. Psychiatry 2004, 7, 66–74. [Google Scholar]
- McLean, B.F.; Mattiske, J.K.; Balzan, R.P. Association of the jumping to conclusions and evidence integration biases with delusions in psychosis: A detailed meta-analyis. Schizophr. Bull. 2017, 43, 344–354. [Google Scholar] [CrossRef]
- Sanford, N.; Veckenstedt, R.; Moritz, S.; Balzan, R.P.; Woodward, T.S. Impaired integration of disambiguating evidence in delusional schizophrenia patients. Psychol. Med. 2014, 44, 2729–2738. [Google Scholar] [CrossRef]
- Georgiou, N.; Delfabbro, P.; Balzan, R. Conspiracy-beliefs and receptivity to disconfirmatory information: A study using the BADE task. SAGE Open 2021, 11, 21582440211006131. [Google Scholar] [CrossRef]
- Hollowell, A.; Ronald, A. Psychotic experiences associate with a Bias Against Disconfirmatory Evidence (BADE) in adolescence. Schizophr. Res. 2020, 218, 304–305. [Google Scholar] [CrossRef]
- Wittorf, A.; Giel, K.E.; Hautzinger, M.; Rapp, A.; Schönenberg, M.; Wolkenstein, L.; Zipfel, S.; Mehl, S.; Fallgatter, A.J.; Klingberg, S. Specificity of jumping to conclusions and attributional biases: A comparison between patients with schizophrenia, depression, and anorexia nervosa. Cogn. Neuropsychiatry 2012, 17, 262–286. [Google Scholar] [CrossRef]
- McKenna, G.; Fox, J.R.E.; Haddock, G. Investigating the “Jumping to conclusions” bias in people with anorexia nervosa. Eur. Eat. Disord. Rev. 2014, 22, 352–359. [Google Scholar] [CrossRef]
- Tenconi, E.; Collantoni, E.; Meregalli, V.; Bonello, E.; Zanetti, T.; Veronese, A.; Meneguzzo, P.; Favaro, A. Clinical and cognitive functioning changes after partial hospitalization in patients with anorexia nervosa. Front. Psychiatry 2021, 12, 653506. [Google Scholar] [CrossRef]
- Tenconi, E.; Santonastaso, P.; Degortes, D.; Bosello, R.; Titton, F.; Mapelli, D.; Favaro, A. Favaro. Set- shifting abilities, central coherence, and handedness in anorexia nervosa patients, their unaffected siblings and healthy controls: Exploring putative endophenotypes. World J. Biol. Psychiatry 2010, 1, 813–823. [Google Scholar] [CrossRef]
- Tenconi, E.; Degortes, D.; Clementi, M.; Collantoni, E.; Pinato, C.; Forzan, M.; Cassina, M.; Santonastaso, P.; Favaro, A. Clinical and genetic correlates of decision making in anorexia nervosa. J. Clin. Exp. Neuropsychol. 2016, 38, 327–337. [Google Scholar] [CrossRef]
- Favaro, A.; Clementi, M.; Manara, R.; Bosello, R.; Forzan, M.; Bruson, A.; Tenconi, E.; Degortes, D.; Titton, F.; Di Salle, F.; et al. Catechol-O-methyltransferase genotype modifies executive functioning and prefrontal functional connectivity in women with anorexia nervosa. J. Psychiatry Neurosci. 2013, 38, 241–248. [Google Scholar] [CrossRef]
- World Medical Association. Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- First, M.B.; Williams, J.B.; Karg, R.S.; Spitzer, R.L. Structured Clinical Interview for DSM-5 Disorders, Clinician Version (SCID-5-CV); American Psychiatric Association: Arlington, VA, USA, 2015. [Google Scholar]
- Derogatis, L.P.; Lipman, R.S.; Rickels, K.; Uhlenhuth, E.H.; Covi, L. The Hopkins Symptom Checklist (HSCL): A self-report symptom inventory. Behav. Sci. 1974, 19, 1–15. [Google Scholar] [CrossRef]
- Garner, D.M.; Olmstead, M.P.; Polivy, J. Development and validation of a multidimensional Eating Disorder Inventory for anorexia nervosa and bulimia. Int. J. Eat. Disord. 1983, 2, 15–35. [Google Scholar] [CrossRef]
- Spielberger, C.D.; Gorsuch, R.I.; Lushene, R.E. Manual for the State-Trait Anxiety Inventory; Spielberger, C.D., Translator; Consulting Psychologists Press: Palo Alto, CA, USA; Forma: Firenze, Italy, 1986; STAI State-Trait Anxiety Inventory. [Google Scholar]
- Oldfield, R.C. The assessment and analysis of handedness: The Edinburgh Inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef]
- Riccaboni, R.; Fresi, F.; Bosia, M.; Buonocore, M.; Leiba, N.; Smeraldi, E.; Cavallaro, R. Patterns of evidence integration in schizophrenia and delusion. Psychiatry Res. 2012, 200, 108–114. [Google Scholar] [CrossRef]
- Eisenacher, S.; Rausch, F.; Mier, D.; Fenske, S.; Veckenstedt, R.; Englisch, S.; Becker, A.; Andreou, C.; Moritz, S.; Meyer-Lindenberg, A.; et al. Bias against disconfirmatory in the “at-risk mental state” and during psychosis. Psychiatry Res. 2016, 238, 242–250. [Google Scholar] [CrossRef]
- Woodward, T.S.; Moritz, S.; Menon, M.; Klinge, R. Belief inflexibility in schizophrenia. Cogn. Neuropsychiatry 2008, 13, 267–277. [Google Scholar] [CrossRef]
- Berg, E.A. A simple objective technique for measuring flexibility in thinking. J. Gen. Psychol. 1948, 39, 15–22. [Google Scholar] [CrossRef]
- Laiacona, M.; Inzaghi, M.G.; De Tanti, A.; Capitani, E. Wisconsin card sorting test: A new global score, with Italians norms, and its relationship with the Weigl sorting test. Neurol. Sci. 2000, 21, 279–291. [Google Scholar] [CrossRef]
- Reitan, R.M. Validity of the Trail Making test as indicator of organic brain damage. Percept. Mot. Skills 1958, 8, 271–276. [Google Scholar] [CrossRef]
- Osterrieth, P. The test of copying a complex figure: A contribution to the study of perception and memory. Arch. Psychol. 1944, 30, 206–356. [Google Scholar]
- Colombo, L.; Sartori, G.; Brivio, C. Stima del quoziente intellettivo tramite l’applicazione del TIB (Test Breve di Intelligenza). GIP 2002, 3, 613–637. [Google Scholar] [CrossRef]
- Wechsler, D. Wechsler Adult Intelligence Scale, 4th ed.; Pearson Assessment: San Antonio, TX, USA, 2008. [Google Scholar] [CrossRef]
- Akavia, N. Writing “The case of Ellen West”: Clinical knowledge and historical representation. Sci. Context 2008, 21, 119–144. [Google Scholar] [CrossRef]
- Favaro, A.; Tenconi, E.; Santonastaso, P. Perinatal factors and the risk of developing anorexia nervosa and bulimia nervosa. Arch. Gen. Psychiatry 2006, 63, 82–88. [Google Scholar] [CrossRef]
- Noordenbos, G.; Aliakbari, N.; Campbell, R. The relationship among critical inner voices, low self esteem, and self criticism in eating disorders. Eat. Disord. 2014, 22, 337–351. [Google Scholar] [CrossRef]
- Solmi, F.; Melamed, D.; Lewis, G.; Kirkbride, J.B. Longitudinal associations between psychotic experiences and disordered eating behaviours in adolescence: A UK population-based study. Lancet Child Adolesc. Health 2018, 2, 591–599. [Google Scholar] [CrossRef]
- Konstantakopoulos, G.; Varsou, E.; Dikeos, D.; Ioannidi, N.; Gonidakis, F.; Papadimitriou, G.; Oulis, P. Delusionality of body image beliefs in eating disorders. Psychiatry Res. 2012, 200, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Phillipou, A.; Mountjoy, R.L.; Rossell, S.L. Overvalued ideas or delusions in anorexia nervosa. Aust. N. Z. J. Psychiatry 2017, 51, 563–564. [Google Scholar] [CrossRef]
- Mountjoy, R.L.; Farhall, J.F.; Rossell, S.L. A phenomenological investigation of overvalued ideas and delusions in clinical and subclinical anorexia nervosa. Psychiatry Res. 2014, 220, 507–512. [Google Scholar] [CrossRef]
- Gadsby, S. Anorexia nervosa and oversized experiences. Philos. Psychol. 2017, 30, 594–615. [Google Scholar] [CrossRef]
- Waite, F.; Freeman, D. Body image and paranoia. Psychiatry Res. 2017, 258, 136–140. [Google Scholar] [CrossRef]
- Buonocore, M.; Bosia, M.; Riccaboni, R.; Bechi, M.; Spangaro, M.; Piantanida, M.; Cocchi, F.; Guglielmino, C.; Bianchi, L.; Smeraldi, E.; et al. Combined neurocognitive and metacognitive rehabilitation in schizophrenia: Effects on bias against disconfirmatory evidence. Eur. Psychiatry 2015, 30, 615–621. [Google Scholar] [CrossRef] [PubMed]
- González, L.E.; López-Carrilero, R.; Barrigón, M.L.; Grasa, E.; Barajas, A.; Pousa, E.; González-Higueras, F.; Ruiz-Delgado, I.; Cid, J.; Lorente-Rovira, E.; et al. Neuropsychological functioning and jumping to conclusions in recent onset psychosis patients. Schizophr. Res. 2018, 195, 366–371. [Google Scholar] [CrossRef]
- Degortes, D.; Tenconi, E.; Santonastaso, P.; Favaro, A. Executive functioning and visuospatial abilities in bulimia nervosa with or without a previous history of anorexia nervosa. Eur. Eat. Disord. Rev. 2016, 24, 139–146. [Google Scholar] [CrossRef]
- Moritz, S.; Andreou, C.; Schneider, B.C.; Wittekind, C.E.; Menonb, M.; Balzan, R.P.; Woodward, T.S. Sowing the seeds of doubt: A narrative review on metacognitive training in schizophrenia. Clin. Psychol. Rev. 2014, 34, 358–366. [Google Scholar] [CrossRef]
- Tchanturia, K.; Lounes, N.; Holttum, S. Cognitive Remediation in Anorexia Nervosa and Related Conditions: A Systematic Review. Eur. Eat. Disord. Rev. 2014, 22, 454–462. [Google Scholar] [CrossRef]

| STATEMENTS | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Jenny can’t fall asleep | ||||||||||||
| 2 | Jenny can’t wait until it is finally morning | ||||||||||||
| 3 | Jenny wonders how many presents she will find under the tree | ||||||||||||
| SENTENCES | |||||||||||||
| 1 | Jenny is excited about Christmas morning | TRUE | |||||||||||
| (Implausible) | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | (Very plausible) | |
| 2 | Jenny is nervous about her exam the next day | NEUTRAL LURE | |||||||||||
| (Implausible) | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | (Very plausible) | |
| 3 | Jenny is worried about her ill mother | EMOTIONAL LURE | |||||||||||
| (Implausible) | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | (Very plausible) | |
| 4 | Jenny loves her bed | ABSURD | |||||||||||
| (Implausible) | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | (Very plausible) | |
| Score | Formula | Meaning |
|---|---|---|
| BADE score | [(MaEL1 + MaNL1)/2] − [(MaEL3 + MaNL3)/2] | Disconfirmatory index |
| BACE score | |MaT3 − MaT1| | Confirmatory index |
| LA score | [(MaA1 + MaA2 + MaA3)/3] | Absurd index |
| Healthy Women (n = 45) | AN Patients (n = 82) | Weight-Recovered AN Patients (n = 21) | |
|---|---|---|---|
| Age | 22.8 (4.5) | 21.0 (7.1) | 21.5 (7.7) |
| Education (years) | 14.5 (2.4) * | 12.5 (2.7) | 12.1 (2.5) |
| Body mass index (kg/m2) | 20.9 (1.8) | 15.9 (1.7) $ | 20.7 (1.8) |
| Age at onset | … | 16.7 (4.6) | 15.7 (3.0) |
| Duration of illness (months) | … | 23.6 (36.1) | 28.1 (63.1) |
| Lowest BMI | 19.1 (1.8) | 14.9 (1.7) $ | 16.2 (2.1) |
| Hand lateralization (Edinburgh score) | 58.1 (47.1) | 57.6 (34.4) | 59.5 (36.3) |
| Healthy Women (n = 45) | AN Patients (n = 82) | |||||
|---|---|---|---|---|---|---|
| BADE | BACE | LA | BADE | BACE | LA | |
| Age | 0.41 ** | −0.36 * | 0.31 * | 0.05 | −0.16 | 0.23 * |
| Education (years) | 0.25 | −0.20 | 0.19 | 0.38 | −0.16 | 0.21 |
| BMI (kg/m2) | −0.20 | −0.11 | 0.29 | 0.07 | 0.01 | −0.05 |
| Age at onset (years) | … | … | … | 0.07 | −0.16 | 0.12 |
| Duration of illness (months) | … | … | … | 0.03 | 0.02 | 0.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tenconi, E.; Meregalli, V.; Buffa, A.; Collantoni, E.; Cavallaro, R.; Meneguzzo, P.; Favaro, A. Belief Inflexibility and Cognitive Biases in Anorexia Nervosa—The Role of the Bias against Disconfirmatory Evidence and Its Clinical and Neuropsychological Correlates. J. Clin. Med. 2023, 12, 1746. https://doi.org/10.3390/jcm12051746
Tenconi E, Meregalli V, Buffa A, Collantoni E, Cavallaro R, Meneguzzo P, Favaro A. Belief Inflexibility and Cognitive Biases in Anorexia Nervosa—The Role of the Bias against Disconfirmatory Evidence and Its Clinical and Neuropsychological Correlates. Journal of Clinical Medicine. 2023; 12(5):1746. https://doi.org/10.3390/jcm12051746
Chicago/Turabian StyleTenconi, Elena, Valentina Meregalli, Adriana Buffa, Enrico Collantoni, Roberto Cavallaro, Paolo Meneguzzo, and Angela Favaro. 2023. "Belief Inflexibility and Cognitive Biases in Anorexia Nervosa—The Role of the Bias against Disconfirmatory Evidence and Its Clinical and Neuropsychological Correlates" Journal of Clinical Medicine 12, no. 5: 1746. https://doi.org/10.3390/jcm12051746
APA StyleTenconi, E., Meregalli, V., Buffa, A., Collantoni, E., Cavallaro, R., Meneguzzo, P., & Favaro, A. (2023). Belief Inflexibility and Cognitive Biases in Anorexia Nervosa—The Role of the Bias against Disconfirmatory Evidence and Its Clinical and Neuropsychological Correlates. Journal of Clinical Medicine, 12(5), 1746. https://doi.org/10.3390/jcm12051746







