Impact of Evolution of Self-Expandable Aortic Valve Design: Peri-Operative and Short-Term Outcomes
Abstract
1. Introduction
2. Materials and Methods
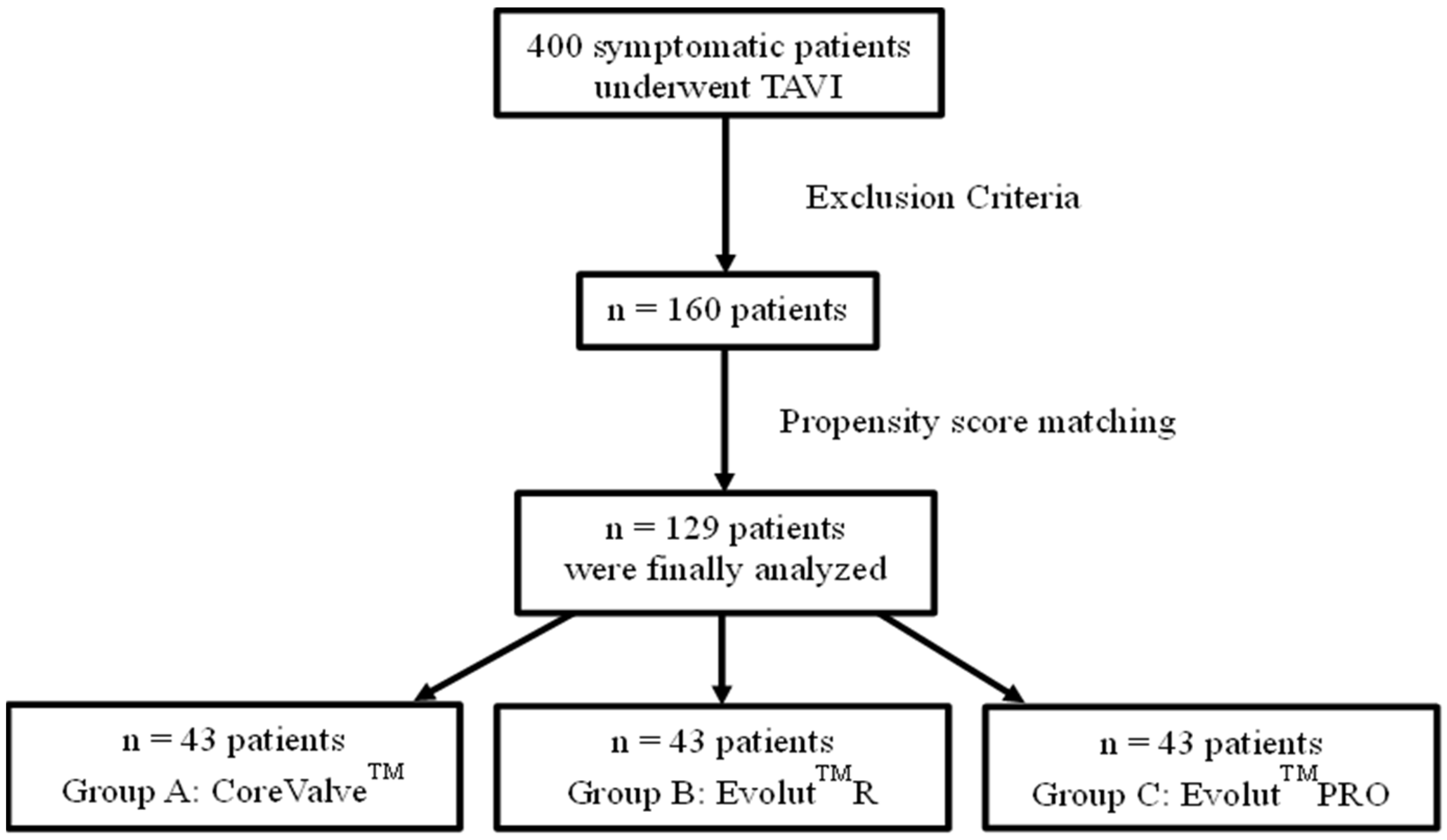
2.1. Study Design and Participants
2.2. Procedural Assessment and Patient Follow-Up
2.3. Outcomes and Extracted Data
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Peri-Procedural Outocomes
3.3. Echocardiographic Characteristics at Discharge and Follow-Up
3.4. Electrocardiographic Characteristics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vahanian, A.; Alfieri, O.; Al-Attar, N.; Antunes, M.; Bax, J.; Cormier, B.; Cribier, A.; De Jaegere, P.; Fournial, G.; Kappetein, A.P.; et al. Transcatheter Valve Implantation for Patients with Aortic Stenosis: A Position Statement from the European Association of Cardio-Thoracic Surgery (EACTS) and the European Society of Cardiology (ESC) in Collaboration with the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur. Heart J. 2008, 29, 1463–1470. [Google Scholar] [CrossRef] [PubMed]
- Leon, M.B.; Smith, C.R.; Mack, M.; Miller, D.C.; Moses, J.W.; Svensson, L.G.; Tuzcu, E.M.; Webb, J.G.; Fontana, G.P.; Makkar, R.R.; et al. Transcatheter Aortic-Valve Implantation for Aortic Stenosis in Patients Who Cannot Undergo Surgery. N. Engl. J. Med. 2010, 363, 1597–1607. [Google Scholar] [CrossRef] [PubMed]
- Voigtländer, L.; Seiffert, M. Expanding TAVI to Low and Intermediate Risk Patients. Front. Cardiovasc. Med. 2018, 5, 92. [Google Scholar] [CrossRef] [PubMed]
- Mack, M.J.; Leon, M.B.; Thourani, V.H.; Makkar, R.; Kodali, S.K.; Russo, M.; Kapadia, S.R.; Malaisrie, S.C.; Cohen, D.J.; Pibarot, P.; et al. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1695–1705. [Google Scholar] [CrossRef]
- Popma, J.J.; Deeb, G.M.; Yakubov, S.J.; Mumtaz, M.; Gada, H.; O’Hair, D.; Bajwa, T.; Heiser, J.C.; Merhi, W.; Kleiman, N.S.; et al. Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1706–1715. [Google Scholar] [CrossRef]
- Popma, J.J.; Reardon, M.J.; Khabbaz, K.; Harrison, J.K.; Hughes, G.C.; Kodali, S.; George, I.; Deeb, G.M.; Chetcuti, S.; Kipperman, R.; et al. Early Clinical Outcomes After Transcatheter Aortic Valve Replacement Using a Novel Self-Expanding Bioprosthesis in Patients with Severe Aortic Stenosis Who Are Suboptimal for Surgery. JACC Cardiovasc. Interv. 2017, 10, 268–275. [Google Scholar] [CrossRef]
- Mahtta, D.; Elgendy, I.Y.; Bavry, A.A. From Corevalve to Evolut PRO: Reviewing the Journey of Self-Expanding Transcatheter Aortic Valves. Cardiol. Ther. 2017, 6, 183–192. [Google Scholar] [CrossRef]
- Forrest, J.K.; Kaple, R.K.; Tang, G.H.L.; Yakubov, S.J.; Nazif, T.M.; Williams, M.R.; Zhang, A.; Popma, J.J.; Reardon, M.J. Three Generations of Self-Expanding Transcatheter Aortic Valves. JACC Cardiovasc. Interv. 2020, 13, 170–179. [Google Scholar] [CrossRef]
- Jilaihawi, H.; Makkar, R.R.; Kashif, M.; Okuyama, K.; Chakravarty, T.; Shiota, T.; Friede, G.; Nakamura, M.; Doctor, N.; Rafique, A.; et al. A Revised Methodology for Aortic-Valvar Complex Calcium Quantification for Transcatheter Aortic Valve Implantation. Eur. Heart J. Cardiovasc. Imaging 2014, 15, 1324–1332. [Google Scholar] [CrossRef]
- Watanabe, Y.; Morice, M.-C.; Bouvier, E.; Leong, T.; Hayashida, K.; Lefèvre, T.; Hovasse, T.; Romano, M.; Chevalier, B.; Donzeau-Gouge, P.; et al. Automated 3-Dimensional Aortic Annular Assessment by Multidetector Computed Tomography in Transcatheter Aortic Valve Implantation. JACC Cardiovasc. Interv. 2013, 6, 955–964. [Google Scholar] [CrossRef]
- VARC-3 Writing Committee; Généreux, P.; Piazza, N.; Alu, M.C.; Nazif, T.; Hahn, R.T.; Pibarot, P.; Bax, J.J.; Leipsic, J.A.; Blanke, P.; et al. Valve Academic Research Consortium 3: Updated Endpoint Definitions for Aortic Valve Clinical Research. Eur. Heart J. 2021, 42, 1825–1857. [Google Scholar] [CrossRef]
- Généreux, P.; Head, S.J.; Hahn, R.; Daneault, B.; Kodali, S.; Williams, M.R.; van Mieghem, N.M.; Alu, M.C.; Serruys, P.W.; Kappetein, A.P.; et al. Paravalvular Leak After Transcatheter Aortic Valve Replacement: The New Achilles’ Heel? A Comprehensive Review of the Literature. J. Am. Coll. Cardiol. 2013, 61, 1125–1136. [Google Scholar] [CrossRef]
- Glikson, M.; Nielsen, J.C.; Kronborg, M.B.; Michowitz, Y.; Auricchio, A.; Barbash, I.M.; Barrabés, J.A.; Boriani, G.; Braunschweig, F.; Brignole, M.; et al. 2021 ESC Guidelines on Cardiac Pacing and Cardiac Resynchronization Therapy. Eur. Heart J. 2021, 42, 3427–3520. [Google Scholar] [CrossRef]
- Vavuranakis, M.; Kariori, M.; Scott, L.; Kalogeras, K.; Siasos, G.; Vrachatis, D.; Lavda, M.; Kalantzis, C.; Vavuranakis, M.; Bei, E.; et al. Impact of “High” Implantation on Functionality of Self-Expandable Bioprosthesis During the Short- and Long-Term Outcome of Patients Who Undergo Transcatheter Aortic Valve Implantation: Is High Implantation Beneficial? Cardiovasc. Ther. 2018, 36, e12330. [Google Scholar] [CrossRef]
- Forrest, J.K.; Mangi, A.A.; Popma, J.J.; Khabbaz, K.; Reardon, M.J.; Kleiman, N.S.; Yakubov, S.J.; Watson, D.; Kodali, S.; George, I.; et al. Early Outcomes with the Evolut PRO Repositionable Self-Expanding Transcatheter Aortic Valve with Pericardial Wrap. JACC Cardiovasc. Interv. 2018, 11, 160–168. [Google Scholar] [CrossRef]
- Rodríguez-Olivares, R.; van Gils, L.; El Faquir, N.; Rahhab, Z.; Di Martino, L.F.M.; van Weenen, S.; de Vries, J.; Galema, T.W.; Geleijnse, M.L.; Budde, R.P.J.; et al. Importance of the Left Ventricular Outflow Tract in the Need for Pacemaker Implantation After Transcatheter Aortic Valve Replacement. Int. J. Cardiol. 2016, 216, 9–15. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Chang, H.-H.; Liao, T.-W.; Leu, H.-B.; Chen, I.-M.; Chen, P.-L.; Lin, S.-M. Membranous Septum Length Predicts Conduction Disturbances Following Transcatheter Aortic Valve Replacement. J. Thorac. Cardiovasc. Surg. 2022, 164, 42–51.e2. [Google Scholar] [CrossRef]
- Kalogeras, K.; Ruparelia, N.; Kabir, T.; Jabbour, R.; Naganuma, T.; Vavuranakis, M.; Nakamura, S.; Wang, B.; Sen, S.; Hadjiloizou, N.; et al. Comparison of the Self-Expanding Evolut-PRO Transcatheter Aortic Valve to Its Predecessor Evolut-R in the Real World Multicenter ATLAS Registry. Int. J. Cardiol. 2020, 310, 120–125. [Google Scholar] [CrossRef]
- Lee, J.J.; Goldschlager, N.; Mahadevan, V.S. Atrioventricular and Intraventricular Block After Transcatheter Aortic Valve Implantation. J. Interv. Card. Electrophysiol. 2018, 52, 315–322. [Google Scholar] [CrossRef]
- Lenders, G.D.; Collas, V.; Hernandez, J.M.; Legrand, V.; Danenberg, H.D.; den Heijer, P.; Rodrigus, I.E.; Paelinck, B.P.; Vrints, C.J.; Bosmans, J.M. Depth of Valve Implantation, Conduction Disturbances and Pacemaker Implantation with CoreValve and CoreValve Accutrak System for Transcatheter Aortic Valve Implantation, a Multi-Center Study. Int. J. Cardiol. 2014, 176, 771–775. [Google Scholar] [CrossRef]
- Rawish, E.; Macherey, S.; Jurczyk, D.; Pätz, T.; Jose, J.; Stiermaier, T.; Eitel, I.; Frerker, C.; Schmidt, T. Reduction of Permanent Pacemaker Implantation by Using the Cusp Overlap Technique in Transcatheter Aortic Valve Replacement: A Meta-Analysis. Clin. Res. Cardiol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Webb, J.G.; Blanke, P.; Meier, D.; Sathananthan, J.; Lauck, S.; Chatfield, A.G.; Jelisejevas, J.; Wood, D.A.; Akodad, M. TAVI in 2022: Remaining Issues and Future Direction. Arch. Cardiovasc. Dis. 2022, 115, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Khokhar, A.A.; Ponticelli, F.; Zlahoda-Huzior, A.; Chandra, K.; Ruggiero, R.; Toselli, M.; Gallo, F.; Cereda, A.; Sticchi, A.; Laricchia, A.; et al. Coronary Access Following ACURATE Neo Implantation for Transcatheter Aortic Valve-in-Valve Implantation: Ex Vivo Analysis in Patient-Specific Anatomies. Front. Cardiovasc. Med. 2022, 9, 902564. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | CoreValveTM | EvolutTMR | EvolutTMPRO | p Value |
|---|---|---|---|---|
| Patients, No | 43 | 43 | 43 | - |
| Age, years | 79.79 ± 5.54 | 81.19 ± 6.54 | 82.47 ± 5.70 | 0.06 |
| Gender (male, %) | 51 | 40 | 49 | 0.52 |
| Body Mass Index, (kg/m2) | 27.40 ± 5.61 | 27.48 ± 3.94 | 28.19 ± 4.87 | 0.17 |
| Hypertension, (%) | 86 | 86 | 93 | 0.48 |
| Diabetes mellites, (%) | 33 | 30 | 33 | 0.94 |
| Dyslipidemia, (%) | 65 | 72 | 67 | 0.78 |
| Coronary artery disease, (%) | 36 | 56 | 44 | 0.17 |
| Previous cardiac surgery, (%) | 21 | 26 | 22 | 0.72 |
| Peripheral artery disease, (%) | 42 | 37 | 23 | 0.18 |
| NYHA classification (>III), (%) | 98 | 98 | 98 | 1.00 |
| Logistic EuroSCORE, (%) | 24.72 ± 8.23 | 21.21 ± 8.76 | 25.17 ± 12.62 | 0.14 |
| Previous pacemaker implantation, (%) | 19 | 28 | 23 | 0.59 |
| Pre-existing LBBB, (%) | 8 | 7 | 16 | 0.29 |
| Pre-existing RBBB, (%) | 10 | 14 | 9 | 0.76 |
| Pre-existing 1st degree AV block, (%) | 5 | 26 | 14 | 0.03 |
| Minimum diameter of LVOT, (mm) | 18.72 ± 3.18 | 17.91 ± 2.79 | 19.02 ± 2.71 | 0.19 |
| Maximum diameter of LVOT, (mm) | 26.98 ± 3.09 | 26.90 ± 4.09 | 27.24 ± 2.87 | 0.89 |
| Minimum diameter of aortic annulus, (mm) | 19.89 ± 3.31 | 19.34 ± 2.26 | 20.33 ± 2.03 | 0.15 |
| Maximum diameter of aortic annulus, (mm) | 25.76 ± 3.24 | 25.23 ± 3.64 | 26.21 ± 2.04 | 0.33 |
| Mean diameter of aortic annulus, (mm) | 23.11 ± 2.82 | 22.31 ± 2.75 | 23.23 ± 1.84 | 0.18 |
| Minimum diameter of sinotubular junction, (mm) | 27.91 ± 3.34 | 27.01 ± 3.61 | 26.24 ± 3.59 | 0.30 |
| Maximum diameter of sinotubular junction, (mm) | 29.14 ± 3.36 | 28.83 ± 3.34 | 28.38 ± 3.70 | 0.75 |
| Maximum diameter of ascending aorta, (mm) | 34.25 ± 3.36 | 33.14 ± 3.35 | 32.22 ± 3.30 | 0.06 |
| Angulation of aorta, (degrees) | 44.60 ± 8.00 | 48.43 ± 9.75 | 46.42 ± 6.84 | 0.15 |
| Calcium Score (mm3) | 1403 (992, 1931) | 1229 (890, 1738) | 1654 (1190, 2108) | 0.073 |
| Characteristic | CoreValveTM | EvolutTMR | EvolutTMPRO | p Value |
|---|---|---|---|---|
| Balloon pre-dilatation, (%) | 79 | 21 | 16 | <0.01 |
| Balloon post-dilatation, (%) | 23 | 42 | 30 | 0.17 |
| Pre-Implantation ID from NCC, (mm) | 6.06 ± 1.11 | 5.22 ± 1.02 | 5.05 ± 1.02 | <0.01 |
| Implantation ID from NCC, (mm) | 3.63 ± 2.77 | 4.96 ± 1.90 | 4.44 ± 2.24 | 0.07 |
| Jump in NCC, (mm) | 2.88 ± 2.33 | 1.48 ± 1.09 | 1.71 ± 1.35 | 0.01 |
| ID over the annulus, (%) | 23 | 5 | 9 | 0.02 |
| Device success, (%) | 98 | 100 | 100 | 0.99 |
| PVL (none and grade 1), (%) | 67 | 58 | 60 | 0.64 |
| Characteristic | CoreValveTM | EvolutTMR | EvolutTMPRO | p Value |
|---|---|---|---|---|
| LBBB immediately after TAVI, (%) | 39 | 23 | 21 | 0.13 |
| LBBB at 1st day after TAVI, (%) | 30 | 21 | 12 | 0.13 |
| RBBB immediately after TAVI, (%) | 8 | 2 | 14 | 0.13 |
| RBBB at 1st day after TAVI, (%) | 3 | 2 | 2 | 0.98 |
| 1st AV block immediately after TAVI, (%) | 33 | 12 | 12 | 0.02 |
| 1st AV block at 1st day after TAVI, (%) | 22 | 30 | 9 | 0.05 |
| Mobitz I after TAVI, (%) | 0 | 0 | 0 | 1.00 |
| Mobitz II after TAVI, (%) | 0 | 2 | 0 | 0.38 |
| Complete AV block immediately after TAVI, (%) | 15 | 5 | 7 | 0.22 |
| Complete AV block at 1st day after TAVI, (%) | 0 | 5 | 0 | 0.16 |
| Pacemaker implantation within the first day, (%) | 33 | 19 | 7 | 0.01 |
| Pacemaker implantation from day 1 to the point of discharge, (%) | 2 | 2 | 5 | 0.77 |
| Overall pacemaker implantation, (%) | 38 | 19 | 9 | <0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bei, E.; Voudris, V.; Kalogeras, K.; Oikonomou, E.; Iakovou, I.; Kosmas, I.; Kalantzis, C.; Vavuranakis, M.-A.; Pantelidis, P.; Lazaros, G.; et al. Impact of Evolution of Self-Expandable Aortic Valve Design: Peri-Operative and Short-Term Outcomes. J. Clin. Med. 2023, 12, 1739. https://doi.org/10.3390/jcm12051739
Bei E, Voudris V, Kalogeras K, Oikonomou E, Iakovou I, Kosmas I, Kalantzis C, Vavuranakis M-A, Pantelidis P, Lazaros G, et al. Impact of Evolution of Self-Expandable Aortic Valve Design: Peri-Operative and Short-Term Outcomes. Journal of Clinical Medicine. 2023; 12(5):1739. https://doi.org/10.3390/jcm12051739
Chicago/Turabian StyleBei, Evangelia, Vasileios Voudris, Konstantinos Kalogeras, Evangelos Oikonomou, Ioannis Iakovou, Ilias Kosmas, Charalampos Kalantzis, Michael-Andrew Vavuranakis, Panteleimon Pantelidis, George Lazaros, and et al. 2023. "Impact of Evolution of Self-Expandable Aortic Valve Design: Peri-Operative and Short-Term Outcomes" Journal of Clinical Medicine 12, no. 5: 1739. https://doi.org/10.3390/jcm12051739
APA StyleBei, E., Voudris, V., Kalogeras, K., Oikonomou, E., Iakovou, I., Kosmas, I., Kalantzis, C., Vavuranakis, M.-A., Pantelidis, P., Lazaros, G., Tousoulis, D., Tsioufis, C., & Vavuranakis, M. (2023). Impact of Evolution of Self-Expandable Aortic Valve Design: Peri-Operative and Short-Term Outcomes. Journal of Clinical Medicine, 12(5), 1739. https://doi.org/10.3390/jcm12051739











