Abstract
Acne vulgaris is a common inflammatory condition that can be associated with profound psychosocial impacts. Conventional treatment includes topical retinoids, benzoyl peroxide, and antimicrobials, and some may cause irritation and skin dryness. In this 8-week open-label study, we examined the effects of a botanical skin care regimen (Codex Labs Shaant Balancing regimen) on mild to moderate facial and truncal acne. Twenty-four male and female subjects between the ages of 12 and 45 years were assessed for eligibility, 20 were enrolled, and 15 completed all study visits. Facial and truncal acne lesion counts, skin hydration, sebum excretion rate, and mood were assessed at baseline, week 4, and week 8. Total facial lesion counts (inflammatory and non-inflammatory lesions) decreased by 20.5% at week 4 (p = 0.06) and by 25.2% at week 8 (p < 0.05). Inflammatory lesion counts on the trunk were found to decrease at week 8 relative to baseline by 48% (p < 0.05). Forehead sebum excretion rate decreased by 40% at week 4 (p = 0.07) and 22% at week 8 (p = 0.08), and cheek skin hydration increased by 27.6% at week 4 (p = 0.14) and 65% at week 8 (p = 0.10). Participants also experienced significant improvement in components of a positive effect, such as feeling “strong” and “inspired”, and a decrease in negative effects, such as feeling “irritable.” Overall, the botanical skin care regimen was found to be well-tolerated. Our study suggests that a botanical skin care regimen may reduce facial and truncal acne lesion counts, increase skin hydration, reduce sebum production, and augment positive effects and moods in those with mild to moderate facial and truncal acne.
1. Introduction
Acne vulgaris is one of the most common dermatologic conditions, affecting over 85% of adolescents and young adults, and it can have major impacts on both physical and mental health [1]. The pathogenesis of acne is multifaceted; prime contributors include the colonization with Cutibacterium acnes (C. acnes), overactive sebum production, follicular hyperkeratinization, and inflammation [2]. Acne is typically present on the face, shoulders, chest, and back with inflammatory lesions such as papules and pustules, along with non-inflammatory lesions such as open or closed comedones [3,4]. As many of these lesions heal, they can leave behind post-inflammatory hyperpigmentation and scarring [5]. Acne is associated with feelings of low self-esteem, decreased confidence, depression, and anxiety, with a quality of life that is impacted negatively overall [6,7].
Common first line treatment options for acne include topical retinoids and topical antimicrobial products [8]. One of the side effects of retinoids is skin irritation [9]. Topical antimicrobials include benzoyl peroxide, clindamycin, and erythromycin [10]. Antibiotics can lead to the development of drug-resistant bacteria [11,12], and the regular use of benzoyl peroxide to reduce the development of drug-resistant bacteria may cause drying and scaling, which can lead to discomfort and irritation [13]. Given these undesirable effects, combination therapy or alternative therapies for acne that include naturally derived ingredients have garnered public interest. While there is a sentiment that botanicals and naturally derived products may not be as efficacious, there is growing evidence that botanicals and naturally derived ingredients can be efficacious in the treatment of acne [14,15,16].
One of the primary drivers for the natural skin care market is the growing awareness about the benefits of botanically derived ingredients [17]. For example, Centella asiatica has been found to offer anti-inflammatory effects in acne, while also improving skin dryness and irritation [18]. Bakuchiol is a plant-derived phytochemical that was found to have comparable effects to topical retinoids in improving wrinkles and hyperpigmentation but with less facial irritation than retinoids [19]. Extracts from biotech amplified Tetraselmis algae have well-characterized antioxidant properties [20]. Additionally, natural ingredients with absorbent properties such as bentonite may be helpful for managing excess sebum production and have demonstrated anti-inflammatory and skin regenerative effects [21]. Based on these findings, we investigated the impact of a botanical-based regimen that contained Centella asiatica, Tetraselmis chui, and bakuchiol on its effect on mild-to-moderate acne and its influence on mood.
2. Methods
2.1. Subjects
This study was conducted between March 2022 and December 2022 as an 8-week open-label study. Institutional Review Board approval was received on 5 March 2022 by Allendale, and the study was listed on clinicaltrials.gov (NCT05271487). Written informed consent was received from all participants prior to enrollment. Subjects from the greater Sacramento region were recruited. Inclusion criteria included males and females between the ages of 12 and 45 years old, with mild-to-moderate acne classified by an investigator global assessment (IGA) of 2 or 3, along with the presence of at least 10 inflammatory lesions and at least 15 total acne lesions. Subjects who had more than two nodules were excluded. Those with severe acne (IGA ≥ 4), women who were pregnant or breastfeeding, those who were current smokers or had a smoking history of >10 pack-years, those unwilling to discontinue facial products except for what is provided in the study, and those who changed their hormonal-based contraception within 3 months prior to enrollment were excluded from the study. Those who had isotretinoin use within the 3 months prior to joining the study and those who were unable to discontinue oral antibiotic, probiotic, topical antibiotics, and topical benzoyl peroxide use were also excluded from the study. Patients were advised to not seek any cosmetic treatments during the study or other medicated acne products.
2.2. Investigational Products
The skin care regimen consisted of a cleanser, oil control cream, exfoliator, toner, spot treatment, clay mask, and body scrub, commercially available as the Shaant collection (Codex Labs, Menlo Park, CA, USA). Compliance was assessed using a product log where participants were asked to make note of each time they used a product on a given day. Participant instructions for product use are outlined in Table 1.

Table 1.
Products used.
2.3. Study Visits and Procedures
Written consent and assent were obtained prior to enrollment. Subjects were asked to undergo a 2-week washout from topical antibiotics or benzoyl peroxide use or a 4-week washout for oral probiotic supplements or oral antibiotic use. The study consisted of a total of 4 visits (a screening visit, a baseline visit, a visit after 4 weeks of product use, and a visit after 8 weeks of product use).
At baseline, week 4, and week 8, facial and truncal lesion count for inflammatory and non-inflammatory lesions was performed by a trained doctor or board-certified dermatologist. During these visits, biophysical features such as stratum corneum hydration and sebum excretion rate were obtained using the MoisturemeterSC® (Delfin Technologies, Kuopio, Finland) and the Sebumeter® (Courage+Khazaka electronic GmbH, Köln, Germany), respectively. Facial photographs were also captured at baseline, week 4, and week 8 using BTBP 3D Clarity Pro® Facial Modeling and Analysis System (Brigh-Tex BioPhotonics, San Jose, CA, USA).
Mood was assessed at baseline, week 4, and week 8 using the validated Positive and Negative Affect Schedule (PANAS) questionnaire. This 20-item survey asked respondents to rate how often they felt each adjective in the prior week using the following 5-point Likert-type scale: 1 = “very slightly or not at all”, 2 = “a little”, 3 = “moderately”, 4 = “quite a bit”, and 5 = “extremely”.
At week 4 and week 8 visits, tolerability was evaluated using an 11-question survey inquiring about potential symptoms (i.e., itching, burning, stinging, scaling, redness, hypo- or hyperpigmentation) experienced with the product use. Participants were asked to rate these symptoms on a 3-point scale, with “0” indicating none, “1” as mild, “2” as moderate, and “3” as severe.
3. Results
Out of 24 eligible participants, 4 did not enroll after screening, and 20 enrolled into the study. A total of 15 completed all visits per-protocol, 2 withdrew due to inability to meet time commitment, and 3 were lost to follow up (Figure 1). The majority of participants were female (23/24), and the mean age was 24.4 ± 7.3 years. The mean IGA severity at enrollment was 2.7.
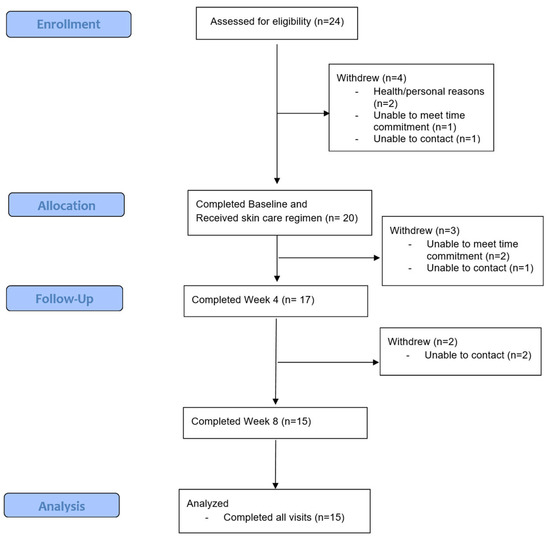
Figure 1.
CONSORT (Consolidated Standards of Reporting Trials) flow diagram.
3.1. Facial and Trunk Lesion Counts
Lesion counts improved from baseline at both week 4 and week 8 in Figure 2. For facial acne (Figure 2A), the number of non-inflammatory lesions decreased by 14.2% at week 4 (p = 0.10) and by 14.8% at week 8 (p = 0.07) relative to baseline. Inflammatory lesions decreased by 10.5% at week 4 (p = 0.44) and by 25.2% at week 8 (p = 0.16). Total lesion counts (inflammatory and non-inflammatory lesions) decreased by 20.5% at week 4 (p = 0.06) and significantly by 25.2% at week 8 (p < 0.05) relative to baseline.
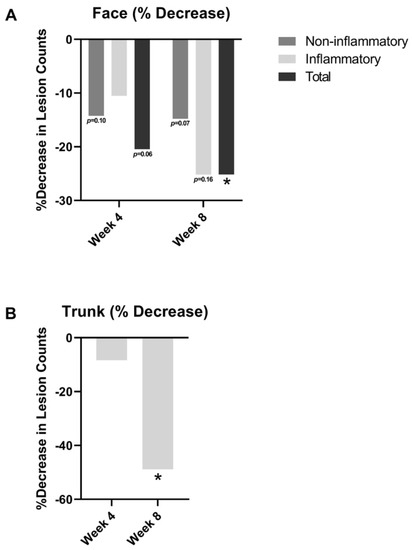
Figure 2.
(A) Facial acne lesion counts and truncal lesion counts; (B) truncal acne inflammatory lesion counts at week 4 and week 8 relative to baseline. * = p < 0.05.
With regards to truncal acne (Figure 2B), there was also an 8.4% reduction in inflammatory lesions at week 4 relative to baseline (p = 0.39) and a 48.9% reduction at week 8 (p < 0.05) relative to baseline.
Representative photo of the results is shown in Figure 3.
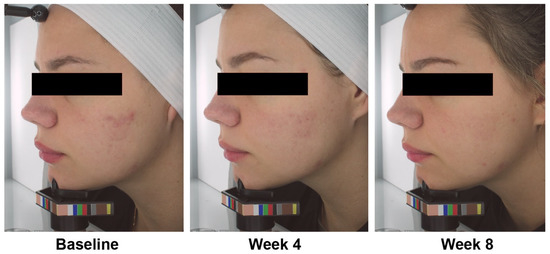
Figure 3.
Acne photographs from a representative participant at baseline, week 4, and week 8.
3.2. Sebum Excretion Rate
The sebum excretion rate was found to have a decreasing trend on the forehead at both follow-up visits relative to baseline (Figure 4). From baseline to week 4, the average percent change was nearly a 40% reduction on the forehead (p = 0.07) and nearly a 35% reduction on the cheeks (p = 0.08). At week 8, there was a decrease in sebum excretion of about 22% on the forehead (p = 0.08).
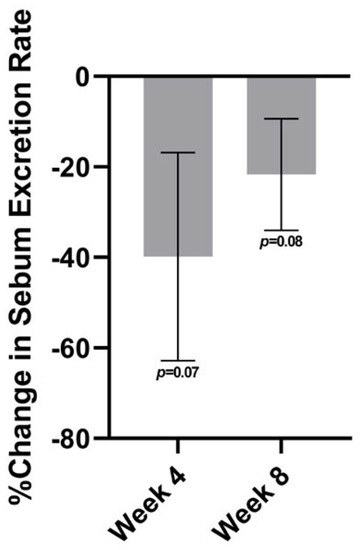
Figure 4.
The sebum excretion rate at week 4 and at week 8 relative to baseline on the forehead.
3.3. Skin Hydration
Skin hydration trended towards an increase at both follow-up visits relative to baseline (Figure 5). By week 4, there was an average increase in skin hydration of 19.1% on the forehead (p = 0.19) and an increase of 27.6% on the cheeks (p = 0.14). At week 8, a 41.1% increase in skin hydration was found on the forehead (p = 0.14), and a 65% increase was found on the cheek (p = 0.10).
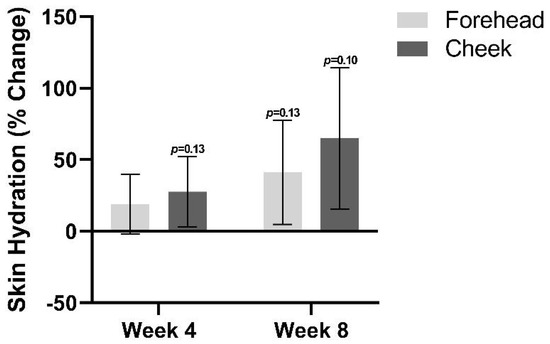
Figure 5.
Skin hydration at week 4 and week 8 relative to baseline.
3.4. Mood and Affect Score
After 8 weeks of use, the acne regimen was found to improve components of positive and negative effects (Figure 6). Overall, participants had an average 0.73 point increase in feeling “strong” (p ≤ 0.05) and a 0.53 increase in feeling “inspired” (p < 0.05). Additionally, at week 8, negative effects decreased, such as feeling “scared” was found to decrease by 0.46 (p = 0.06), while feeling “irritable” decreased by 1 point (p < 0.05).
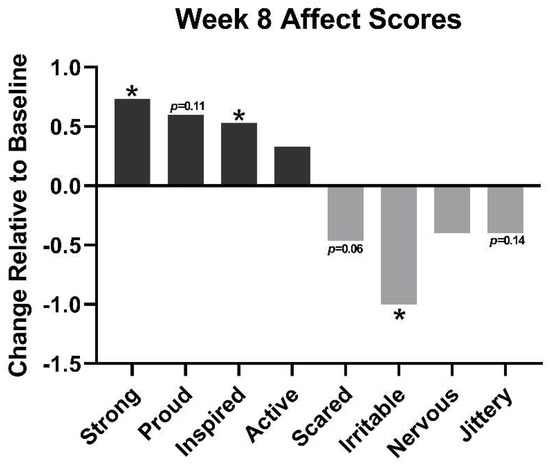
Figure 6.
Components of positive effects (strong, proud, inspired, active) and negative effects (scared, irritable, nervous, jittery) at week 8 relative to baseline. * = p < 0.05.
3.5. Tolerability
Overall, the products were found to be well-tolerated (Figure 7). On average, after 8 weeks of product use, participants rated symptoms of itching, burning, and stinging at 0.16, 0.16, and 0.25, respectively, on a 3.0 scale where 0 = no symptoms and 3 = severe. Scaling, erythema, hypopigmentation, and hyperpigmentation were almost never observed, on average, while using the product. No adverse effects were reported.
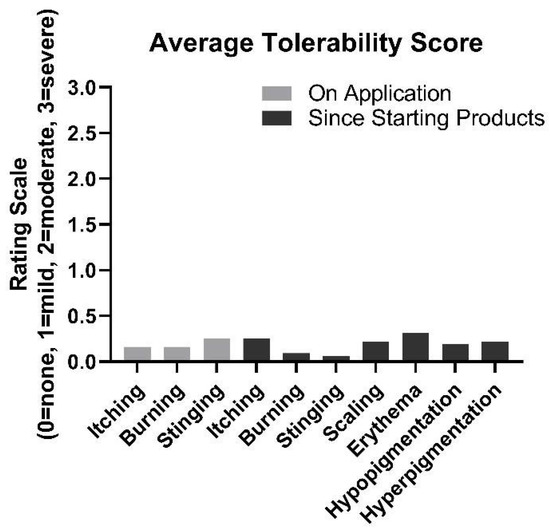
Figure 7.
Average tolerability ratings from participants at week 8.
4. Discussion
There is a growing interest in natural, plant-based products for acne, yet few studies have sought to understand the effects of botanical compounds to help prevent and treat this common skin condition. Most clinical trials have focused on individual ingredients rather than a formulation or skincare regimen. This study examined the effect of a combination of botanical products intended to target various components of acne pathophysiology such as inflammation, C. acnes colonization, oil production, and follicular hyperkeratinization.
In this open-label clinical trial, a botanical-based skin care regimen improved the lesion count in those with mild-to-moderate acne. Both non-inflammatory and inflammation improved with this regimen, showing that both the comedones and papules of acne may improve with treatment. Moreover, there was a trend towards increased skin hydration and a trend towards decreased sebum excretion rate, which suggests that these products may help to control acne for oily skin types without drying the skin. This trend contrasts that of benzoyl peroxide, which generates free radicals in the skin and may damage the cutaneous barrier [22]. A study found that benzoyl peroxide-induced oxidative stress was attenuated when mice were pre-treated with spearmint extracted from the Mentha spicata plant [23]. This suggests that plant-based ingredients, especially those with antioxidant and anti-inflammatory properties, may help to counteract unwanted side effects associated with mainstay treatment options for acne. Although benzoyl peroxide was not tested in this particular regimen, it would be interesting to see if this regimen may allow for benzoyl peroxide to be used along with this regimen.
Many plant-based ingredients have been shown to synergistically target multiple pathways contributing to acne development and help explain the improvement in both the inflammatory and non-inflammatory lesions noted in this study. Tannins, such as those found in Hamamelis virginiana (or witch hazel), which is in the study toner product, act as an astringent with anti-inflammatory properties and have been shown to inhibit C. acnes-induced inflammation [24]. In addition, bakuchiol, derived from the leaves and seeds of the Psoralea corylifolia plant, is found in the balancing oil control cream and targets the inflammatory pathways in acne while also reducing skin discoloration [25]. For example, one study found that a cream containing 0.5% bakuchiol reduced the number of inflammatory lesions while improving existing post-inflammatory hyperpigmentation in a cohort of subjects with Fitzpatrick skin types III-VI with mild-to-moderate acne [25]. Because bakuchiol may have retinoid-like properties [26], it may target follicular hyperkeratinization as well. Bakuchiol is typically well tolerated at doses of 1%, as used in the botanical regimen studied here. The botanical regimen also utilizes Tetraselmis chui, which is an algae that has been reported to reduce sebum production on the face [27].
Another important aspect of the study regimen and study results worth highlighting is the Shaant Balancing Body Scrub, which was applied to the back, chest, and shoulders. Studies suggest that, while approximately 50% of facial acne patients also experience truncal acne, the trunk region tends to be overlooked in clinical trials [28,29]. The standard treatment option for truncal acne has been topical benzoyl peroxide, but there may be concern around the risk of bleaching clothes and the tendency toward skin irritation [29,30]. The results here suggest another option for those that are seeking treatment for truncal acne without an oral or systemic treatment.
With regards to the spot treatment, one of the main ingredients is sulfur. This can sometimes be found in combination with sodium sulfacetamide or may also be used alone. Mainly used in formulations for its anti-inflammatory benefits, research has shown that a sodium sulfacetamide/sulfur emollient foam can markedly reduce the C. acnes colony count in vitro [31].
Additionally, an ingredient commonly found in all of the products is patchouli extract from the Pogostemon cablin plant [32]. Aside from its pleasant smell, patchouli has many therapeutic potentials. Commonly used in aromatherapy, patchouli has been shown to exert antidepressant-like activity by decreasing cortisol and increasing dopamine and serotonin, and it may be a potential contributing mechanism by which positive and negative effects and mood were improved in this study [33]. The effect of aromatherapy on mood also points to another potential advantage of incorporating botanical ingredients in topical formulations. Given that acne is associated with feelings of low self-esteem, embarrassment, and limitations on daily activities of living and social interactions, more research should be conducted to understand how the scent offered by botanicals in topical products may impact the skin–brain axis [33,34].
Of note, this was an open-label study and there was no comparator group. A placebo control is not possible with a regimen as utilized here. While there was no way to assess their individual effects, their various effects have potential to be synergistic. This allows for targeting multiple pathways of a condition simultaneously that may include the skin directly as well as the mind–body connection. However, study results warrant future studies that may utilize a head-to-head design against other regimens/therapies or as an adjuvant to pharmaceutical therapies.
There are several limitations to this study. This study was a pilot study, and the results found here should be investigated with a larger sample size. The study duration was 8 weeks, which allowed us to assess early changes in acne; however, future studies could utilize the results here to justify a longer trial of 12 weeks or longer. Since the products were utilized as a regimen, we can only comment on the effect of the regimen as a whole and not the individual products’ effects. The majority of participants were female. Nevertheless, the efficacy results warrant further study with expansion of the study population to include more males. While this study did not assess the prevalence of depression or anxiety, we were still able to measure statistically significant shifts in mood based on positive and negative effect scores over study duration.
5. Conclusions
The use of a botanical skin care regimen (Codex Labs Shaant Balancing regimen) may help to improve the lesion count on the face and trunk in individuals with mild-to-moderate acne, while also improving various aspects of mood. Additional factors such as skin hydration and sebum production may also improve or stabilize during treatment. Further research with larger sample sizes is needed to better understand the benefits and mechanisms of botanical skincare regimens in the prevention and treatment of mild-to-moderate acne.
Author Contributions
Conceptualization, R.K.S. and J.M.; methodology, J.M. and R.K.S.; formal analysis, Y.N., W.B. and R.K.S.; investigation, Y.N., N.G. and J.M.; resources, R.K.S.; data curation, Y.N., N.G. and J.M.; writing—original draft preparation, Y.N., N.G., J.R. and J.M.; writing—review and editing, R.K.S. and J.M.; visualization, W.B., Y.N., R.K.S. and J.M.; supervision, R.K.S. and J.M.; project administration, Y.N., N.G., R.K.S. and J.M.; funding acquisition, R.K.S. All authors have read and agreed to the published version of the manuscript.
Funding
Codex Labs Corp. The source of funding had no role in design of the study, drafting of the manuscript, or the decision to submit for publication.
Institutional Review Board Statement
The Allendale IRB reviewed this protocol (CB_Acne_01) on 5 March 2022. All participants provided written consent or assent in the cases of minors with written consent from their parent/guardian.
Informed Consent Statement
Written informed consent and assent where applicable were obtained from all subjects involved in the study.
Data Availability Statement
No publicly archived datasets.
Conflicts of Interest
J.M. serves as a consultant to Codex Labs Corp. R.K.S. serves as a scientific advisor for LearnHealth, Codex Labs Corp, and Arbonne and as a consultant to Burt’s Bees, Novozymes, Nutrafol, Incyte, Fotona, Biogena, Bristol Myer Squibb, Novartis, Element Apothec, Abbvie, Leo, UCB, Sun, Sanofi, Regeneron Pharmaceuticals.
References
- Bhate, K.; Williams, H.C. Epidemiology of acne vulgaris. Br. J. Dermatol. 2013, 168, 474–485. [Google Scholar] [CrossRef] [PubMed]
- Zaenglein, A.L.; Pathy, A.L.; Schlosser, B.J.; Alikhan, A.; Baldwin, H.E.; Berson, D.S.; Bowe, W.P.; Graber, E.M.; Harper, J.C.; Kang, S.; et al. Guidelines of care for the management of acne vulgaris. J. Am. Acad. Dermatol. 2016, 74, 945–973.e933. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.U.; Schlosser, B.J.; Paller, A.S. A review of diagnosis and treatment of acne in adult female patients. Int. J. Womens Dermatol. 2018, 4, 56–71. [Google Scholar] [CrossRef] [PubMed]
- Masterson, K.N. Acne Basics: Pathophysiology, Assessment, and Standard Treatment Options. J. Dermatol. Nurses Assoc. 2018, 10, S2–S10. [Google Scholar] [CrossRef]
- Connolly, D.; Vu, H.L.; Mariwalla, K.; Saedi, N. Acne Scarring-Pathogenesis, Evaluation, and Treatment Options. J. Clin. Aesthet. Dermatol. 2017, 10, 12–23. [Google Scholar]
- Gorelick, J.; Daniels, S.R.; Kawata, A.K.; Degboe, A.; Wilcox, T.K.; Burk, C.T.; Douse-Dean, T. Acne-Related Quality of Life among Female Adults of Different Races/Ethnicities. J. Dermatol. Nurses Assoc. 2015, 7, 154–162. [Google Scholar] [CrossRef]
- Sood, S.; Jafferany, M.; Vinaya Kumar, S. Depression, psychiatric comorbidities, and psychosocial implications associated with acne vulgaris. J. Cosmet. Dermatol. 2020, 19, 3177–3182. [Google Scholar] [CrossRef]
- Hauk, L. Acne Vulgaris: Treatment Guidelines from the AAD. Am. Fam. Physician. 2017, 95, 740–741. [Google Scholar]
- Thielitz, A.; Abdel-Naser, M.B.; Fluhr, J.W.; Zouboulis, C.C.; Gollnick, H. Topical retinoids in acne--an evidence-based overview. J. Dtsch. Dermatol. Ges. 2008, 6, 1023–1031. [Google Scholar] [CrossRef]
- Kraft, J.; Freiman, A. Management of acne. CMAJ 2011, 183, E430–E435. [Google Scholar] [CrossRef]
- Cunliffe, W.J.; Holland, K.T.; Bojar, R.; Levy, S.F. A randomized, double-blind comparison of a clindamycin phosphate/benzoyl peroxide gel formulation and a matching clindamycin gel with respect to microbiologic activity and clinical efficacy in the topical treatment of acne vulgaris. Clin. Ther. 2002, 24, 1117–1133. [Google Scholar] [CrossRef]
- Simonart, T.; Dramaix, M. Treatment of acne with topical antibiotics: Lessons from clinical studies. Br. J. Dermatol. 2005, 153, 395–403. [Google Scholar] [CrossRef]
- Sevimli Dikicier, B. Topical treatment of acne vulgaris: Efficiency, side effects, and adherence rate. J. Int. Med. Res. 2019, 47, 2987–2992. [Google Scholar] [CrossRef]
- Fisk, W.A.; Lev-Tov, H.A.; Sivamani, R.K. Botanical and phytochemical therapy of acne: A systematic review. Phytother. Res. 2014, 28, 1137–1152. [Google Scholar] [CrossRef] [PubMed]
- Chilicka, K.; Rusztowicz, M.; Rogowska, A.M.; Szygula, R.; Asanova, B.; Nowicka, D. Efficacy of Hydrogen Purification and Cosmetic Acids in the Treatment of Acne Vulgaris: A Preliminary Report. J. Clin. Med. 2022, 11, 6269. [Google Scholar] [CrossRef]
- Chilicka, K.; Rogowska, A.M.; Szygula, R.; Taradaj, J. Examining Quality of Life After Treatment with Azelaic and Pyruvic Acid Peels in Women with Acne Vulgaris. Clin. Cosmet. Investig. Dermatol. 2020, 13, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Natural Skin Care Products Market Size, Share & Trends Analysis Report By Type (Mass, Premium), By Product (Facial Care, Body Care), By End-Use (Men, Women), By Distribution Channel, By Region, And Segment Forecasts, 2022–2030. Available online: https://www.grandviewresearch.com/industry-analysis/natural-skin-care-products-market (accessed on 3 February 2023).
- Park, K.S. Pharmacological Effects of Centella asiatica on Skin Diseases: Evidence and Possible Mechanisms. Evid. Based Complement. Alternat. Med. 2021, 2021, 5462633. [Google Scholar] [CrossRef] [PubMed]
- Dhaliwal, S.; Rybak, I.; Ellis, S.R.; Notay, M.; Trivedi, M.; Burney, W.; Vaughn, A.R.; Nguyen, M.; Reiter, P.; Bosanac, S.; et al. Prospective, randomized, double-blind assessment of topical bakuchiol and retinol for facial photoageing. Br. J. Dermatol. 2019, 180, 289–296. [Google Scholar] [CrossRef]
- Jo, W.S.; Yang, K.M.; Park, H.S.; Kim, G.Y.; Nam, B.H.; Jeong, M.H.; Choi, Y.J. Effect of Microalgal Extracts of Tetraselmis suecica against UVB-Induced Photoaging in Human Skin Fibroblasts. Toxicol. Res. 2012, 28, 241–248. [Google Scholar] [CrossRef]
- Lee, J.Y.; Suh, H.N.; Choi, K.Y.; Song, C.W.; Hwang, J.H. Regenerative and anti-inflammatory effect of a novel bentonite complex on burn wounds. Vet. Med. Sci. 2022, 8, 2422–2433. [Google Scholar] [CrossRef]
- Slaga, T.J.; Klein-Szanto, A.J.P.; Triplett, L.L.; Yotti, L.P. Skin Tumor-Promoting Activity of Benzoyl Peroxide, a Widely Used Free Radical-Generating Compound. Science 1981, 213, 1023–1025. [Google Scholar] [CrossRef]
- Saleem, M.; Alam, A.; Sultana, S. Attenuation of benzoyl peroxide-mediated cutaneous oxidative stress and hyperproliferative response by the prophylactic treatment of mice with spearmint (Mentha spicata). Food Chem. Toxicol. 2000, 38, 939–948. [Google Scholar] [CrossRef] [PubMed]
- Piazza, S.; Martinelli, G.; Vrhovsek, U.; Masuero, D.; Fumagalli, M.; Magnavacca, A.; Pozzoli, C.; Canilli, L.; Terno, M.; Angarano, M.; et al. Anti-Inflammatory and Anti-Acne Effects of Hamamelis virginiana Bark in Human Keratinocytes. Antioxidants 2022, 11, 1119. [Google Scholar] [CrossRef] [PubMed]
- Brownell, L.; Geen, S.; Lee, W.L. A Clinical Study Evaluating the Efficacy of Topical Bakuchiol (UP256) Cream on Facial Acne. J. Drugs. Dermatol. 2021, 20, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, R.K.; Bojanowski, K. Bakuchiol: A retinol-like functional compound revealed by gene expression profiling and clinically proven to have anti-aging effects. Int. J. Cosmet. Sci. 2014, 36, 221–230. [Google Scholar] [CrossRef]
- Herrmann, M.; Gaebler, S.; Stuhlmann, D.; Welsoh, A.-C.; Meyer, I. Tetraselmis Extract. February 27 2019. Available online: https://patents.google.com/patent/WO2019166520A1/fr (accessed on 3 February 2023).
- Del Rosso, J.Q.; Bikowski, J.B.; Baum, E.; Smith, J.; Hawkes, S.; Benes, V.; Bhatia, N. A closer look at truncal acne vulgaris: Prevalence, severity, and clinical significance. J. Drugs Dermatol. 2007, 6, 597–600. [Google Scholar] [PubMed]
- Tan, J.K.L.; Dirschka, T. A New Era for Truncal Acne: Emerging from a Legacy of Neglect. Dermatol. Ther. 2021, 11, 665–668. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhang, Y.; Lazic Mosler, E.; Hu, J.; Li, H.; Zhang, Y.; Liu, J.; Zhang, Q. Topical benzoyl peroxide for acne. Cochrane. Database. Syst. Rev. 2020, 3, Cd011154. [Google Scholar] [CrossRef]
- Del Rosso, J.Q. The use of sodium sulfacetamide 10%-sulfur 5% emollient foam in the treatment of acne vulgaris. J. Clin. Aesthet. Dermatol. 2009, 2, 26–29. [Google Scholar]
- Muhammad, S.; H.P.S., A.K.; Abd Hamid, S.; Danish, M.; Marwan, M.; Yunardi, Y.; Abdullah, C.K.; Faisal, M.; Yahya, E.B. Characterization of Bioactive Compounds from Patchouli Extracted via Supercritical Carbon Dioxide (SC-CO2) Extraction. Molecules 2022, 27, 6025. [Google Scholar] [CrossRef]
- Astuti, P.; Khairan, K.; Marthoenis, M.; Hasballah, K. Antidepressant-like Activity of Patchouli Oil var. Tapak Tuan (Pogostemon cablin Benth) via Elevated Dopamine Level: A Study Using Rat Model. Pharmaceuticals 2022, 15, 608. [Google Scholar] [CrossRef] [PubMed]
- Hazarika, N.; Archana, M. The Psychosocial Impact of Acne Vulgaris. Indian. J. Dermatol. 2016, 61, 515–520. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).