Graphene-Doped Polymethyl Methacrylate (PMMA) as a New Restorative Material in Implant-Prosthetics: In Vitro Analysis of Resistance to Mechanical Fatigue
Abstract
:1. Introduction
2. Materials and Methods
3. Results
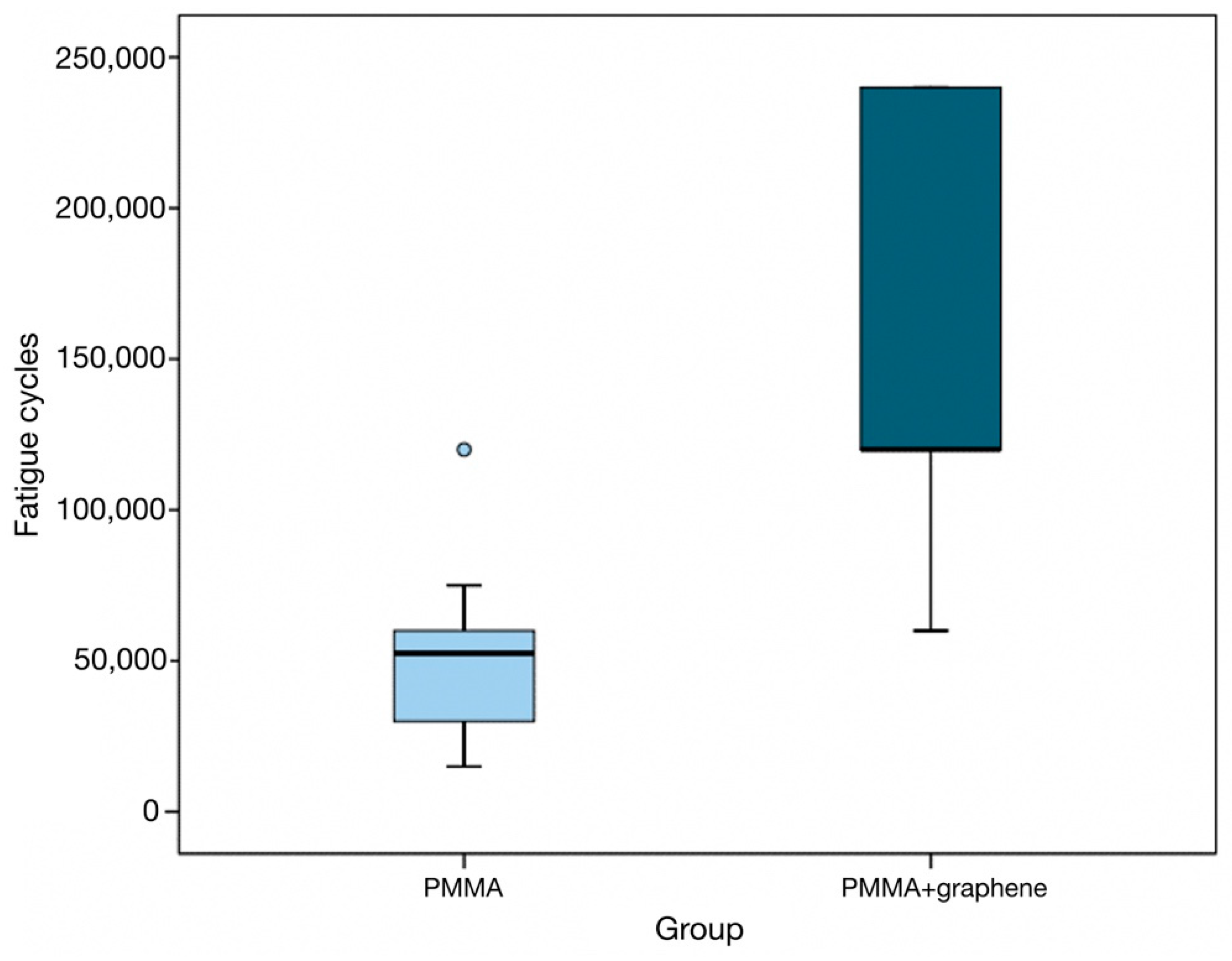
- In this regard, the specimens in the PMMA group recorded a median of 52,500 load applications (interquartile range [IQR]: 30,000–60,000) versus 120,000 (IQR: 120,000–240,000) in the PMMA-G group (Table 3).
| GROUP | |||
|---|---|---|---|
| Total | PMMA | PMMA + Graphene | |
| N | 44 | 22 | 22 |
| Mean | 103,295.5 | 51,136.4 | 155,454.5 |
| Standard deviation | 72,928.3 | 22,516.2 | 68,433.3 |
| Minimum | 15,000.0 | 15,000.0 | 60,000.0 |
| Maximum | 240,000.0 | 120,000.0 | 240,000.0 |
| Percentile 25 | 52,500.0 | 30,000.0 | 120,000.0 |
| Median | 60,000.0 | 52,500.0 | 120,000.0 |
| Percentile 75 | 120,000.0 | 60,000.0 | 240,000.0 |
- In descriptive terms, the difference between the two groups is significant, and the box plots show the number of cycles to be significantly greater in the graphene-doped specimen group (p < 0.001) (Figure 2).
- Likewise, the number of specimens that exceeded 120,000 load applications without fracture was significantly greater in the PMMA-G group than in the PMMA group (p < 0.001), and the corresponding fracture rate was significantly lower (p = 0.009).
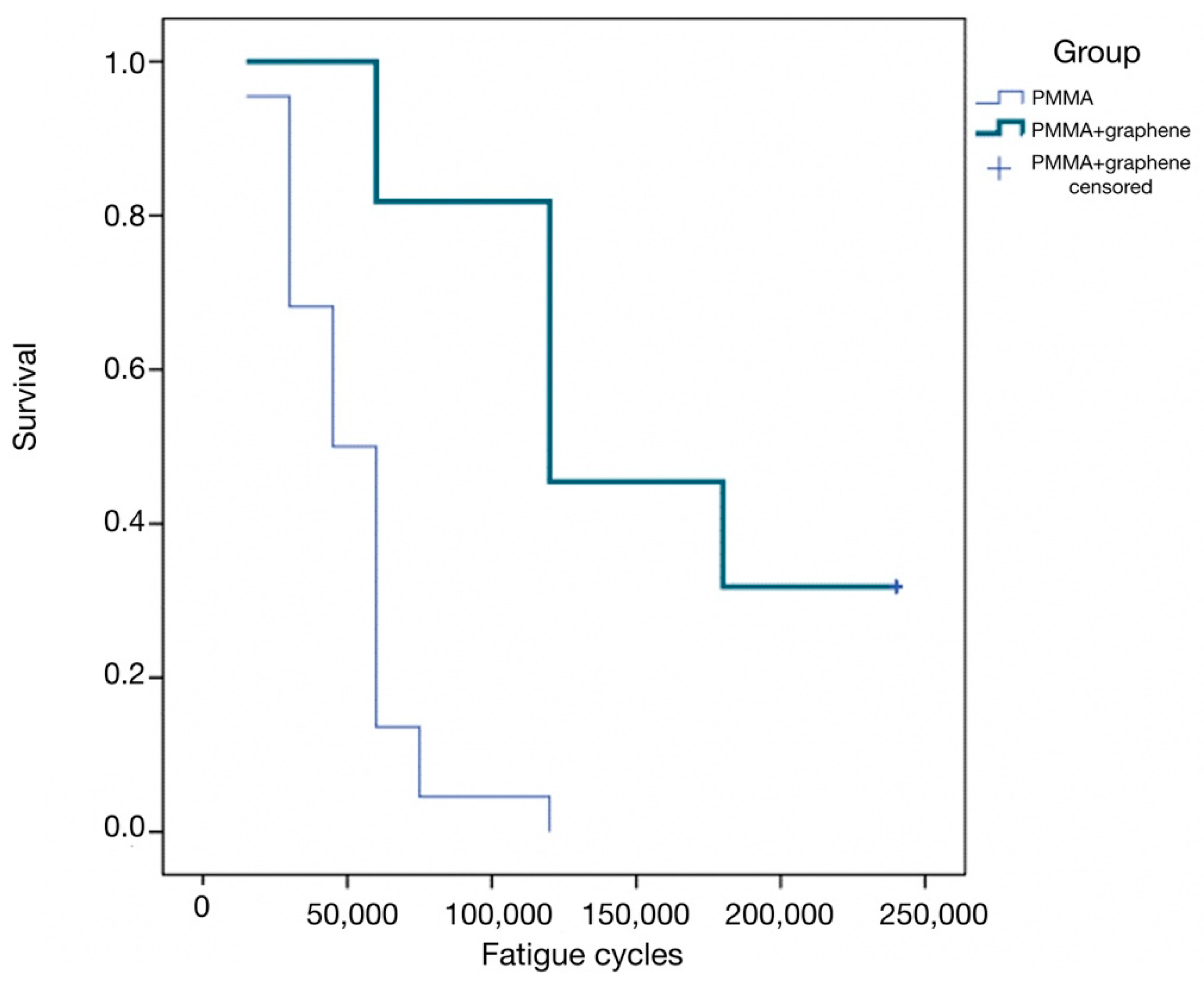
- Lastly, the cumulative survival curves corresponding to the number of cycles up to fracture showed significant differences between the two groups (p < 0.001, log-rank test). Specifically, in the PMMA group, the median survival was 45,000 cycles (95% confidence interval [95%CI]: 33,508–56,491), versus 120,000 in the PMMA-G group (95%CI: 70,063–169,937) (Figure 3). This survival curve simulates a clinical situation, because the aim of the study was to analyze the resistance of this material in a fatigue load test. The great dispersion in the resistance values highlights the unpredictability of the behavior of the material, as it fractures at very different values. However, PMMA-G has, on average, better fracture resistance values.
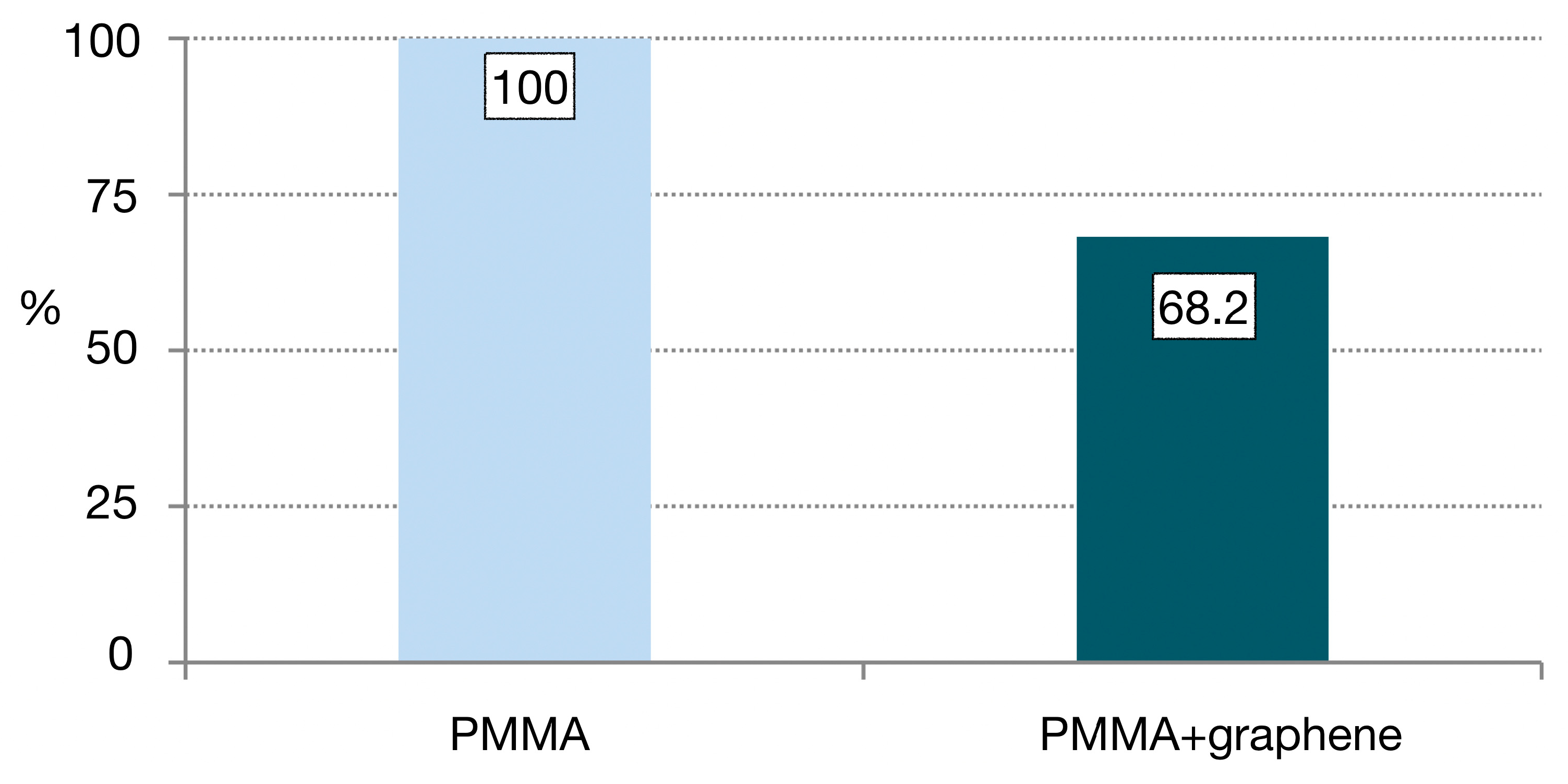
- Most fractures occurred in both groups in the area of the junction with the titanium abutment, where the thickness of the PMMA was less than 2 mm (Figure 4 and Figure 5). The fatigue study was carried out either until the fracture of the material (which occurred in 100% of the PMMA samples) or up to 240,000 cycles, and 68.2% of the PMMA samples doped with graphene were fractured.

4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Romanos:, G.E.; Gaertner, K.; Nentwig, G.H. Long-term evaluation of immediately loaded implants in the edentulous mandible using fixed bridges and platform shifting. Clin. Implant. Dent. Relat. Res. 2014, 16, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.; Ishiura, Y.; Tanaka, S.; Baba, K. Influence of the rigidity of a provisional restoration supported on four immediately loaded implants in the edentulous maxilla on biomechanical bone-implant interactions under simulated bruxism conditions: A three-dimensional finite element analysis. Int. J. Prosthodont. 2014, 27, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Montero, J.; Guadilla, Y.; Flores, J.; Pardal-Peláez, B.; Quispe-López, N.; Gómez-Polo, C.; Dib, A. Patient-Centered Treatment Outcomes with Full-Arch PEEK Rehabilitation Supported on Four Immediate or Conventionally Loaded Implants. A Randomized Clinical Trial. J. Clin. Med. 2021, 10, 4589. [Google Scholar] [CrossRef] [PubMed]
- Morton, D.; Jaffin, R.; Weber, H.-P. Immediate restoration and loading of dental implants: Clinical considerations and protocols. Int. J. Oral Maxillofac. Implants. 2004, 19, 103–108. [Google Scholar]
- Ostman, P.-O.; Hellman, M.; Sennerby, L. Direct implant loading in the edentulous maxilla using a bone density-adapted surgical protocol and primary implant stability criteria for inclusion. Clin. Implant Dent. Relat. Res. 2005, 7 (Suppl. 1), S60–S69. [Google Scholar] [CrossRef]
- Drago, C.J.; del Castillo, R.A. Treatment of edentulous and partially edentulous patients with CAD/CAM frameworks: A pilot case study. Pract. Proced. Aesthet. Dent. 2006, 18, 665–671. [Google Scholar]
- Raszewski, Z. Acrylic resins in the CAD/CAM technology: A systematic literature review. Dent. Med. Probl. 2020, 57, 449–454. [Google Scholar] [CrossRef]
- Srinivasan, M.; Gjengedal, H.; Cattani-Lorente, M.; Moussa, M.; Durual, S.; Schimmel, M.; Müller, F. CAD/CAM milled complete removable dental prostheses: An in vitro evaluation of biocompatibility, mechanical properties, and surface roughness. Dent. Mater. J. 2018, 37, 526–533. [Google Scholar] [CrossRef] [PubMed]
- Drago, C. Ratios of Cantilever Lengths and Anterior-Posterior Spreads of Definitive Hybrid Full-Arch, Screw-Retained Prostheses: Results of a Clinical Study. J. Prosthodont. 2018, 27, 402–408. [Google Scholar] [CrossRef]
- Li, B.B.; Xu, J.B.; Cui, H.Y.; Lin, Y.; Di, P. In vitro evaluation of the flexural properties of All-on-Four provisional fixed denture base resin partially reinforced with fibers. Dent. Mater. J. 2016, 35, 264–269. [Google Scholar] [CrossRef]
- Paes-Junior, T.A.; Tribst, J.P.; Dal Piva, A.O.; Amaral, M.; Borges, A.L.; Gonçalves, F.D. Stress distribution of complete-arch implant-supported prostheses reinforced with silica-nylon mesh. J Clin Exp Dent. 2019, 11, e1163–e1169. [Google Scholar] [CrossRef]
- Collaert, B.; De Bruyn, H. Immediate functional loading of TiOblast dental implants in full-arch edentulous maxillae: A 3-year prospective study. Clin. Oral Implants Res. 2008, 19, 1254–1260. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, F.C.P.; Amaral, M.; Borges, A.L.S.; Gonçalves, L.F.M.; Paes-Junior, T.J.A. Fracture load of complete-arch implant-supported prostheses reinforced with nylon-silica mesh: An in vitro study. J. Prosthet. Dent. 2018, 119, 606–610. [Google Scholar] [CrossRef]
- Radunovic, M.; Pavic, A.; Ivanovic, V.; Milivojevic, M.; Radovic, I.; Di Carlo, R.; Pilato, S.; Fontana, A.; Piattelli, A.; Petrovic, S. Biocompatibility and antibiofilm activity of graphene-oxide functionalized titanium discs and collagen membranes. Dent. Mater. 2022, 38, 1117–1127. [Google Scholar] [CrossRef]
- Ionescu, A.C.; Brambilla, E.; Pires, P.M.; López-Castellano, A.; Alambiaga-Caravaca, A.M.; Lenardi, C.; Sauro, S. Physical-chemical and microbiological performances of graphene-doped PMMA for CAD/CAM applications before and after accelerated aging protocols. Dent. Mater. 2022, 38, 1470–1481. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Jo, J.K.; Kim, D.A.; Patel, K.D.; Kim, H.W.; Lee, H.H. Nano-graphene oxide incorporated into PMMA resin to prevent microbial adhesion. Dent. Mater. 2018, 34, e63–e72. [Google Scholar] [CrossRef] [PubMed]
- Ciocan, L.T.; Ghitman, J.; Vasilescu, V.G.; Iovu, H. Mechanical Properties of Polymer-Based Blanks for Machined Dental Restorations. Materials 2021, 14, 7293. [Google Scholar] [CrossRef]
- Lorusso, F.; Inchingolo, F.; Lucchina, A.G.; Scogna, G.; Scarano, A. Graphene-doped Poly(methyl-methacrylate) as an enhanced biopolymer for medical device and dental implant. J. Biol. Regul. Homeost. Agents 2021, 35, 195–204. [Google Scholar] [CrossRef]
- Guzmán, P.C.; Freitas, F.F.A.; Ferreira, P.M.; de Freitas, C.A.; Reis, K.R. Influence of different cantilever extensions and glass or polyaramide reinforcement fibers on fracture strength of implant-supported temporary. J. Appl. Oral Sci. 2008, 16, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, B.; Alp, G.; Seidt, J.; Johnston, W.M.; Vitter, R.; McGlumphy, E.A. Fracture analysis of CAD-CAM high-density polymers used for interim implant-supported fixed, cantilevered prostheses. J. Prosthet. Dent. 2018, 120, 79–84. [Google Scholar] [CrossRef]
- Angelara, K.; Bratos, M.; Sorensen, J.A. Comparison of strength of milled and conventionally processed PMMA complete-arch implant-supported immediate interim fixed dental prostheses. J. Prosthet. Dent. 2021, 19, S0022-3913. [Google Scholar] [CrossRef]
- Jacques, L.B.; Moura, M.S.; Suedam, V.; Souza, E.A.; Rubo, J.H. Effect of cantilever length and framework alloy on the stress distribution of mandibular-cantilevered implant-supported prostheses. Clin. Oral. Implants Res. 2009, 20, 737–741. [Google Scholar] [CrossRef]
- Romanos, G.E.; Gupta, B.; Eckert, S.E. Distal cantilevers and implant dentistry. Int. J. Oral Maxillofac. Implants. 2012, 27, 1131–1136. [Google Scholar] [PubMed]
- Suarez-Feito, J.-M.; Sicilia, A.; Angulo, J.; Banerji, S.; Cuesta, I.; Millar, B. Clinical performance of provisional screw-retained metal-free acrylic restorations in an immediate loading implant protocol: A 242 consecutive patients’ report. Clin. Oral Implants. Res. 2010, 21, 1360–1369. [Google Scholar] [CrossRef]
- Jemt, T. Failures and complications in 391 consecutively inserted fixed prostheses supported by Brånemark implants in edentulous jaws: A study of treatment from the time of prosthesis placement to the first annual checkup. Int. J. Oral Maxillofac. Implants. 1991, 6, 270–276. [Google Scholar]
- Albosefi, A.; Finkelman, M.; Zandparsa, R. An in Vitro Comparison of Fracture Load of Zirconia Custom Abutments with Internal Connection and Different Angulations and Thickness: Part I. J. Prosthodont. 2014, 23, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Rosentritt, M.; Siavikis, G.; Behr, M.; Kolbeck, C.; Handel, G. Approach for valuating the significance of laboratory simulation. J. Dent. 2008, 36, 1048–1053. [Google Scholar] [CrossRef]
- Shen, H.; Di, P.; Luo, J.; Lin, Y. Clinical assessment of implant-supported full-arch immediate prostheses over 6 months of function. Clin. Implant Dent. Relat. Res. 2019, 21, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Drago, C.; del Castillo, R.; Peterson, T. Immediate occlusal loading in edentulous jaws, CT-guided surgery and fixed provisional prosthesis: A maxillary arch clinical report. J. Prosthodont. 2011, 20, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Sartori, I.A.M.; Alcântara, P.R.; Vieira, R.A.; Suzuki, D.; Gasparini Kiatake Fontão, F.; Rodrigo Tiossi, R. Implant stability measurements of two immediate loading protocols for the edentulous mandible: Rigid and semi-rigid splinting of the implants. Implant Dent. 2012, 21, 486–490. [Google Scholar] [CrossRef]
- Del Fabbro, M.; Testori, T.; Francetti, L.; Taschieri, S.; Weinstein, R. Systematic review of survival rates for immediately loaded dental implants. Int. J. Periodontics Restor. Dent. 2006, 26, 249–263. [Google Scholar]
- Misch, C.M. Immediate loading of definitive implants in the edentulous mandible using a fixed provisional prosthesis: The denture conversion technique. J. Oral Maxillofac. Surg. 2004, 62, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Maló, P.; Rangert, B.; Nobre, M. All-on-4 immediate-function concept with Brånemark System implants for completely edentulous maxillae: A 1-year retrospective clinical study. Clin. Implant Dent. Relat. Res. 2005, 1, S88–S94. [Google Scholar] [CrossRef]
- Pitta, J.; Burkhardt, F.; Mekki, M.; Fehmer, V.; Mojon, P.; Sailer, I. Effect of airborne-particle abrasion of a titanium base abutment on the stability of the bonded interface and retention forces of crowns after artificial aging. J. Prosthet. Dent. 2021, 126, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Steiner, M.; Mitsias, M.E.; Ludwig, K.; Kern, M. In vitro evaluation of a mechanical testing chewing simulator. Dent. Mater. 2009, 25, 494–499. [Google Scholar] [CrossRef] [PubMed]
- Soriano-Valero, S.; Román-Rodriguez, J.L.; Agustín-Panadero, R.; Bellot-Arcís, C.; Fons-Font, A.; Fernández-Estevan, L. Systematic review of chewing simulators: Reality and reproducibility of in vitro studies. J. Clin. Exp. Dent. 2020, 12, e1189–e1195. [Google Scholar] [CrossRef]
- Zurdo, J.; Romao, C.; Wennström, J.L. Survival and complication rates of implant-supported fixed partial dentures with cantilevers: A systematic review. Clin. Oral Impl. Res. 2009, 20, 59–66. [Google Scholar] [CrossRef] [PubMed]

| Phototopic | Binding Energy (eV) | Binding Type | Percentage (% at) |
|---|---|---|---|
| C1s | 284.5 | C-C, C=C | 84 |
| 285.9 | C-O | 10 | |
| 287.2 | C=O | 4 | |
| 290 | COOR | 2 | |
| O1s | 532 | C=O | 19 |
| 533.8 | C-O | 52 | |
| 535.3 | OH | 29 | |
| Si2p | 104 | SiO2 | 46 |
| 105.5 | Si-O | 54 |
| Characteristics | Properties | Graphene Nanofibers (GNF) |
|---|---|---|
| Characteristics Textural | Surface area (m2/g) | 70–250 |
| Micropore area (m2/g) a | 2–50 (2–20) | |
| Total pore volume (cm3/g) | 0.3–1.6 | |
| Degree of Graffiti | DRX: npg b | 10–25 (npg of graphite ≈ 95) |
| Raman: ID/IG c | 0.95–1.05 (ID/of graphite ≈ 0.6) | |
| Characteristics Physical/Chemical | Fiber diameter (nm) d | 5–160 |
| Fiber length (nm) d | >20 | |
| Catalyst content (%) (CNF gross, unpurified) | 12–20 | |
| Elemental analysis of the product (free of catalyst residues) (% mole) | C (75–93) O (2.5–22) H (4.5–5.5) | |
| Characteristics Thermal | Oxidation temperature (°C) e | 350–680 (520–640) |
| Decomposition products/Thermal oxidation | CO, CO2 mainly |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Selva-Otaolaurruchi, E.J.; Fernández-Estevan, L.; Solá-Ruiz, M.F.; García-Sala-Bonmati, F.; Selva-Ribera, I.; Agustín-Panadero, R. Graphene-Doped Polymethyl Methacrylate (PMMA) as a New Restorative Material in Implant-Prosthetics: In Vitro Analysis of Resistance to Mechanical Fatigue. J. Clin. Med. 2023, 12, 1269. https://doi.org/10.3390/jcm12041269
Selva-Otaolaurruchi EJ, Fernández-Estevan L, Solá-Ruiz MF, García-Sala-Bonmati F, Selva-Ribera I, Agustín-Panadero R. Graphene-Doped Polymethyl Methacrylate (PMMA) as a New Restorative Material in Implant-Prosthetics: In Vitro Analysis of Resistance to Mechanical Fatigue. Journal of Clinical Medicine. 2023; 12(4):1269. https://doi.org/10.3390/jcm12041269
Chicago/Turabian StyleSelva-Otaolaurruchi, Eduardo J., Lucía Fernández-Estevan, María Fernanda Solá-Ruiz, Fernando García-Sala-Bonmati, Inmaculada Selva-Ribera, and Rubén Agustín-Panadero. 2023. "Graphene-Doped Polymethyl Methacrylate (PMMA) as a New Restorative Material in Implant-Prosthetics: In Vitro Analysis of Resistance to Mechanical Fatigue" Journal of Clinical Medicine 12, no. 4: 1269. https://doi.org/10.3390/jcm12041269
APA StyleSelva-Otaolaurruchi, E. J., Fernández-Estevan, L., Solá-Ruiz, M. F., García-Sala-Bonmati, F., Selva-Ribera, I., & Agustín-Panadero, R. (2023). Graphene-Doped Polymethyl Methacrylate (PMMA) as a New Restorative Material in Implant-Prosthetics: In Vitro Analysis of Resistance to Mechanical Fatigue. Journal of Clinical Medicine, 12(4), 1269. https://doi.org/10.3390/jcm12041269







