Elevated Circulating Endocan Levels Are Associated with Increased Levels of Endothelial and Inflammation Factors in Postprandial Lipemia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects and Study Groups
2.2. Oral High-Fat Meal Test
2.3. Biochemical Parameters
2.4. Assessment of Endothelial Factors
2.5. Assessment of Inflammatory Factors
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jackson, K.G.; Sally, D.P.; Minihane, A.M. Postprandial lipemia and cardiovascular disease risk: Interrelationships between dietary, physiological and genetic determinants. Atherosclerosis 2012, 1, 22–33. [Google Scholar] [CrossRef]
- Orem, A.; Yaman, S.O.; Altinkaynak, B.; Kural, B.V.; Yucesan, F.B.; Altinkaynak, Y.; Orem, C. Relationship between postprandial lipemia and atherogenic factors in healthy subjects by considering gender differences. Clin. Chim. Acta. 2018, 480, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Nordestgaard, B.G. Triglyceride-rich lipoproteins and atherosclerotic cardiovascular disease: New insights from epidemiology, genetics, and biology. Circ. Res. 2016, 118, 547–563. [Google Scholar] [CrossRef]
- Bansal, S.; Buring, J.E.; Rifai, N.; Mora, S.; Sacks, F.M.; Ridke, P.M. Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. JAMA 2007, 298, 309–316. [Google Scholar] [CrossRef] [PubMed]
- van Oostrom, A.J.; Sijmonsma, T.P.; Verseyden, C.; Jansen, E.H.; Koning, E.J.; Rabelink, T.J.; Cabezas, M.C. Postprandial recruitment of neutrophils may contribute to endothelial dysfunction. J. Res. 2003, 44, 576–583. [Google Scholar] [CrossRef]
- Norata, G.D.; Grigore, L.; Raselli, S.; Redaelli, L.; Hamsten, A.; Maggi, F.; Eriksson, P.; Catapano, A.L. Post-prandial endothelial dysfunction in hypertriglyceridemic subjects: Molecular mechanisms and gene expression studies. Atherosclerosis 2007, 193, 321–327. [Google Scholar] [CrossRef]
- Alipour, A.; Elte, J.W.; van Zaanen, H.C.; Rietveld, A.P.; Cabezas, M.C. Postprandial inflammation and endothelial dysfuction. Biochem. Soc. Trans. 2007, 35, 466–469. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, L.; Yang, S.; Liu, G.; Pan, L.; Gu, C.; Wang, Y.; Li, D.; Zhao, R.; Wu, M. Mechanisms of atherosclerosis induced by postprandial lipemia. Front. Cardiovasc. Med. 2021, 8, 636947. [Google Scholar] [CrossRef] [PubMed]
- Yaman, S.O.; Yucesan, F.B.; Orem, A.; Orem, C.; Kural, B.V.; Yaman, H. An increased disulfide/native thiol ratio and oxidative stress index in metabolic syndrome patients with postprandial lipemia. Int. J. Diabetes Dev. Ctries. 2022, 1–9. [Google Scholar] [CrossRef]
- Balta, S.; Mikhailidis, D.P.; Demirkol, S.; Ozturk, C.; Celik, T.; Iyisoy, A. Endocan: A novel inflammatory indicator in cardiovascular disease? Atherosclerosis 2015, 243, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Kali, A.; Shetty, K.S. Endocan: A novel circulating proteoglycan. Indian J. Pharmacol. 2014, 46, 579–583. [Google Scholar] [CrossRef]
- Altintas, N.; Mutlu, L.C.; Akkoyun, D.C.; Aydin, M.; Bilir, B. Effect of CPAP on new endothelial dysfunction marker, endocan, in people with obstructive sleep apnea. Angiology 2016, 67, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, M.I.; Siriopol, D.; Saglam, M.; Kurt, Y.G.; Unal, H.U.; Eyileten, T. Plasma endocan levels associate with inflammation, vascular abnormalities, cardiovascular events, and survival in chronic kidney disease. Kidney Int. 2014, 86, 1213–1220. [Google Scholar] [CrossRef]
- Zhang, S.; Zuo, L.; Zhou, Q.; Gui, S.; Shi, R.; Wu, Q. Expression and distribution of endocan in human tissues. Biotech. Histochem. 2012, 87, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Bicer, M.; Guler, A.; Kocabas, U.; Imamoglu, G.; Baloglu, C. Endocan is a predictor of increased cardiovascular risk in women with polycystic ovary syndrome. Endocr. Res. 2017, 42, 145–153. [Google Scholar] [CrossRef]
- Sarrazin, S.; Maurage, C.A.; Delmas, D.; Lassalle, P.; Delehedde, M. Endocan as a biomarker of endothelial dysfunction in cancer. J. Cancer Sci. Ther. 2010, 2, 047–052. [Google Scholar]
- Canpolat, U.; Kocyigit, D.; Yildirim, A. Role of Endothelia Dysfunction and Endocan in Atherosclerosis: Point of Origin or End Point? Angiology 2020, 71, 477. [Google Scholar] [CrossRef]
- Chen, J.; Jiang, L.; Yu, X.H.; He, P.; Ouyang, X.; Hu, M.; Zhang, Y.K.; Liu, X.; He, P.; Ouyang, X. Endocan: A key player of cardiovascular disease. Front. Cardiovasc. Med. 2022, 8, 2079. [Google Scholar] [CrossRef] [PubMed]
- Bessa, J.; Albino-Teixeira, A.; Reina-Couto, M.; Sousa, T. Endocan: A novel biomarker for risk stratification, prognosis and therapeutic monitoring in human cardiovascular and renal diseases. Clin. Chim. Acta. 2020, 509, 310–335. [Google Scholar] [CrossRef]
- Çimen, T.; Akyel, A.; Efe, T.H. Reply to the Letter to the Editor Entitled “Role of Endothelial Dysfunction and Endocan in Atherosclerosis: Point of Origin or End Point?”. Angiology 2016, 71, 0003319716664285. [Google Scholar] [CrossRef] [PubMed]
- Celık, T.; Balta, S.; Karaman, M.; Ahmet, S.A.; Demırkol, S.; Ozturk, C.; Dınc, M. Endocan, a novel marker of endothelial dysfunction in patients with essential hypertension: Comparative effects of amlodipine and valsartan. Blood Press. 2015, 24, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.S.; Yang, W.; Luo, T.; Wang, J.M.; Jing, Y.Y. Serum endocan levels are correlated with the presence and severity of coronary artery disease in patients with hypertension. Genet Test Mol. Biomark. 2015, 19, 124–127. [Google Scholar] [CrossRef] [PubMed]
- Arman, Y.; Akpinar, T.S.; Kose, M.; Emet, S.; Yuruyen, G.; Akarsu, M. Effect of Glycemic Regulation on Endocan Levels in Patients With Diabetes: A Preliminary Study. Angiology 2016, 67, 239–244. [Google Scholar] [CrossRef]
- Klisic, A.; Kavaric, N.; Stanisic, V.; Vujcic, S.; Spasojevic-Kalimanovska, V.; Ninic, A.; Kotur-Stevuljevic, J. Endocan and a novel score for dyslipidemia, oxidative stress and inflammation (DOI score) are independently correlated with glycated hemoglobin (HbA1c) in patients with prediabetes and type 2 diabetes. Arch. Med. Sci. 2020, 16, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Ko, G.J.; Kim, Y.G.; Lee, S.Y.; Lee, D.Y.; Jeong, K.H.; Lee, S.H. Plasma endocan as a predictor of cardiovascular event in patients with end-stage renal disease on hemodialysis. J. Clin. Med. 2020, 9, 4086. [Google Scholar] [CrossRef]
- Kolovou, G.D.; Mikhailidis, D.P.; Kovar, J.; Lairon, D.; Nordestgaard, B.G.; Ooi, T.C.; Perez-Martinez, P.; Bilianou, H.; Anagnostopoulou, K.; Panotopoulos, G. Assessment and clinical relevance of non-fasting and postprandial triglycerides: An expert panel statement. Curr. Vasc. Pharmacol. 2011, 9, 258–270. [Google Scholar] [CrossRef]
- Yaman, S.O.; Orem, A.; Yucesan, F.B.; Kural, B.V.; Orem, C. Evaluation of circulating miR-122, miR-30c and miR-33a levels and their association with lipids, lipoproteins in postprandial lipemia. Life Sci. 2021, 264, 118585. [Google Scholar] [CrossRef]
- Katsuki, A.; Sumida, Y.; Gabazza, E.C.; Murashima, S.; Furuta, M.; Araki-Sasaki, R.; Hori, Y.; Yano, Y.; Adachi, Y. Homeostasis model International Journal of Diabetes in Developing Countries assessment is a reliable indicator of insulin resistance during follow-up of patients with type 2 diabetes. Diab. Care. 2001, 24, 362–365. [Google Scholar] [CrossRef]
- Langsted, A.; Freiberg, J.J.; Tybjaerg-Hansen, A.; Schnohr, P.; Jensen, G.B.; Nordestgaard, B.G. Nonfasting cholesterol and triglycerides and association with risk of myocardial infarction and total mortality: The Copenhagen City heart study with 31years of follow-up. J. Intern. Med. 2010, 270, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.T.; Gao, Y.; Zheng, Y.Y.; Ma, Y.T.; Xie, X. Atherogenic index of plasma (AIP): A novel predictive indicator for the coronary artery disease in postmenopausal women. Lipids Health Dis. 2018, 17, 197. [Google Scholar] [CrossRef]
- He, X.W.; Ke, S.F.; Bao, Y.Y.; Hong, W.J.; Shen, Y.G.; Li, C. Serum levels of endocan and endoglin are associated with large-artery atherosclerotic stroke. Clin. Chim. Acta 2018, 478, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Kose, M.; Emet, S.; Akpinar, T.S.; Kocaaga, M.; Cakmak, R.; Akarsu, M.; Tukek, T. Serum endocan level and the severity of coronary artery disease: A pilot study. Angiology 2015, 66, 727–731. [Google Scholar] [CrossRef]
- Zhao, T.; Kecheng, Y.; Zhao, X.; Hu, X.; Zhu, J.; Wang, Y.; Ni, J. The higher serum endocan levels may be a risk factor for the onset of cardiovascular disease: A metaanalysis. Medicine 2018, 97, e13407. [Google Scholar] [CrossRef] [PubMed]
- Kundi, H.; Balun, A.; Cicekcioglu, H.; Karayigit, O.; Topcuoglu, C.; Kilinckaya, M.F.; Kiziltunc, E.; Cetin, M.; Ornek, E. Admission endocan level may be a useful pre dictor for in-hospital mortality and coronary severity index in patients with ST segment elevation myocardial infarction. Angiology 2017, 68, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Bar, A.; Targosz-Korecka, M.; Suraj, J.; Proniewski, B.; Jasztal, A.; Marczyk, B. Degradation of glycocalyx and multiple manifestations of endothelial dysfunction coincide in the early phase of endothelial dysfunction before atherosclerotic plaque development in apolipoprotein E/lowdensity lipoprotein receptor-deficient mice. J. Am. Heart Assoc. 2019, 8, e011171. [Google Scholar] [CrossRef]
- Pawlak, K.; Mysliwiec, M.; Pawlak, D. Endocan—The new endothelial activation marker independently associated with soluble endothelial adhesion molecules in uraemic patients with cardiovascular disease. Clin. Biochem. 2015, 48, 425–430. [Google Scholar] [CrossRef]
- Tadzic, R.; Mihalj, M.; Vcev, A.; Ennen, J.; Tadzic, A.; Drenjancevic, I. The effects of arterial blood pressure reduction on endocan and soluble endothelial cell adhesion molecules (CAMs) and CAMs ligands expression in hypertensive patients on Ca channel blocker therapy. Kidney Blood Press. Res. 2013, 37, 103–115. [Google Scholar] [CrossRef]

| Parametreler | Control n:28 | PPL n:54 | p |
|---|---|---|---|
| Age (years) | 42.0 ± 6.76 | 45.6 ± 8.42 | 0.786 |
| BMI (kg/m2) | 23.8 ± 2.68 | 27.3 ± 4.75 | 0.0001 |
| WHR | 0.802 ± 0.071 | 0.865 ± 0.096 | 0.022 |
| WHtR | 0.477 ± 0.052 | 0.549 ± 0.078 | 0.0001 |
| Glucose (mmol/L) | 4.96 ± 0.361 | 5.30 ± 0.512 | 0.012 |
| Glucose TG 4th h (mmol/L) | 5.07 ± 0.373 | 5.76 ± 0.546 | 0.376 |
| Insulin (pmol/L) | 38.7 (21.7–67.1) | 45.0 (30.7–54.0) | 0.286 * |
| Insulin TG 4th h (pmol/L) | 43.3 (28.6–49.3) | 49.6 (34.3–58.5) | 0.122 * |
| HOMA-IR | 1.44 (0.821–2.76) | 2.08 (1.22–2.83) | 0.129 * |
| TG fasting (mmol/L) | 0.965 (0.711–1.16) | 2.20 (1.55–2.89) | 0.0001 * |
| Postprandial TG 4th h (mmol/L) | 1.46 (1.20–1.74) | 2.94 (2.09–3.74) | 0.0001 * |
| TC (mmol/L) | 4.65 ± 0.701 | 5.50 ± 0.938 | 0.0001 |
| HDL-C (mmol/L) | 1.51 ± 0.380 | 1.25 ± 0.311 | 0.005 |
| LDL-C (mmol/L) | 2.80 ± 0.451 | 3.25 ± 0.635 | 0.0001 |
| RLP-C (mmol/L) | 0.518 (0.206–0.725) | 0.958 (0.458–1.79) | 0.002 * |
| AIP (mmol/L) | 0.176 (−0.026–0.255) | 0.583 (0.420–0.769) | 0.0001 |
| AUC of fasting and 4th h TG | 428 ± 116 | 920 ± 230 | 0.0001 |
| AUC of Fasting and 4 h TG Tertiles | |||||||
|---|---|---|---|---|---|---|---|
| Control 426 ± 116 (n:28) | PPL 920 ± 230 (n:54) | p | 1 539 ± 158 (n:18) | 2 958 ± 93 (n:18) | 3 1284 ± 114 (n:18) | p | |
| Endothelial factors | |||||||
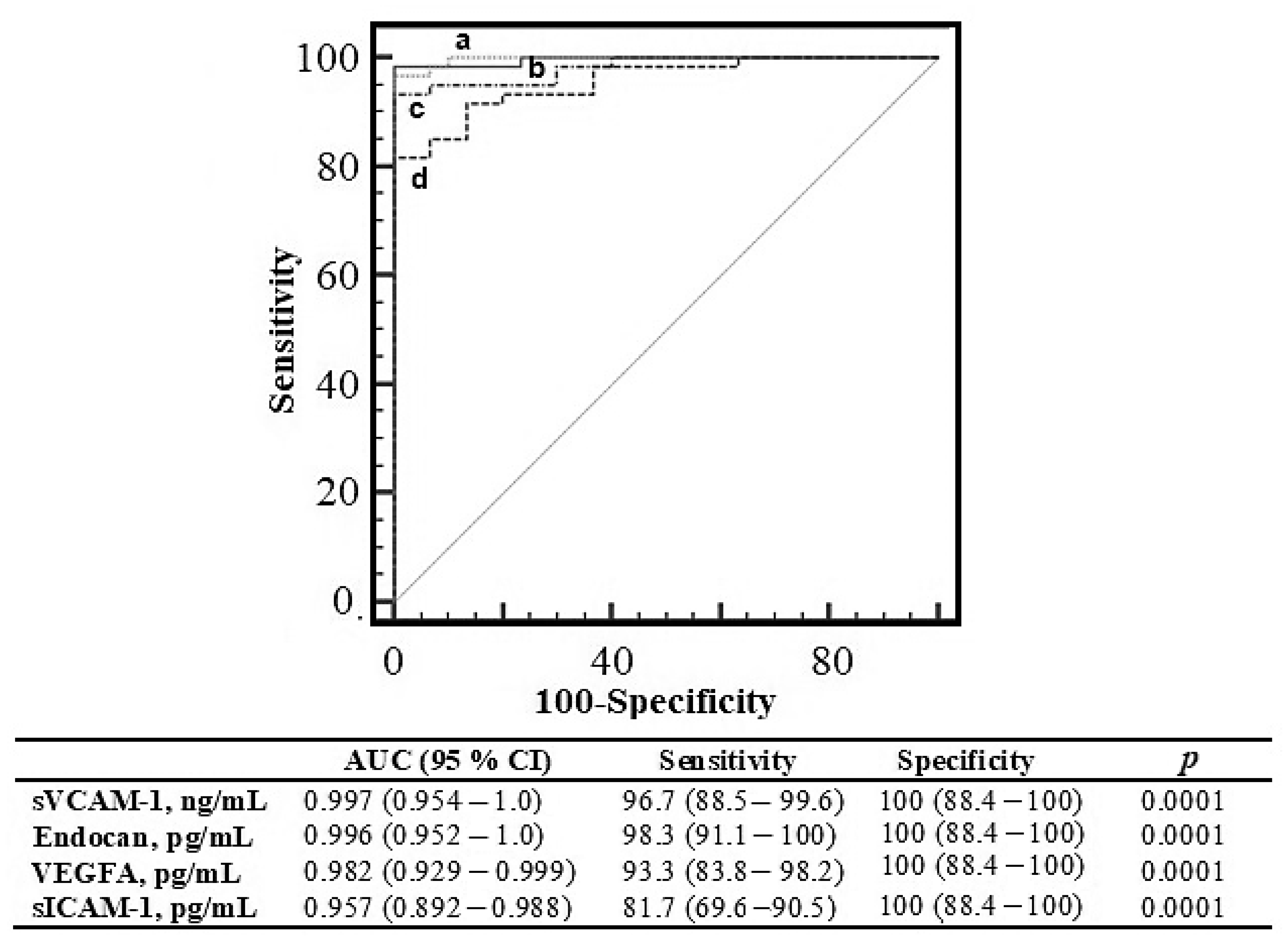
| Endocan, pg/mL | 43.1 (32.3–52.8) | 145 (107–188) | 0.0001 | 101 (83.0–118) a | 140 (120–162) a,b | 201 (177–281) a,b,c | 0.0001 * |
| VEGFA, pg/mL | 37.2 (33.0–53.1) | 141 (93.6–190) | 0.0001 | 79.9 (71.4–93.8) a | 168 (136–194) a,b | 189 (145–228) a,b | 0.0001 * |
| sICAM-1, pg/mL | 135 (110–181) | 529 (301–960) | 0.0001 | 236 (189–301) a | 562 (447–799) a,b | 1176 (841–1259) a,b, c | 0.0001 * |
| sVCAM-1, ng/mL | 8.77 (5.97–8.72) | 22.1 (14.3–31.3) | 0.0001 | 12.0 (10.59–15.6) a | 22.1 (16.4–25.2) a,b | 36.9 (31.2–46.7) a,b,c | 0.0001 * |
| Inflammatory factors | |||||||
| IL-6, pg/mL | 11.3 (10.0–14.5) | 24.4 (15.7–25.7) | 0.0001 | 15.0 (12.0–15.8) a | 24.8 (23.7–25.9) a,b | 25.5 (25.1–31.8) a,b | 0.001 * |
| LFA-1α, ng/mL | 0.236 (0.230–0.243) | 0.326 (0.281–0.388) | 0.0001 | 0.247 (0.242–0.283) a | 0.342 (0.316–0.388) a,b | 0.384 (0.336–0.401) a,b | 0.001 * |
| PPL (n:54) | ||||
|---|---|---|---|---|
| AUC of Fasting and 4 h TG | Endocan | |||
| Spearman’s Rho | p | Spearman’s Rho | p | |
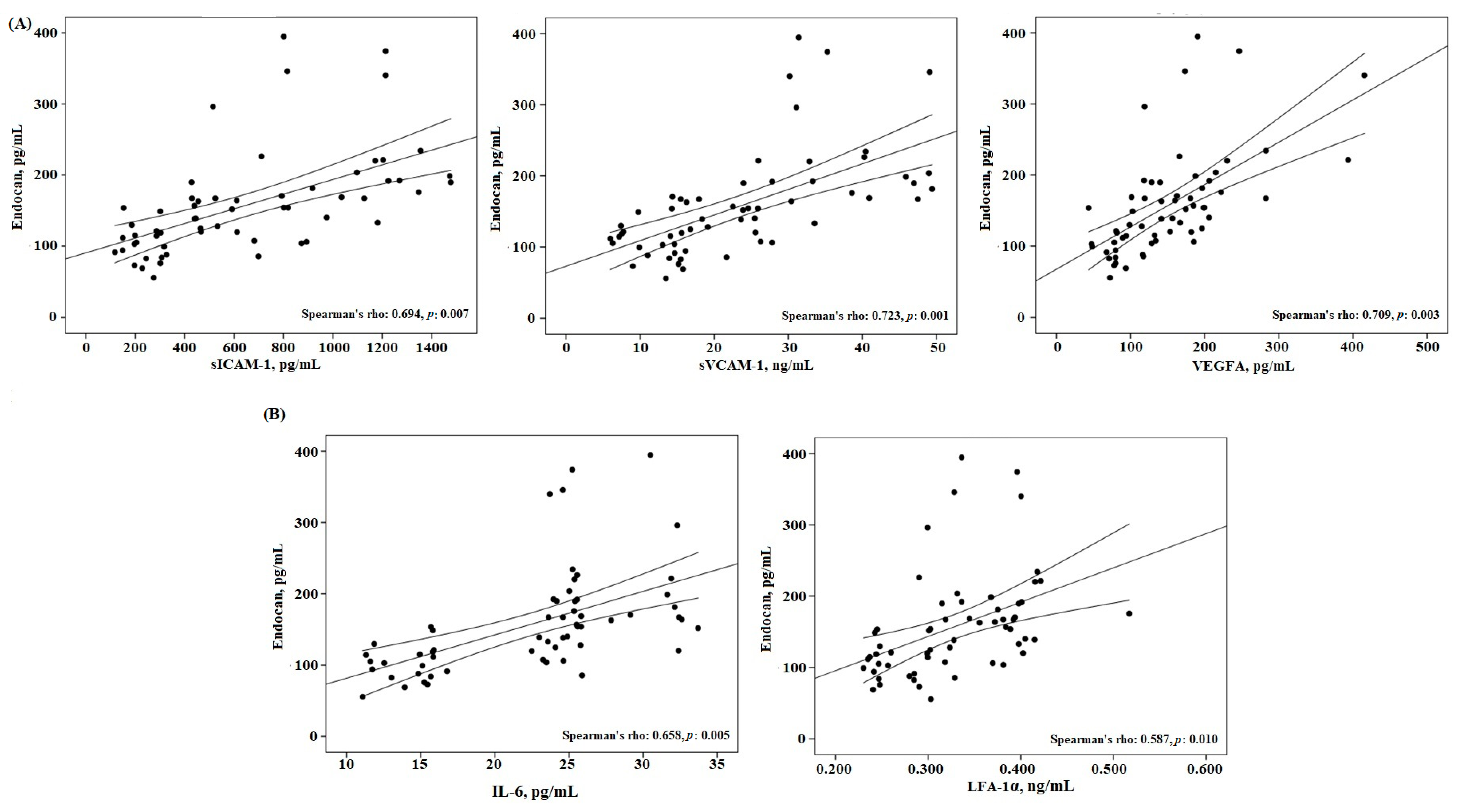
| Endothelial factors | ||||
| Endocan, pg/mL | 0.768 | 0.003 | ||
| sICAM-1, pg/mL | 0.856 | 0.001 | 0.694 | 0.007 |
| sVCAM-1, ng/mL | 0.883 | 0.001 | 0.723 | 0.001 |
| VEGFA, pg/mL | 0.622 | 0.008 | 0.709 | 0.003 |
| Inflammatory factors | ||||
| IL-6, pg/mL | 0.652 | 0.006 | 0.658 | 0.005 |
| LFA-1α, ng/mL | 0.735 | 0.004 | 0.587 | 0.010 |
| Atherogenic factors | ||||
| RLP-C | 0.465 | 0.037 | 0.503 | 0.031 |
| AIP | 0.757 | 0.001 | 0.817 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ozer Yaman, S.; Balaban Yucesan, F.; Orem, C.; Vanizor Kural, B.; Orem, A. Elevated Circulating Endocan Levels Are Associated with Increased Levels of Endothelial and Inflammation Factors in Postprandial Lipemia. J. Clin. Med. 2023, 12, 1267. https://doi.org/10.3390/jcm12041267
Ozer Yaman S, Balaban Yucesan F, Orem C, Vanizor Kural B, Orem A. Elevated Circulating Endocan Levels Are Associated with Increased Levels of Endothelial and Inflammation Factors in Postprandial Lipemia. Journal of Clinical Medicine. 2023; 12(4):1267. https://doi.org/10.3390/jcm12041267
Chicago/Turabian StyleOzer Yaman, Serap, Fulya Balaban Yucesan, Cihan Orem, Birgul Vanizor Kural, and Asım Orem. 2023. "Elevated Circulating Endocan Levels Are Associated with Increased Levels of Endothelial and Inflammation Factors in Postprandial Lipemia" Journal of Clinical Medicine 12, no. 4: 1267. https://doi.org/10.3390/jcm12041267
APA StyleOzer Yaman, S., Balaban Yucesan, F., Orem, C., Vanizor Kural, B., & Orem, A. (2023). Elevated Circulating Endocan Levels Are Associated with Increased Levels of Endothelial and Inflammation Factors in Postprandial Lipemia. Journal of Clinical Medicine, 12(4), 1267. https://doi.org/10.3390/jcm12041267







