Effectiveness of Benralizumab in OCS-Dependent Severe Asthma: The Impact of 2 Years of Therapy in a Real-Life Setting
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
3.1. Blood Eosinophil Counts
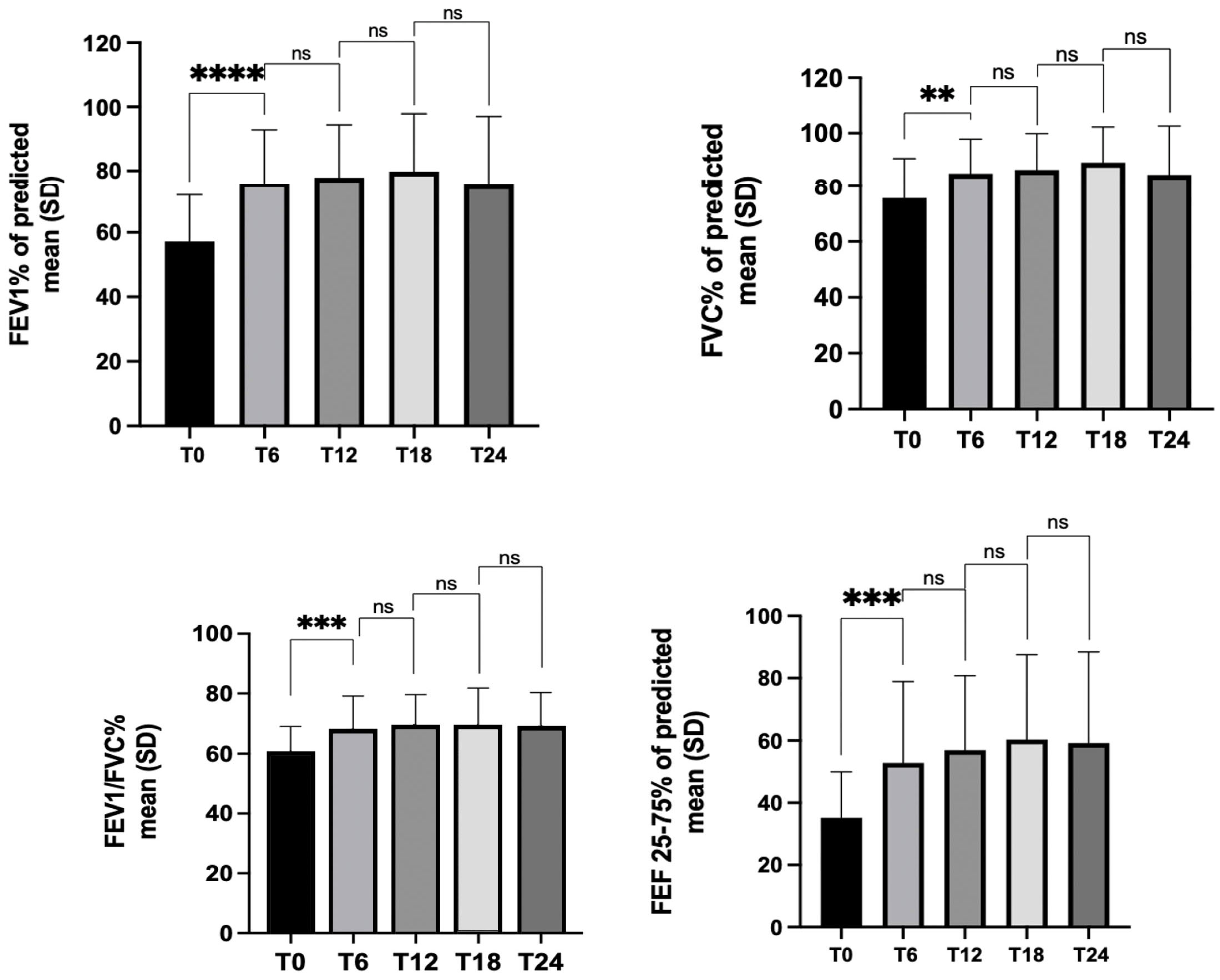
3.2. Lung Function
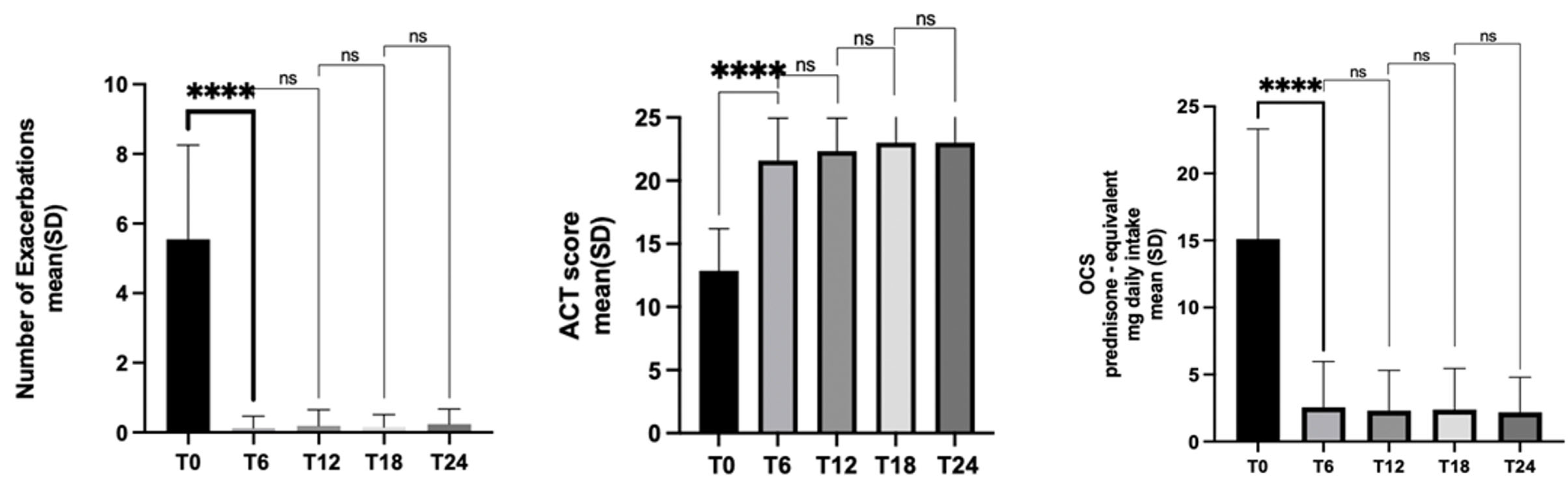
3.3. Exacerbations
3.4. Asthma Control
3.5. OCS Use
3.6. Safety
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- 2022 GINA Main Report. Available online: https://ginasthma.org/gina-reports/ (accessed on 14 December 2022).
- Chung, K.F.; Wenzel, S.E.; Brozek, J.L.; Bush, A.; Castro, M.; Sterk, P.J.; Adcock, I.M.; Bateman, E.D.; Bel, E.H.; Bleecker, E.R.; et al. International ERS/ATS Guidelines on Definition, Evaluation and Treatment of Severe Asthma. Eur. Respir. J. 2014, 43, 343–373. [Google Scholar] [CrossRef]
- Bleecker, E.R.; Menzies-Gow, A.N.; Price, D.B.; Bourdin, A.; Sweet, S.; Martin, A.L.; Alacqua, M.; Tran, T.N. Systematic Literature Review of Systemic Corticosteroid Use for Asthma Management. Am. J. Respir. Crit. Care Med. 2020, 201, 276–293. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Kim, H.-J.; Park, C.S.; Park, S.Y.; Park, S.-Y.; Lee, H.; Kim, S.-H.; Cho, Y.S. Clinical Characteristics and Disease Burden of Severe Asthma According to Oral Corticosteroid Dependence: Real-World Assessment From the Korean Severe Asthma Registry (KoSAR). Allergy Asthma Immunol. Res. 2022, 14, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Volmer, T.; Effenberger, T.; Trautner, C.; Buhl, R. Consequences of Long-Term Oral Corticosteroid Therapy and Its Side-Effects in Severe Asthma in Adults: A Focused Review of the Impact Data in the Literature. Eur. Respir. J. 2018, 52, 1800703. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Ryu, J.; Nam, E.; Chung, S.J.; Yeo, Y.; Park, D.W.; Park, T.S.; Moon, J.-Y.; Kim, T.-H.; Sohn, J.W.; et al. Increased Mortality in Patients with Corticosteroid-Dependent Asthma: A Nationwide Population-Based Study. Eur. Respir. J. 2019, 54, 1900804. [Google Scholar] [CrossRef]
- Barry, L.E.; Sweeney, J.; O’Neill, C.; Price, D.; Heaney, L.G. The Cost of Systemic Corticosteroid-Induced Morbidity in Severe Asthma: A Health Economic Analysis. Respir. Res. 2017, 18, 129. [Google Scholar] [CrossRef] [PubMed]
- Vatrella, A.; Maglio, A.; Pellegrino, S.; Pelaia, C.; Stellato, C.; Pelaia, G.; Vitale, C. Phenotyping Severe Asthma: A Rationale for Biologic Therapy. Expert Rev. Precis. Med. Drug Dev. 2020, 5, 265–274. [Google Scholar] [CrossRef]
- Kuruvilla, M.E.; Lee, F.E.-H.; Lee, G.B. Understanding Asthma Phenotypes, Endotypes, and Mechanisms of Disease. Clin. Rev. Allergy Immunol. 2019, 56, 219–233. [Google Scholar] [CrossRef]
- Pelaia, C.; Pelaia, G.; Crimi, C.; Maglio, A.; Stanziola, A.A.; Calabrese, C.; Terracciano, R.; Longhini, F.; Vatrella, A. Novel Biological Therapies for Severe Asthma Endotypes. Biomedicines 2022, 10, 1064. [Google Scholar] [CrossRef]
- Vatrella, A.; Maglio, A.; Pelaia, C.; Ciampo, L.; Pelaia, G.; Vitale, C. Eosinophilic Inflammation: An Appealing Target for Pharmacologic Treatments in Severe Asthma. Biomedicines 2022, 10, 2181. [Google Scholar] [CrossRef]
- Principe, S.; Porsbjerg, C.; Bolm Ditlev, S.; Kjaersgaard Klein, D.; Golebski, K.; Dyhre-Petersen, N.; van Dijk, Y.E.; van Bragt, J.J.M.H.; Dankelman, L.L.H.; Dahlen, S.-E.; et al. Treating Severe Asthma: Targeting the IL-5 Pathway. Clin. Exp. Allergy 2021, 51, 992–1005. [Google Scholar] [CrossRef] [PubMed]
- Bagnasco, D.; Ferrando, M.; Varricchi, G.; Puggioni, F.; Passalacqua, G.; Canonica, G.W. Anti-Interleukin 5 (IL-5) and IL-5Ra Biological Drugs: Efficacy, Safety, and Future Perspectives in Severe Eosinophilic Asthma. Front. Med. 2017, 4, 135. [Google Scholar] [CrossRef] [PubMed]
- Pelaia, C.; Calabrese, C.; Vatrella, A.; Busceti, M.T.; Garofalo, E.; Lombardo, N.; Terracciano, R.; Pelaia, G. Benralizumab: From the Basic Mechanism of Action to the Potential Use in the Biological Therapy of Severe Eosinophilic Asthma. BioMed Res. Int. 2018, 2018, 4839230. [Google Scholar] [CrossRef] [PubMed]
- Bleecker, E.R.; FitzGerald, J.M.; Chanez, P.; Papi, A.; Weinstein, S.F.; Barker, P.; Sproule, S.; Gilmartin, G.; Aurivillius, M.; Werkström, V.; et al. Efficacy and Safety of Benralizumab for Patients with Severe Asthma Uncontrolled with High-Dosage Inhaled Corticosteroids and Long-Acting Β2-Agonists (SIROCCO): A Randomised, Multicentre, Placebo-Controlled Phase 3 Trial. Lancet Lond. Engl. 2016, 388, 2115–2127. [Google Scholar] [CrossRef] [PubMed]
- FitzGerald, J.M.; Bleecker, E.R.; Nair, P.; Korn, S.; Ohta, K.; Lommatzsch, M.; Ferguson, G.T.; Busse, W.W.; Barker, P.; Sproule, S.; et al. Benralizumab, an Anti-Interleukin-5 Receptor α Monoclonal Antibody, as Add-on Treatment for Patients with Severe, Uncontrolled, Eosinophilic Asthma (CALIMA): A Randomised, Double-Blind, Placebo-Controlled Phase 3 Trial. Lancet Lond. Engl. 2016, 388, 2128–2141. [Google Scholar] [CrossRef] [PubMed]
- Nair, P.; Wenzel, S.; Rabe, K.F.; Bourdin, A.; Lugogo, N.L.; Kuna, P.; Barker, P.; Sproule, S.; Ponnarambil, S.; Goldman, M.; et al. Oral Glucocorticoid-Sparing Effect of Benralizumab in Severe Asthma. N. Engl. J. Med. 2017, 376, 2448–2458. [Google Scholar] [CrossRef]
- Korn, S.; Bourdin, A.; Chupp, G.; Cosio, B.G.; Arbetter, D.; Shah, M.; Gil, E.G. Integrated Safety and Efficacy Among Patients Receiving Benralizumab for Up to 5 Years. J. Allergy Clin. Immunol. Pract. 2021, 9, 4381–4392.e4. [Google Scholar] [CrossRef]
- Menzies-Gow, A.; Gurnell, M.; Heaney, L.G.; Corren, J.; Bel, E.H.; Maspero, J.; Harrison, T.; Jackson, D.J.; Price, D.; Lugogo, N.; et al. Oral Corticosteroid Elimination via a Personalised Reduction Algorithm in Adults with Severe, Eosinophilic Asthma Treated with Benralizumab (PONENTE): A Multicentre, Open-Label, Single-Arm Study. Lancet Respir. Med. 2022, 10, 47–58. [Google Scholar] [CrossRef]
- Kavanagh, J.E.; Hearn, A.P.; Dhariwal, J.; d’Ancona, G.; Douiri, A.; Roxas, C.; Fernandes, M.; Green, L.; Thomson, L.; Nanzer, A.M.; et al. Real-World Effectiveness of Benralizumab in Severe Eosinophilic Asthma. Chest 2021, 159, 496–506. [Google Scholar] [CrossRef]
- Pelaia, C.; Busceti, M.T.; Vatrella, A.; Rago, G.F.; Crimi, C.; Terracciano, R.; Pelaia, G. Real-Life Rapidity of Benralizumab Effects in Patients with Severe Allergic Eosinophilic Asthma: Assessment of Blood Eosinophils, Symptom Control, Lung Function and Oral Corticosteroid Intake after the First Drug Dose. Pulm. Pharmacol. Ther. 2019, 58, 101830. [Google Scholar] [CrossRef]
- Pelaia, C.; Busceti, M.T.; Vatrella, A.; Ciriolo, M.; Garofalo, E.; Crimi, C.; Terracciano, R.; Lombardo, N.; Pelaia, G. Effects of the First Three Doses of Benralizumab on Symptom Control, Lung Function, Blood Eosinophils, Oral Corticosteroid Intake, and Nasal Polyps in a Patient with Severe Allergic Asthma. SAGE Open Med. Case Rep. 2020, 8, 2050313X20906963. [Google Scholar] [CrossRef] [PubMed]
- Menzella, F.; Bargagli, E.; Aliani, M.; Bracciale, P.; Brussino, L.; Caiaffa, M.F.; Caruso, C.; Centanni, S.; D’Amato, M.; Del Giacco, S.; et al. ChAracterization of ItaliaN Severe Uncontrolled Asthmatic PatieNts Key Features When Receiving Benralizumab in a Real-Life Setting: The Observational REtrospective ANANKE Study. Respir. Res. 2022, 23, 36. [Google Scholar] [CrossRef] [PubMed]
- Sposato, B.; Scalese, M.; Camiciottoli, G.; Carpagnano, G.E.; Pelaia, C.; Santus, P.; Pelaia, G.; Palmiero, G.; Di Tomassi, M.; Ronchi, M.C.; et al. Severe Asthma and Long-Term Benralizumab Effectiveness in Real-Life. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 7461–7473. [Google Scholar] [CrossRef]
- Hew, M.; Chung, K.F. Corticosteroid Insensitivity in Severe Asthma: Significance, Mechanisms and Aetiology. Intern. Med. J. 2010, 40, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Adcock, I.M.; Barnes, P.J. Molecular Mechanisms of Corticosteroid Resistance. Chest 2008, 134, 394–401. [Google Scholar] [CrossRef]
- Nolasco, S.; Crimi, C.; Pelaia, C.; Benfante, A.; Caiaffa, M.F.; Calabrese, C.; Carpagnano, G.E.; Ciotta, D.; D’Amato, M.; Macchia, L.; et al. Benralizumab Effectiveness in Severe Eosinophilic Asthma with and without Chronic Rhinosinusitis with Nasal Polyps: A Real-World Multicenter Study. J. Allergy Clin. Immunol. Pract. 2021, 9, 4371–4380.e4. [Google Scholar] [CrossRef]
- Pelaia, C.; Busceti, M.T.; Crimi, C.; Carpagnano, G.E.; Lombardo, N.; Terracciano, R.; Vatrella, A.; Pelaia, G. Real-Life Effects of Benralizumab on Exacerbation Number and Lung Hyperinflation in Atopic Patients with Severe Eosinophilic Asthma. Biomed. Pharmacother. Biomedecine Pharmacother. 2020, 129, 110444. [Google Scholar] [CrossRef]
- Maglio, A.; Vitale, C.; Pellegrino, S.; Calabrese, C.; D’Amato, M.; Molino, A.; Pelaia, C.; Triggiani, M.; Pelaia, G.; Stellato, C.; et al. Real-Life Effectiveness of Mepolizumab on Forced Expiratory Flow between 25% and 75% of Forced Vital Capacity in Patients with Severe Eosinophilic Asthma. Biomedicines 2021, 9, 1550. [Google Scholar] [CrossRef]
- Jackson, D.J.; Humbert, M.; Hirsch, I.; Newbold, P.; Garcia Gil, E. Ability of Serum IgE Concentration to Predict Exacerbation Risk and Benralizumab Efficacy for Patients with Severe Eosinophilic Asthma. Adv. Ther. 2020, 37, 718–729. [Google Scholar] [CrossRef]
- Harrison, T.W.; Chanez, P.; Menzella, F.; Canonica, G.W.; Louis, R.; Cosio, B.G.; Lugogo, N.L.; Mohan, A.; Burden, A.; McDermott, L.; et al. Onset of Effect and Impact on Health-Related Quality of Life, Exacerbation Rate, Lung Function, and Nasal Polyposis Symptoms for Patients with Severe Eosinophilic Asthma Treated with Benralizumab (ANDHI): A Randomised, Controlled, Phase 3b Trial. Lancet Respir. Med. 2021, 9, 260–274. [Google Scholar] [CrossRef]
- Bleecker, E.R.; Wechsler, M.E.; FitzGerald, J.M.; Menzies-Gow, A.; Wu, Y.; Hirsch, I.; Goldman, M.; Newbold, P.; Zangrilli, J.G. Baseline Patient Factors Impact on the Clinical Efficacy of Benralizumab for Severe Asthma. Eur. Respir. J. 2018, 52, 1800936. [Google Scholar] [CrossRef] [PubMed]
- FitzGerald, J.M.; Bleecker, E.R.; Menzies-Gow, A.; Zangrilli, J.G.; Hirsch, I.; Metcalfe, P.; Newbold, P.; Goldman, M. Predictors of Enhanced Response with Benralizumab for Patients with Severe Asthma: Pooled Analysis of the SIROCCO and CALIMA Studies. Lancet Respir. Med. 2018, 6, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Lipworth, B.; Misirovs, R.; Chan, R. Adrenal Insufficiency in Patients Taking Benralizumab as Corticosteroid Sparing Therapy. Lancet Respir. Med. 2022, 10, e7. [Google Scholar] [CrossRef] [PubMed]
- Broide, J.; Soferman, R.; Kivity, S.; Golander, A.; Dickstein, G.; Spirer, Z.; Weisman, Y. Low-Dose Adrenocorticotropin Test Reveals Impaired Adrenal Function in Patients Taking Inhaled Corticosteroids. J. Clin. Endocrinol. Metab. 1995, 80, 1243–1246. [Google Scholar] [CrossRef]
- Upham, J.W.; Le Lievre, C.; Jackson, D.J.; Masoli, M.; Wechsler, M.E.; Price, D.B. Delphi Panel Defining a Severe Asthma Super-Responder: Findings from a Delphi Process. J. Allergy Clin. Immunol. Pract. 2021, 9, 3997–4004. [Google Scholar] [CrossRef]
| Patients, n | 44 |
|---|---|
| Age (mean ± SD) | 59.4 ± 9.5 |
| Gender (female) | 27 F (61.3%) |
| Smokers/former smokers/non-smokers | 2/26/16 |
| Body mass index (kg/m2, mean ± SD) | 27.1 ± 7.32 |
| Asthma duration median (IQR) | 21.5 (16.5) |
| Age at asthma onset (mean ± SD) | 36.3 ± 13.5 |
| Atopy, n | 24 (54.5%) |
| OCS-dependent patients | 44 (100%) |
| Comorbidities | |
| Obesity (BMI ⩾ 30 kg/m2) | 6 (13.6%) |
| Chronic rhinosinusitis with nasal polyposis | 18 (41%) |
| Gastroesophageal reflux disease | 19 (43.1%) |
| T0 | T6 | T12 | T18 | T24 | |
|---|---|---|---|---|---|
| Patients, n | 44 | 44 | 39 | 36 | 32 |
| ACT score, mean ± SD | 12.8 ± 3.3 | 21.6 ± 3.3 | 22.3 ± 2.6 | 23 ± 2.3 | 23 ± 2.2 |
| Blood eosinophils (cells/μL), median (IQR) | 695 (508) | 0 | 0 | 0 | 0 |
| Exacerbation history, previous year (n/y), mean ± SD | 5.54 ± 2.7 | 0.12 ± 0.34 | 0.18 ± 0.46 | 0.15 ± 0.36 | 0.23 ± 0.44 |
| OCS users, n | 44 (100%) | 19(43.1%) | 17 (43.5%) | 14 (38.8%) | 13 (40.6%) |
| OCS (prednisone-equivalent mg/day), mean ± SD | 15.2 ± 12.5 | 2.3 ± 3 | 2.3 ± 2.9 | 2.4 ± 3 | 2.2 ± 2.6 |
| FEV1, %th mean ± SD | 57.2 ± 14.5 | 75.6 ± 17.2 | 77.8 ± 27.6 | 79.8 ± 38.1 | 75 ± 37.8 |
| FVC, %th mean ± SD | 75.7 ± 15.1 | 85.3 ± 12.5 | 86.7 ± 28.2 | 89.4 ± 40.4 | 85 ± 40.1 |
| FEV1/FVC, %th mean ± SD | 59.7 ± 9.2 | 67.5 ± 11.6 | 68.6 ± 23.1 | 68.5 ± 32.8 | 68.5 ± 31.6 |
| FEF 25–75%, %th mean ± SD | 35.2 ± 14.7 | 52.8 ± 26 | 56.9 ± 28.1 | 60.3 ± 35.3 | 59.2 ± 29.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vitale, C.; Maglio, A.; Pelaia, C.; D’Amato, M.; Ciampo, L.; Pelaia, G.; Molino, A.; Vatrella, A. Effectiveness of Benralizumab in OCS-Dependent Severe Asthma: The Impact of 2 Years of Therapy in a Real-Life Setting. J. Clin. Med. 2023, 12, 985. https://doi.org/10.3390/jcm12030985
Vitale C, Maglio A, Pelaia C, D’Amato M, Ciampo L, Pelaia G, Molino A, Vatrella A. Effectiveness of Benralizumab in OCS-Dependent Severe Asthma: The Impact of 2 Years of Therapy in a Real-Life Setting. Journal of Clinical Medicine. 2023; 12(3):985. https://doi.org/10.3390/jcm12030985
Chicago/Turabian StyleVitale, Carolina, Angelantonio Maglio, Corrado Pelaia, Maria D’Amato, Luigi Ciampo, Giulia Pelaia, Antonio Molino, and Alessandro Vatrella. 2023. "Effectiveness of Benralizumab in OCS-Dependent Severe Asthma: The Impact of 2 Years of Therapy in a Real-Life Setting" Journal of Clinical Medicine 12, no. 3: 985. https://doi.org/10.3390/jcm12030985
APA StyleVitale, C., Maglio, A., Pelaia, C., D’Amato, M., Ciampo, L., Pelaia, G., Molino, A., & Vatrella, A. (2023). Effectiveness of Benralizumab in OCS-Dependent Severe Asthma: The Impact of 2 Years of Therapy in a Real-Life Setting. Journal of Clinical Medicine, 12(3), 985. https://doi.org/10.3390/jcm12030985









