The Advantage of Growth Hormone Alone as an Adjuvant Therapy in Advanced Age and BMI ≥ 24 kg/m2 with In Vitro Fertilization Failure Due to Poor Embryo Quality
Abstract
1. Introduction
2. Materials and Methods
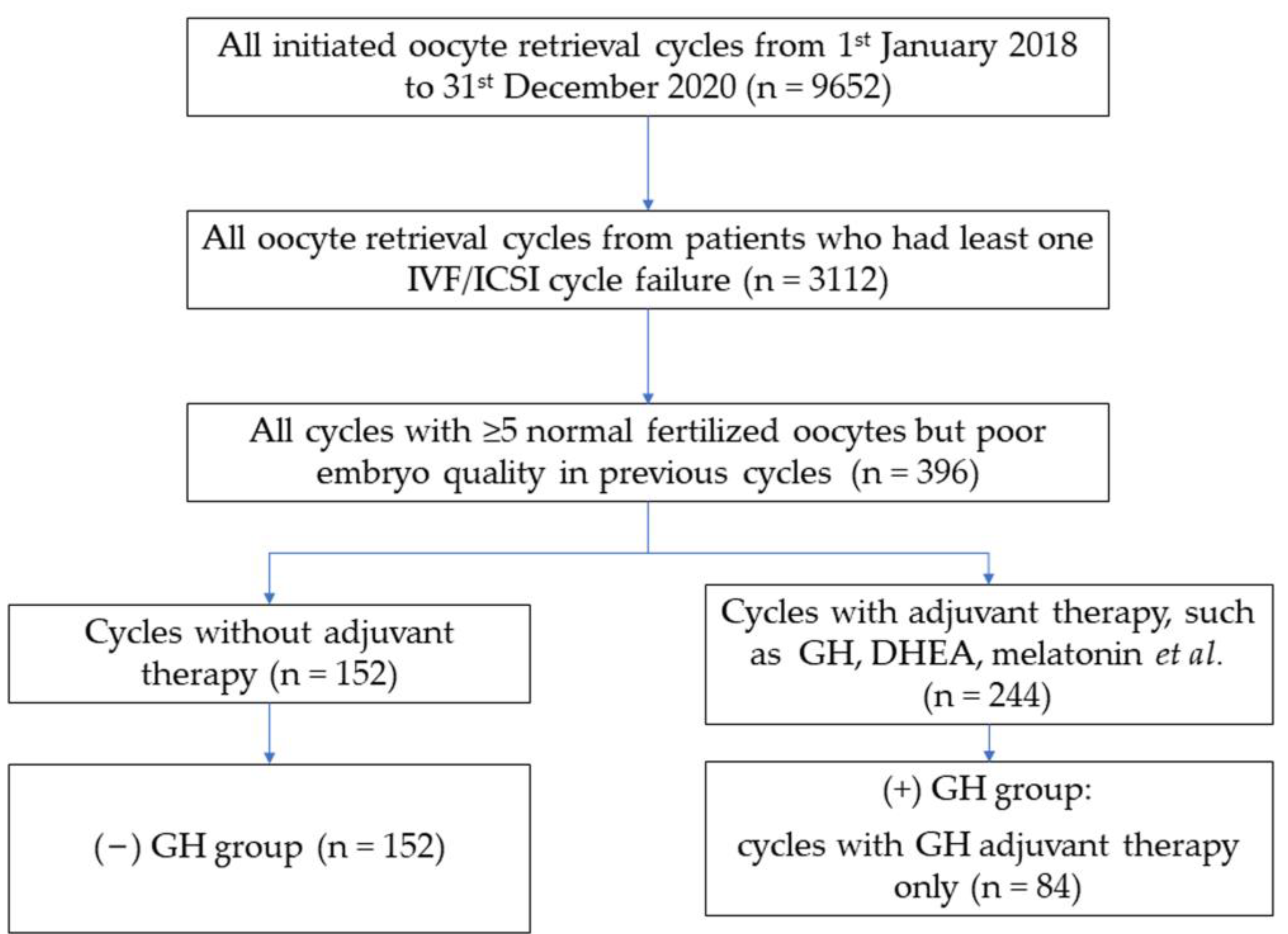
2.1. Study Design and Patient
2.2. Clinical Management
2.3. Fertilization, Embryo Culture, and Embryo Transfer
2.4. Outcome Assessments
2.5. Statistical Analysis
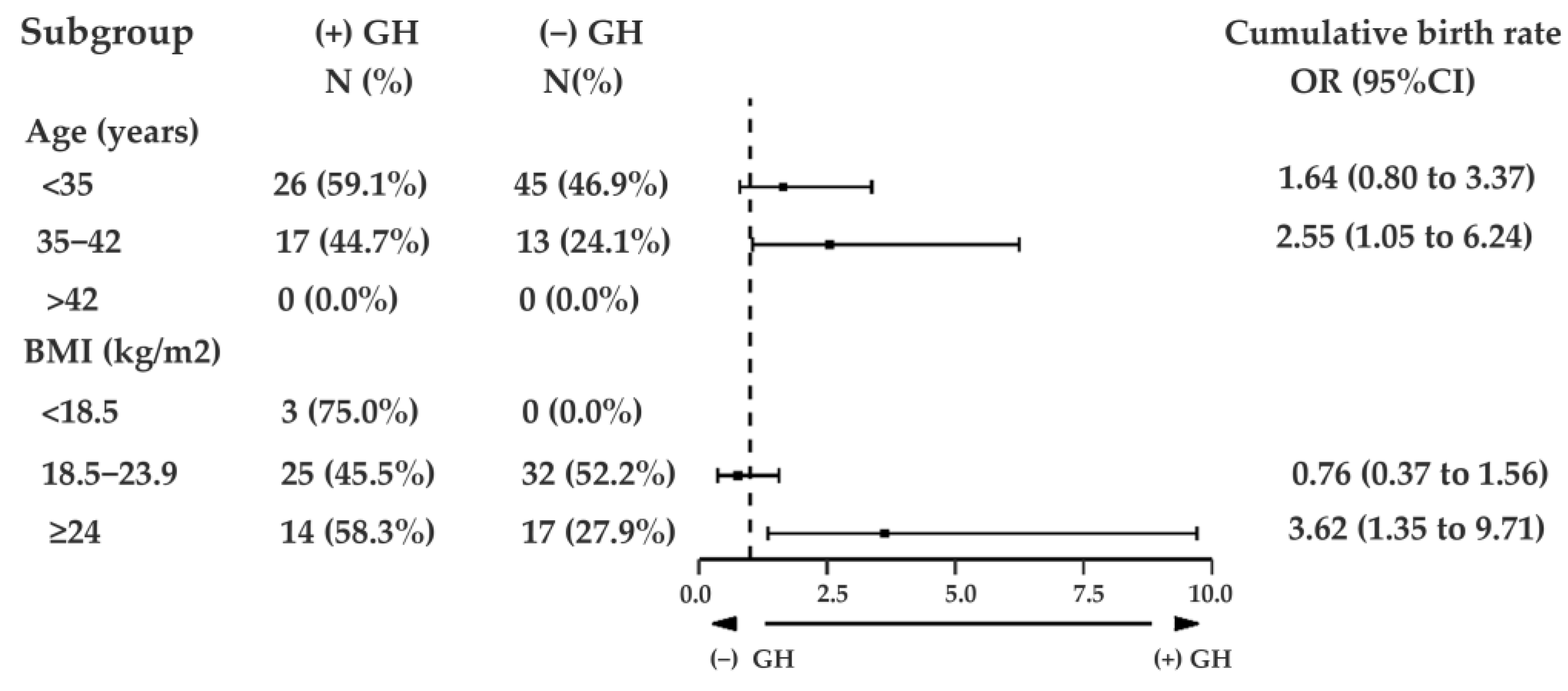
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Giorgetti, C.; Terriou, P.; Auquier, P.; Hans, E.; Spach, J.L.; Salzmann, J.; Roulier, R. Embryo score to predict implantation after in-vitro fertilization: Based on 957 single embryo transfers. Hum. Reprod. 1995, 10, 2427–2431. [Google Scholar] [CrossRef] [PubMed]
- Awadalla, M.; Kim, A.; Vestal, N.; Ho, J.; Bendikson, K. Effect of Age and Embryo Morphology on Live Birth Rate After Transfer of Unbiopsied Blastocysts. JBRA Assist. Reprod. 2021, 25, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Meldrum, D.R.; Casper, R.F.; Diez-Juan, A.; Simon, C.; Domar, A.D.; Frydman, R. Aging and the environment affect gamete and embryo potential: Can we intervene? Fertil. Steril. 2016, 105, 548–559. [Google Scholar] [CrossRef] [PubMed]
- Moller, N.; Jorgensen, J.O. Effects of growth hormone on glucose, lipid, and protein metabolism in human subjects. Endocr. Rev. 2009, 30, 152–177. [Google Scholar] [CrossRef] [PubMed]
- Caballero-Villarraso, J.; Aguado, R.; Canete, M.D.; Roldan, L.; Canete, R.; Santamaria, M. Hormone replacement therapy in children with growth hormone deficiency: Impact on immune profile. Arch. Physiol. Biochem. 2021, 127, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Abir, R.; Garor, R.; Felz, C.; Nitke, S.; Krissi, H.; Fisch, B. Growth hormone and its receptor in human ovaries from fetuses and adults. Fertil. Steril. 2008, 90, 1333–1339. [Google Scholar] [CrossRef]
- Frank, S.J. Classical and novel GH receptor signaling pathways. Mol. Cell Endocrinol. 2020, 518, 110999. [Google Scholar] [CrossRef]
- Chhabra, Y.; Lee, C.M.M.; Muller, A.F.; Brooks, A.J. GHR signalling: Receptor activation and degradation mechanisms. Mol. Cell Endocrinol. 2021, 520, 111075. [Google Scholar] [CrossRef]
- Cui, N.; Li, A.M.; Luo, Z.Y.; Zhao, Z.M.; Xu, Y.M.; Zhang, J.; Yang, A.M.; Wang, L.L.; Hao, G.M.; Gao, B.L. Effects of growth hormone on pregnancy rates of patients with thin endometrium. J. Endocrinol. Investig. 2019, 42, 27–35. [Google Scholar] [CrossRef]
- Van den Eijnden, M.J.; Strous, G.J. Autocrine growth hormone: Effects on growth hormone receptor trafficking and signaling. Mol. Endocrinol. 2007, 21, 2832–2846. [Google Scholar] [CrossRef]
- Safdarian, L.; Aghahosseini, M.; Alyasin, A.; Samaei Nouroozi, A.; Rashidi, S.; Shabani Nashtaei, M.; Najafian, A.; Lak, P. Growth Hormone (GH) Improvement of Ovarian Responses and Pregnancy Outcome in Poor Ovarian Responders: A Randomized Study. Asian Pac. J. Cancer Prev. 2019, 20, 2033–2037. [Google Scholar] [CrossRef] [PubMed]
- Regan, S.L.P.; Knight, P.G.; Yovich, J.L.; Arfuso, F.; Dharmarajan, A. Growth hormone during in vitro fertilization in older women modulates the density of receptors in granulosa cells, with improved pregnancy outcomes. Fertil. Steril. 2018, 110, 1298–1310. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Zhang, K.; Xiong, D.; Wei, J.; Tan, H.; Qin, S. Growth hormone alleviates oxidative stress and improves the IVF outcomes of poor ovarian responders: A randomized controlled trial. Reprod. Biol. Endocrinol. 2020, 18, 91. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, Q.; Wang, J.; Huang, G.; Ye, H. Does growth hormone supplementation improve oocyte competence and IVF outcomes in patients with poor embryonic development? A randomized controlled trial. BMC Pregnancy Childbirth 2020, 20, 310. [Google Scholar] [CrossRef] [PubMed]
- Tesarik, J.; Hazout, A.; Mendoza, C. Improvement of delivery and live birth rates after ICSI in women aged >40 years by ovarian co-stimulation with growth hormone. Hum. Reprod. 2005, 20, 2536–2541. [Google Scholar] [CrossRef] [PubMed]
- Bassiouny, Y.A.; Dakhly, D.M.R.; Bayoumi, Y.A.; Hashish, N.M. Does the addition of growth hormone to the in vitro fertilization/intracytoplasmic sperm injection antagonist protocol improve outcomes in poor responders? A randomized, controlled trial. Fertil. Steril. 2016, 105, 697–702. [Google Scholar] [CrossRef]
- Mohammad, E.H.; Abou El Serour, A.G.; Mohamed, E.A.H.; Abbasy, A.H.; Zaatar, M.; Rageh, K.A.; Shafeek, M.M.; Issak, E.R. Efficacy of growth hormone supplementation with ultrashort GnRH antagonist in IVF/ICSI for poor responders; randomized controlled trial. Taiwan J. Obstet. Gynecol. 2021, 60, 51–55. [Google Scholar] [CrossRef]
- Sood, A.; Mohiyiddeen, G.; Ahmad, G.; Fitzgerald, C.; Watson, A.; Mohiyiddeen, L. Growth hormone for in vitro fertilisation (IVF). Cochrane Database Syst Rev. 2021, 11, CD000099. [Google Scholar] [CrossRef]
- Sciorio, R.; Miranian, D.; Smith, G.D. Non-invasive oocyte quality assessment. Biol. Reprod. 2022, 106, 274–290. [Google Scholar] [CrossRef]
- Lasiene, K.; Vitkus, A.; Valanciute, A.; Lasys, V. Morphological criteria of oocyte quality. Medicina 2009, 45, 509–515. [Google Scholar] [CrossRef]
- Moghadam, A.R.E.; Moghadam, M.T.; Hemadi, M.; Saki, G. Oocyte quality and aging. JBRA Assist. Reprod. 2022, 26, 105–122. [Google Scholar] [CrossRef] [PubMed]
- Drakopoulos, P.; Errazuriz, J.; Santos-Ribeiro, S.; Tournaye, H.; Vaiarelli, A.; Pluchino, N.; Blockeel, C.; Polyzos, N.P. Cumulative live birth rates in in-vitro fertilization. Minerva Ginecol. 2019, 71, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Maheshwari, A.; McLernon, D.; Bhattacharya, S. Cumulative live birth rate: Time for a consensus? Hum. Reprod. 2015, 30, 2703–2707. [Google Scholar] [CrossRef]
- Alpha Scientists in Reproductive Medicine and ESHRE Special Interest Group of Embryology. The Istanbul consensus workshop on embryo assessment: Proceedings of an expert meeting. Hum. Reprod. 2011, 26, 1270–1283. [Google Scholar] [CrossRef] [PubMed]
- Meseguer, M.; Herrero, J.; Tejera, A.; Hilligsoe, K.M.; Ramsing, N.B.; Remohi, J. The use of morphokinetics as a predictor of embryo implantation. Hum. Reprod. 2011, 26, 2658–2671. [Google Scholar] [CrossRef]
- Nagler, H.M.; Virji, N.J.J.o.U. A Textbook of In Vitro Fertilization and Assisted Reproduction: The Bourn Hall Guide to Clinical and Laboratory Practice, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2000; Volume 164, pp. 264–265. [Google Scholar]
- Gardner, D.K.; Lane, M.; Stevens, J.; Schlenker, T.; Schoolcraft, W.B. Blastocyst score affects implantation and pregnancy outcome: Towards a single blastocyst transfer. Fertil. Steril. 2000, 73, 1155–1158. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, C.; Shu, J.; Guo, J.; Chang, H.M.; Leung, P.C.K.; Sheng, J.Z.; Huang, H. Adjuvant treatment strategies in ovarian stimulation for poor responders undergoing IVF: A systematic review and network meta-analysis. Hum. Reprod. Update 2020, 26, 247–263. [Google Scholar] [CrossRef] [PubMed]
- Adashi, E.Y.; Resnick, C.E.; D’Ercole, A.J.; Svoboda, M.E.; Van Wyk, J.J. Insulin-like growth factors as intraovarian regulators of granulosa cell growth and function. Endocr. Rev. 1985, 6, 400–420. [Google Scholar] [CrossRef]
- Bergh, C.; Carlstrom, K.; Selleskog, U.; Hillensjo, T. Effect of growth hormone on follicular fluid androgen levels in patients treated with gonadotropins before in vitro fertilization. Eur. J. Endocrinol. 1996, 134, 190–196. [Google Scholar] [CrossRef]
- Yoshimura, Y.; Ando, M.; Nagamatsu, S.; Iwashita, M.; Adachi, T.; Sueoka, K.; Miyazaki, T.; Kuji, N.; Tanaka, M. Effects of insulin-like growth factor-I on follicle growth, oocyte maturation, and ovarian steroidogenesis and plasminogen activator activity in the rabbit. Biol. Reprod. 1996, 55, 152–160. [Google Scholar] [CrossRef]
- Wang, J.; Wu, J.; Zhang, Y.; Zhang, J.; Xu, W.; Wu, C.; Zhou, P. Growth hormone protects against ovarian granulosa cell apoptosis: Alleviation oxidative stress and enhancement mitochondrial function. Reprod. Biol. 2021, 21, 100504. [Google Scholar] [CrossRef] [PubMed]
- Labarta, E.; de Los Santos, M.J.; Herraiz, S.; Escriba, M.J.; Marzal, A.; Buigues, A.; Pellicer, A. Autologous mitochondrial transfer as a complementary technique to intracytoplasmic sperm injection to improve embryo quality in patients undergoing in vitro fertilization-a randomized pilot study. Fertil. Steril. 2019, 111, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Bai, H.; Xie, J.; Shi, J. Growth hormone co-treatment on controlled ovarian stimulation in normal ovarian response women can improve embryo quality. Gynecol. Endocrinol. 2019, 35, 787–791. [Google Scholar] [CrossRef] [PubMed]
- Keane, K.N.; Yovich, J.L.; Hamidi, A.; Hinchliffe, P.M.; Dhaliwal, S.S. Single-centre retrospective analysis of growth hormone supplementation in IVF patients classified as poor-prognosis. BMJ Open 2017, 7, e018107. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.H.; Gao, L.Z.; Liang, X.Y.; Fang, C.; Wu, Y.Q.; Yang, X. The Effect of Growth Hormone on the Clinical Outcomes of Poor Ovarian Reserve Patients Undergoing in vitro Fertilization/Intracytoplasmic Sperm Injection Treatment: A Retrospective Study Based on POSEIDON Criteria. Front. Endocrinol. 2019, 10, 775. [Google Scholar] [CrossRef]
- Giustina, A.; Veldhuis, J.D. Pathophysiology of the neuroregulation of growth hormone secretion in experimental animals and the human. Endocr. Rev. 1998, 19, 717–797. [Google Scholar] [CrossRef]
- Liu, C.; Li, S.; Li, Y.; Tian, J.; Sun, X.; Song, T.; Yan, G.; Ding, L.; Sun, H. Growth hormone ameliorates the age-associated depletion of ovarian reserve and decline of oocyte quality via inhibiting the activation of Fos and Jun signaling. Aging 2021, 13, 6765–6781. [Google Scholar] [CrossRef]
- Yovich, J.L.; Regan, S.L.P.; Zaidi, S.; Keane, K.N. The Concept of Growth Hormone Deficiency Affecting Clinical Prognosis in IVF. Front. Endocrinol. 2019, 10, 650. [Google Scholar] [CrossRef]
- Rasmussen, M.H. Obesity, growth hormone and weight loss. Mol. Cell Endocrinol. 2010, 316, 147–153. [Google Scholar] [CrossRef]
- Steyn, F.J.; Xie, T.Y.; Huang, L.; Ngo, S.T.; Veldhuis, J.D.; Waters, M.J.; Chen, C. Increased adiposity and insulin correlates with the progressive suppression of pulsatile GH secretion during weight gain. J. Endocrinol. 2013, 218, 233–244. [Google Scholar] [CrossRef]
- Rasmussen, M.H.; Hvidberg, A.; Juul, A.; Main, K.M.; Gotfredsen, A.; Skakkebaek, N.E.; Hilsted, J.; Skakkebae, N.E. Massive weight loss restores 24-hour growth hormone release profiles and serum insulin-like growth factor-I levels in obese subjects. J. Clin. Endocrinol. Metab. 1995, 80, 1407–1415. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Huang, L.; Waters, M.J.; Chen, C. Insulin and Growth Hormone Balance: Implications for Obesity. Trends Endocrinol. Metab. 2020, 31, 642–654. [Google Scholar] [CrossRef] [PubMed]
- Bredella, M.A.; Gerweck, A.V.; Lin, E.; Landa, M.G.; Torriani, M.; Schoenfeld, D.A.; Hemphill, L.C.; Miller, K.K. Effects of GH on body composition and cardiovascular risk markers in young men with abdominal obesity. J. Clin. Endocrinol. Metab. 2013, 98, 3864–3872. [Google Scholar] [CrossRef] [PubMed]
- Kopchick, J.J.; Berryman, D.E.; Puri, V.; Lee, K.Y.; Jorgensen, J.O.L. The effects of growth hormone on adipose tissue: Old observations, new mechanisms. Nat. Rev. Endocrinol. 2020, 16, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Wu, R.; Zhang, H. The effect of growth hormone supplementation in poor ovarian responders undergoing IVF or ICSI: A meta-analysis of randomized controlled trials. Reprod. Biol. Endocrinol. 2020, 18, 76. [Google Scholar] [CrossRef]
- Ob’edkova, K.; Kogan, I.; Krikheli, I.; Dzhemlikhanova, L.; Muller, V.; Mekina, I.; Lesik, E.; Komarova, E.; Mazilina, M.; Niauri, D.; et al. Growth hormone co-treatment in IVF/ICSI cycles in poor responders. Gynecol. Endocrinol. 2017, 33, 15–17. [Google Scholar] [CrossRef]
- Cozzolino, M.; Cecchino, G.N.; Troiano, G.; Romanelli, C. Growth hormone cotreatment for poor responders undergoing in vitro fertilization cycles: A systematic review and meta-analysis. Fertil. Steril. 2020, 114, 97–109. [Google Scholar] [CrossRef]
- Lattes, K.; Brassesco, M.; Gomez, M.; Checa, M.A. Low-dose growth hormone supplementation increases clinical pregnancy rate in poor responders undergoing in vitro fertilisation. Gynecol. Endocrinol. 2015, 31, 565–568. [Google Scholar] [CrossRef]
- Martinez-Moreno, C.G.; Calderon-Vallejo, D.; Harvey, S.; Aramburo, C.; Quintanar, J.L. Growth Hormone (GH) and Gonado tropin-Releasing Hormone (GnRH) in the Central Nervous System: A Potential Neurological Combinatory Therapy? Int. J. Mol. Sci. 2018, 19, 375. [Google Scholar] [CrossRef]
- Busardo, F.P.; Frati, P.; Sanzo, M.D.; Napoletano, S.; Pinchi, E.; Zaami, S.; Fineschi, V. The impact of nandrolone decanoate on the central nervous system. Curr. Neuropharmacol. 2015, 13, 122–131. [Google Scholar] [CrossRef]
- Reed, M.L.; Merriam, G.R.; Kargi, A.Y. Adult growth hormone deficiency—Benefits, side effects, and risks of growth hormone replacement. Front. Endocrinol. 2013, 4, 64. [Google Scholar] [CrossRef] [PubMed]
- Patterson, B.C.; Chen, Y.; Sklar, C.A.; Neglia, J.; Yasui, Y.; Mertens, A.; Armstrong, G.T.; Meadows, A.; Stovall, M.; Robison, L.L.; et al. Growth hormone exposure as a risk factor for the development of subsequent neoplasms of the central nervous system: A report from the childhood cancer survivor study. J. Clin. Endocrinol. Metab. 2014, 99, 2030–2037. [Google Scholar] [CrossRef] [PubMed]
| (−) GH | (+) GH | p-Value | |
|---|---|---|---|
| n = 152 | n = 84 | ||
| Age (year) | 32.00 (29.00–36.00) | 34.00 (30.25–37.00) | 0.062 |
| BMI (kg/m2) | 23.40 (20.60–25.40) | 22.00 (20.40–24.00) | 0.070 |
| Duration of infertility (years) | 3.50 (2.33–5.73) | 3.42 (2.08–5.42) | 0.483 |
| Cause of infertility | 0.902 | ||
| 1—male factor | 10 (6.58%) | 7 (8.33%) | |
| 2—anovulation | 14 (9.21%) | 6 (7.14%) | |
| 3—tubal block | 71 (46.71%) | 36 (42.86%) | |
| 4—chromosomal abnormality | 8 (5.26%) | 7 (8.33%) | |
| 5—unexplained | 28 (18.42%) | 15 (17.86%) | |
| 6—combined | 21 (13.82%) | 13 (15.48%) | |
| AFC (n) | 14.00 (10.00–23.50) | 10.00 (8.00–15.00) | 0.001 * |
| Basal FSH (IU/L) | 6.90 (5.61–8.08) | 7.06 (6.08–8.32) | 0.239 |
| AMH (ng/mL) | 3.62 (2.28–5.64) | 2.85 (1.82–4.85) | 0.021 * |
| (−) GH | (+) GH | p-Value | |
|---|---|---|---|
| Cycles conducted with antagonist protocol (n) | 77 (50.65%) | 46 (54.76%) | 0.546 |
| Cycles conducted with long protocol (n) | 35 (23.03%) | 20 (23.81%) | 0.892 |
| Cycles conducted with other protocols (n) | 40 (26.32%) | 18 (21.43%) | 0.404 |
| Cycles conducted PGT | 25 (16.44%) | 13 (15.48%) | 0.846 |
| Length of Gn stimulation (day) | 9.00 (8.00–10.00) | 9.00 (8.00–9.75) | 0.001 * |
| Total dose of Gn stimulation (IU/mL) | 2250.00 (1800.00–2775.00) | 1950.00 (1575.00–2643.75) | 0.002 * |
| No. of follicles ≥16 mm at the day of trigger (n) | 8.00 (5.00–10.00) | 7.00 (6.00–9.00) | 0.694 |
| Endometrial thickness at the day of trigger (mm) | 10.00 (9.00–12.00) | 11.00 (8.50–12.75) | 0.455 |
| Peak E2 at the day of trigger (pg/mL) | 2964.00 (2069.25–5085.50) | 3480.50 (1760.00–4852.50) | 0.983 |
| No. of oocytes (n) | 12.00 (7.00–15.75) | 11.00 (7.00–15.00) | 0.747 |
| Fertilization type | 0.201 | ||
| IVF (n) | 71 (46.71%) | 32 (38.10%) | |
| ICSI (n) | 81 (53.29%) | 52 (61.90%) | |
| No. of 2PN (n) | 7.00 (4.00–10.75) | 8.00 (6.00–10.00) | 0.547 |
| No. of transferable embryos (n) | 6.00 (3.00–9.00) | 6.00 (4.00–9.00) | 0.265 |
| No. of good and fair quality embryos (n) | 2.00 (1.00–4.00) | 2.00 (0.25–5.00) | 0.449 |
| (−) GH | (+) GH | p-Value | |
|---|---|---|---|
| Fresh ET cycles (n) | 21 | 13 | |
| Fresh ET implantation rate, n (%) | 10/42 (23.8) | 3/24 (12.5) | 0.266 |
| Fresh ET chemical pregnancy rate, n (%) | 10/21 (47.6) | 3/13 (23.1) | 0.601 |
| Fresh ET clinical pregnancy rate, n (%) | 8/21 (38.1) | 3/13 (23.1) | 0.363 |
| Fresh ET miscarriage rate, n (%) | 1/21 (4.8) | 0/13 (0.0) | 0.425 |
| Fresh ET live birth rate, n (%) | 7/21 (33.3) | 3/13 (23.1) | 0.524 |
| Frozen ET cycles (n) | 122 | 68 | |
| Frozen ET implantation rate, n (%) | 71/182 (39.0) | 48/82 (58.5) | 0.003 * |
| Frozen ET chemical pregnancy rate, n (%) | 66/122 (54.1) | 48/68 (70.6) | 0.026 * |
| Frozen ET clinical pregnancy rate, n (%) | 65/122 (53.3) | 44/68 (64.7) | 0.127 |
| Frozen ET miscarriage rate, n (%) | 14/122 (11.5) | 4/68 (5.9) | 0.207 |
| Frozen ET live birth rate, n (%) | 51/122(41.8) | 40/68 (58.8) | 0.024 * |
| (−) GH | (+) GH | p-Value | |
|---|---|---|---|
| Clinical pregnancy | |||
| Fresh cycle, n (%) | 8/21 (38.1) | 3/13 (23.1) | 0.363 |
| Frozen cycle, n (%) | 65/122 (53.3) | 44/68 (64.7) | 0.127 |
| Clinical pregnancy rate per transfer cycle | 73/143 (51.0) | 47/81 (58.0) | 0.315 |
| Live birth | |||
| Fresh cycle, n (%) | 7/21 (33.3) | 3/13 (23.1) | 0.524 |
| Frozen cycle, n (%) | 51/122 (41.8) | 40/68 (58.8) | 0.024 * |
| Cumulative live birth rate per transfer cycle | 58/143 (40.6) | 43/81 (53.1) | 0.07 |
| Cumulative live birth rate per oocyte retrieval cycle | 58/152 (38.2) | 43/84 (51.2) | 0.053 |
| Cumulative Live Birth OR (95% CI) | ||||
|---|---|---|---|---|
| Univariate Analysis | p-Value | Multivariate Analysis | p-Value | |
| GH | ||||
| (−) GH | 1.00 | - | 1.00 | - |
| (+) GH | 1.70 (0.99–2.91) | 0.054 | 1.96 (1.06–3.64) | 0.032 * |
| Age | 0.88 (0.82–0.93) | <0.001 | 0.87 (0.81–0.93) | <0.001 * |
| BMI | 0.94 (0.87–1.02) | 0.115 | ||
| AMH | 1.02 (0.95–1.11) | 0.587 | ||
| FSH | 1.00 (0.89–1.13) | 0.988 | ||
| AFC | 1.00 (0.95–1.05) | 0.891 | ||
| No. of follicles ≥16 mm at the day of trigger (n) | 1.12 (1.04–1.20) | 0.004 | 1.08 (0.95–1.22) | 0.221 |
| Endometrial thickness at the day of trigger | 1.01 (0.91–1.12) | 0.830 | ||
| Number of oocytes | 1.06 (1.02–1.11) | 0.005 | 0.92 (0.83–1.03) | 0.131 |
| Number of 2PN | 1.12 (1.05–1.19) | 0.001 | 0.97 (0.80–1.17) | 0.722 |
| Number of transferable embryos | 1.18 (1.10–1.27) | <0.001 | 1.24 (1.02–1.51) | 0.033 * |
| Number of good quality embryos | 1.20 (1.08–1.33) | 0.001 | 1.03 (0.89–1.19) | 0.720 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, S.; Fu, L.; Zhang, W.; Zuo, N.; Guan, W.; Sun, H.; Wang, X. The Advantage of Growth Hormone Alone as an Adjuvant Therapy in Advanced Age and BMI ≥ 24 kg/m2 with In Vitro Fertilization Failure Due to Poor Embryo Quality. J. Clin. Med. 2023, 12, 955. https://doi.org/10.3390/jcm12030955
Jiang S, Fu L, Zhang W, Zuo N, Guan W, Sun H, Wang X. The Advantage of Growth Hormone Alone as an Adjuvant Therapy in Advanced Age and BMI ≥ 24 kg/m2 with In Vitro Fertilization Failure Due to Poor Embryo Quality. Journal of Clinical Medicine. 2023; 12(3):955. https://doi.org/10.3390/jcm12030955
Chicago/Turabian StyleJiang, Shuyi, Lingjie Fu, Wei Zhang, Na Zuo, Wenzheng Guan, Hao Sun, and Xiuxia Wang. 2023. "The Advantage of Growth Hormone Alone as an Adjuvant Therapy in Advanced Age and BMI ≥ 24 kg/m2 with In Vitro Fertilization Failure Due to Poor Embryo Quality" Journal of Clinical Medicine 12, no. 3: 955. https://doi.org/10.3390/jcm12030955
APA StyleJiang, S., Fu, L., Zhang, W., Zuo, N., Guan, W., Sun, H., & Wang, X. (2023). The Advantage of Growth Hormone Alone as an Adjuvant Therapy in Advanced Age and BMI ≥ 24 kg/m2 with In Vitro Fertilization Failure Due to Poor Embryo Quality. Journal of Clinical Medicine, 12(3), 955. https://doi.org/10.3390/jcm12030955






