Elective Tracheotomy in Patients Receiving Mandibular Reconstructions: Reduced Postoperative Ventilation Time and Lower Incidence of Hospital-Acquired Pneumonia
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
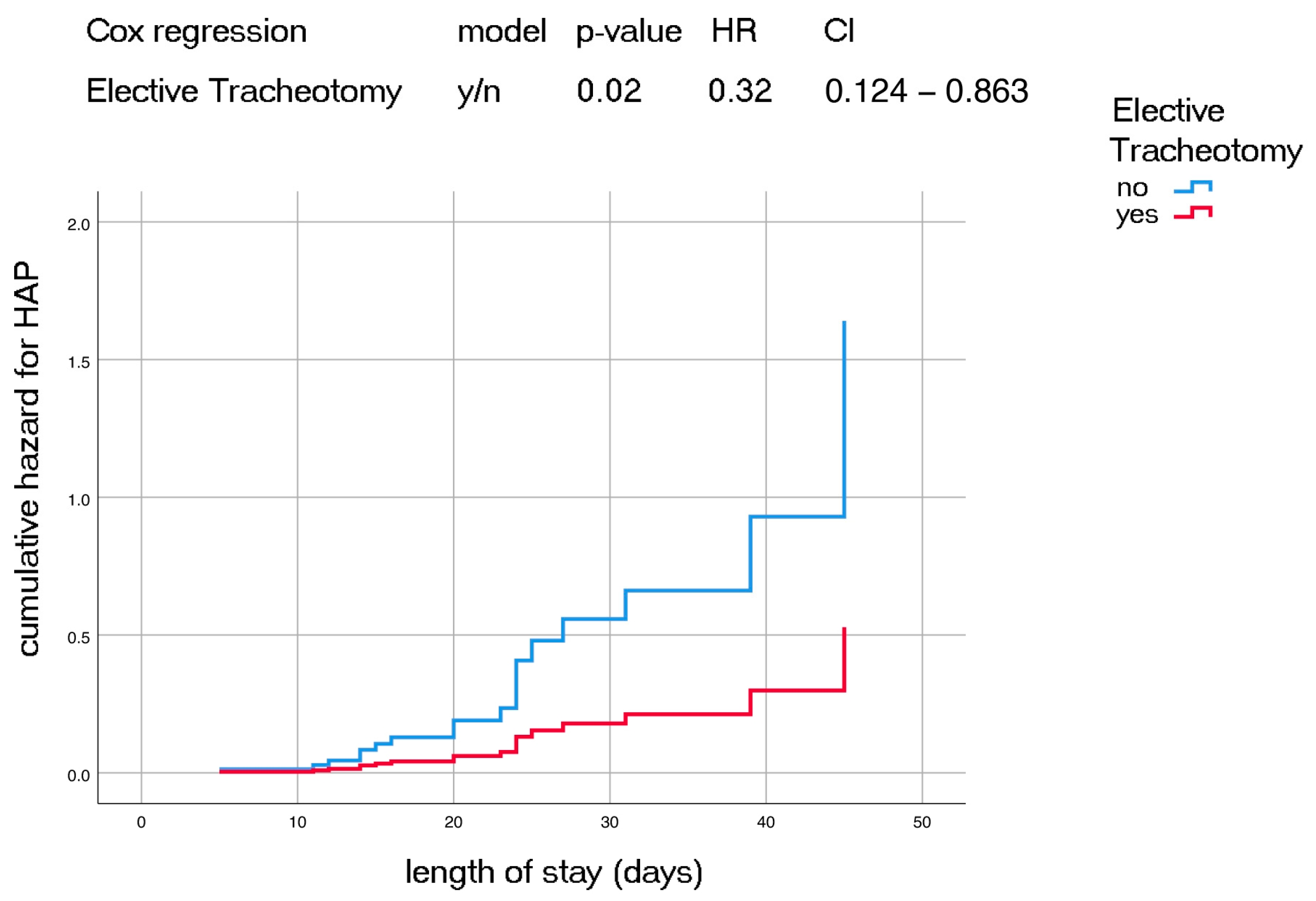
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vincent, A.; Kohlert, S.; Lee, T.S.; Inman, J.; Ducic, Y. Free-Flap Reconstruction of the Tongue. Semin. Plast. Surg. 2019, 33, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Torroni, A.; Marianetti, T.M.; Romandini, M.; Gasparini, G.; Cervelli, D.; Pelo, S. Mandibular Reconstruction with Different Techniques. J. Craniofac. Surg. 2015, 26, 885–890. [Google Scholar] [CrossRef] [PubMed]
- Meier, J.; Wunschel, M.; Angermann, A.; Ettl, T.; Metterlein, T.; Klingelhöffer, C.; Reichert, T.E.; Ritzka, M. Influence of early elective tracheostomy on the incidence of postoperative complications in patients undergoing head and neck surgery. BMC Anesthesiol. 2019, 19, 43. [Google Scholar] [CrossRef] [PubMed]
- Leiser, Y.; Barak, M.; Ghantous, Y.; Yehudai, N.; Abu el-naaj, I. Indications for Elective Tracheostomy in Reconstructive Surgery in Patients with Oral Cancer. J. Craniofac. Surg. 2017, 28, e18–e22. [Google Scholar] [CrossRef] [PubMed]
- Cramer, J.D.; Samant, S.; Greenbaum, E.; Patel, U.A. Association of Airway Complications with Free Tissue Transfer to the Upper Aerodigestive Tract with or without Tracheotomy. JAMA Otolaryngol. Head Neck Surg. 2016, 142, 1177–1183. [Google Scholar] [CrossRef]
- Lapis, P.N.; DeLacure, M.D.; Givi, B. Factors in Successful Elimination of Elective Tracheotomy in Mandibular Reconstruction with Microvascular Tissue. JAMA Otolaryngol. Head Neck Surg. 2016, 142, 46–51. [Google Scholar] [CrossRef]
- Moubayed, S.P.; Barker, D.A.; Razfar, A.; Nabili, V.; Blackwell, K.E. Microvascular Reconstruction of Segmental Mandibular Defects without Tracheostomy. Otolaryngol. Head Neck Surg. 2015, 152, 250–254. [Google Scholar] [CrossRef]
- Lin, H.-S.; Wang, D.; Fee, W.E.; Goode, R.L.; Terris, D.J. Airway Management after Maxillectomy: Routine Tracheostomy Is Unnecessary. Laryngoscope 2003, 113, 929–932. [Google Scholar] [CrossRef]
- Brickman, D.S.; Reh, D.D.; Schneider, D.S.; Bush, B.; Rosenthal, E.L.; Wax, M.K. Airway management after maxillectomy with free flap reconstruction. Head Neck 2013, 35, 1061–1065. [Google Scholar] [CrossRef]
- Brown, J.S.; Barry, C.; Ho, M.; Shaw, R. A new classification for mandibular defects after oncological resection. Lancet Oncol. 2016, 17, e23–e30. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Committee on Standards and Practice Parameters; Apfelbaum, J.L.; Hagberg, C.A.; Caplan, R.A.; Blitt, C.D.; Connis, R.T.; Nickinovich, D.G. Practice Guidelines for Management of the Difficult Airway: An Updated Report by the American Society of Anesthesiologists Task Force on Management of the Difficult Airway. Anesthesiology 2013, 118, 251–270. [Google Scholar] [CrossRef] [PubMed]
- Dalhoff, K.; Abele-Horn, M.; Andreas, S.; Deja, M.; Ewig, S.; Gastmeier, P.; Gatermann, S.; Gerlash, H.; Grabein, B.; Heuβel, C.P.; et al. Epidemiologie, Diagnostik und Therapie erwachsener Patienten mit nosokomialer Pneumonie—Update 2017. Pneumologie 2018, 72, 15–63. [Google Scholar] [CrossRef] [PubMed]
- Coyle, M.J.; Tyrrell, R.; Godden, A.; Hughes, C.W.; Perkins, C.; Thomas, S.; Godden, D. Replacing tracheostomy with overnight intubation to manage the airway in head and neck oncology patients: Towards an improved recovery. Br. J. Oral Maxillofac. Surg. 2013, 51, 493–496. [Google Scholar] [CrossRef]
- Singh, T.; Sankla, P.; Smith, G. Tracheostomy or delayed extubation after maxillofacial free-flap reconstruction? Br. J. Oral Maxillofac. Surg. 2016, 54, 878–882. [Google Scholar] [CrossRef]
- Gupta, K.; Mandlik, D.; Patel, D.; Patel, P.; Shah, B.; Vijay, D.G.; Kothari, J.M.; Toprani, R.B.; Patel, K.D. Clinical assessment scoring system for tracheostomy (CASST) criterion: Objective criteria to predict pre-operatively the need for a tracheostomy in head and neck malignancies. J. Cranio-Maxillofac. Surg. 2016, 44, 1310–1313. [Google Scholar] [CrossRef] [PubMed]
- Cameron, M.; Corner, A.; Diba, A.; Hankins, M. Development of a tracheostomy scoring system to guide airway management after major head and neck surgery. Int. J. Oral Maxillofac. Surg. 2009, 38, 846–849. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, K.K.; Baker, D.; Quinn, B. The epidemiology of nonventilator hospital-acquired pneumonia in the United States. Am. J. Infect. Control 2018, 46, 322–327. [Google Scholar] [CrossRef]
- Allak, A.; Nguyen, T.N.; Shonka, D.C.; Reibel, J.F.; Levine, P.A.; Jameson, M.J. Immediate postoperative extubation in patients undergoing free tissue transfer: Immediate Extubation after Free Flap Surgery. Laryngoscope 2011, 121, 763–768. [Google Scholar] [CrossRef]
- Tamplen, M.L.; Ricceri, S.; Hemmat, S.; Seth, R.; Ryan, W.R.; Knott, P.D. Benefits of Immediate Extubation Following Free Tissue Transfer for Head and Neck Reconstruction. J. Reconstr. Microsurg. 2016, 32, 533–539. [Google Scholar] [CrossRef]
- Xu, S.; Wang, K.; Liu, K.; Liu, Y.; Huang, Y.; Zhang, Y.; Wang, X.; Xu, Z.; Liu, S.; Liu, J. Predictive Nomogram for the Necessity of Tracheotomy During Oral and Oropharyngeal Cancer Surgery. Laryngoscope 2021, 131, E1489–E1495. [Google Scholar] [CrossRef]
- Schuderer, J.G.; Spörl, S.; Spanier, G.; Gottsauner, M.; Gessner, A.; Hitzenbichler, F.; Meier, J.K.; Reichert, T.E.; Ettl, T. Surgical and remote site infections after reconstructive surgery of the head and neck: A risk factor analysis. J. Cranio-Maxillofac. Surg. 2021, 50, 178–187. [Google Scholar] [CrossRef] [PubMed]
- McDevitt, J.; de Camargo Cancela, M.; Kelly, M.; Comber, H.; Sharp, L. Tracheostomy and infection prolong length of stay in hospital after surgery for head and neck cancer: A population based study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2016, 121, 22–28.e1. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Feng, L.; Lewis, C.M. A data review of airway management in patients with oral cavity or oropharyngeal cancer: A single-institution experience. BMC Anesthesiol. 2019, 19, 92. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-J.; Huang, E.-Y.; Hsu, H.-C.; Chen, H.-C.; Fang, F.-M.; Hsiung, C.-Y. The Degree and Time-Course Assessment of Radiation-Induced Trismus Occurring after Radiotherapy for Nasopharyngeal Cancer. Laryngoscope 2005, 115, 1458–1460. [Google Scholar] [CrossRef]
- Deng, J.; Ridner, S.H.; Dietrich, M.S.; Wells, N.; Wallston, K.A.; Sinard, R.J.; Cmelak, A.J.; Murphy, B.A. Prevalence of Secondary Lymphedema in Patients With Head and Neck Cancer. J. Pain Symptom. Manag. 2012, 43, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Kruse-Lösler, B.; Langer, E.; Reich, A.; Joos, U.; Kleinheinz, J. Score system for elective tracheotomy in major head and neck tumour surgery. Acta Anaesthesiol. Scand. 2005, 49, 654–659. [Google Scholar] [CrossRef]
- Chughtai, M.; Gwam, C.U.; Mohamed, N.; Khlopas, A.; Newman, J.M.; Khan, R.; Nadhim, A.; Shaffiy, S.; Mont, M.A. The Epidemiology and Risk Factors for Postoperative Pneumonia. J. Clin. Med. Res. 2017, 9, 466–475. [Google Scholar] [CrossRef]
- Scheld, W.M.; Mandell, G.L. Nosocomial Pneumonia: Pathogenesis and Recent Advances in Diagnosis and Therapy. Clin. Infect. Dis. 1991, 13, S743–S751. [Google Scholar] [CrossRef]
- Loeffelbein, D.J.; Julinek, A.; Wolff, K.-D.; Kochs, E.; Haller, B.; Haseneder, R. Perioperative risk factors for postoperative pulmonary complications after major oral and maxillofacial surgery with microvascular reconstruction: A retrospective analysis of 648 cases. J. Cranio-Maxillofac. Surg. 2016, 44, 952–957. [Google Scholar] [CrossRef]
- Xu, J.; Hu, J.; Yu, P.; Wang, W.; Hu, X.; Hou, J.; Fang, S.; Liu, X. Perioperative risk factors for postoperative pneumonia after major oral cancer surgery: A retrospective analysis of 331 cases. PLoS ONE 2017, 12, e0188167. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yuan, W.; Zhang, S.; Wang, K.; Ruan, H. Analysis of Risk Factors for Pneumonia in 482 Patients Undergoing Oral Cancer Surgery with Tracheotomy. J. Oral Maxillofac. Surg. 2016, 74, 415–419. [Google Scholar] [CrossRef] [PubMed]
- Georges, H.; Leroy, O.; Guery, B.; Alfandari, S.; Beaucaire, G. Predisposing Factors for Nosocomial Pneumonia in Patients Receiving Mechanical Ventilation and Requiring Tracheotomy. Chest J. 2000, 118, 767–774. [Google Scholar] [CrossRef] [PubMed]
- El Solh, A.A.; Pietrantoni, C.; Bhat, A.; Bhora, M.; Berbary, E. Indicators of potentially drug resistant bacteria in severe nursing home acquired pneumonia. Chest J. 2004, 126, 845S. [Google Scholar] [CrossRef] [PubMed]
- Wagner, C.; Marchina, S.; Deveau, J.A.; Frayne, C.; Sulmonte, K.; Kumar, S. Risk of Stroke-Associated Pneumonia and Oral Hygiene. CED 2016, 41, 35–39. [Google Scholar] [CrossRef]
- Bøje, C.R.; Dalton, S.O.; Primdahl, H.; Kristensen, C.A.; Andersen, E.; Johansen, J.; Anderson, L.J.; Overgaard, J. Evaluation of comorbidity in 9388 head and neck cancer patients: A national cohort study from the DAHANCA database. Radiother. Oncol. 2014, 110, 91–97. [Google Scholar] [CrossRef]
- Kashlan, K.N.; Williams, A.M.; Chang, S.S.; Yaremchuk, K.L.; Mayerhoff, R. Analysis of patient factors associated with 30-day mortality after tracheostomy. Laryngoscope 2019, 129, 847–851. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Bahethi, R.; Yang, A.; Gray, M.; Wong, K.; Courey, M. Effect of Patient Demographics and Tracheostomy Timing and Technique on Patient Survival. Laryngoscope 2021, 131, 1468–1473. [Google Scholar] [CrossRef]
- Katna, R.; Kalyani, N.; Agarwal, S.; Singh, S.; Deshpande, A.; Bhosale, B.; Mumbai Oncology Group—Head and Neck. Impact of comorbidities on perioperative outcomes for carcinoma of oral cavity. Ann. R. Coll. Surg. Engl. 2020, 102, 232–235. [Google Scholar] [CrossRef]
- Shiiba, M.; Takei, M.; Nakatsuru, M.; Bukawa, H.; Yokoe, H.; Uzawa, K.; Tanzawa, H. Clinical observations of postoperative delirium after surgery for oral carcinoma. Int. J. Oral Maxillofac. Surg. 2009, 38, 661–665. [Google Scholar] [CrossRef]
- Gazda, A.J.; Kwak, M.J.; Jani, P.; Dinh, K.; Hussain, R.; Dronavalli, G.; Warner, M.; De Amas, I.S.; Kumar, S.; Natham, S.; et al. Association Between Early Tracheostomy and Delirium in Older Adults in the United States. J. Cardiothorac. Vasc. Anesth. 2021, 35, 1974–1980. [Google Scholar] [CrossRef] [PubMed]
- Taxis, J.; Spoerl, S.; Broszio, A.; Eichberger, J.; Grau, E.; Schuderer, J.; Ludwig, N.; Gottsauner, M.; Spanier, G.; Bundscherer, A.; et al. Postoperative Delirium after Reconstructive Surgery in the Head and Neck Region. J. Clin. Med. 2022, 11, 6630. [Google Scholar] [CrossRef] [PubMed]
| Ventilation ≤ 48 h N = 71 | Ventilation > 48 h N = 52 | p-Value | No HAP N = 104 | HAP N = 19 | p-Value | ||
|---|---|---|---|---|---|---|---|
| Sex | Men | 45 (63.4%) | 36 (69.2%) | 70 (67.3%) | 11 (57.9%) | ||
| Women | 26 (36.6%) | 16 (30.8%) | 34 (32.7%) | 8 (42.1%) | |||
| Age | years | 57.3 ± 13.9 | 56.7 ± 10.9 | 56.7 ± 13.1 | 58.7 ± 9.9 | ||
| Previous surgery head neck | Yes | 43 (60.6%) | 30 (57.7%) | 60 (57.7%) | 13 (68.4%) | ||
| Diagnosis | |||||||
| Tumor | 44 (62%) | 35 (67.3%) | 71 (68.3%) | 8 (42.1%) | |||
| RONJ | 16 (22.5%) | 15 (28.8%) | 23 (22.1%) | 8 (42.1%) | |||
| MRONJ | 2 (2.8%) | 1 (1.9%) | 2 (1.9%) | 1 (5.3%) | |||
| Osteomyelitis | 8 (11.3%) | 1 (1.9%) | 7 (6.7%) | 2 (10.5%) | |||
| Trauma | 1 (1.4%) | 1 (1%) | |||||
| Overall Tracheotomy | Yes | 45 (63.4%) | 37 (71.2%) | 68 (65.4%) | 14 (73.7%) | ||
| Elective tracheotomy | Yes | 44 (62%) | 26 (50%) | 60 (57.7%) | 10 (52.6%) | ||
| Unplanned tracheotomy | Yes | 1 (1.4%) | 11 (21.2%) | <0.001 | 8 (7.7%) | 4 (21.1%) | |
| No tracheotomy | Yes | 26 (36.6%) | 15 (28.8%) | 36 (34.6%) | 5 (26.3%) | ||
| Closure of tracheotomy | days | 15.1 ± 10.2 | 23.6 ± 19.1 | 0.03 | 18.8 ± 15.9 | 21.6 ± 14.1 | |
| Complication Tracheostoma site | Yes | 10 (14.1%) | 11 (21.2%) | 17 (16.3%) | 4 (21.1%) | ||
| Difficult airway | Yes | 23 (32.4%) | 21 (40.4%) | 33 (31.7%) | 11 (57.9%) | 0.03 | |
| Oral intake after surgery | days | 12.7 ± 6.5 | 18.1 ± 7.3 | 0.04 | 15.1 ± 6.8 | 15.8 ± 8.7 | |
| Neck dissection | Yes | 47 (66.2%) | 42 (80.8%) | 76 (73.1%) | 13 (68.4%) | ||
| Brown Classification | |||||||
| I | 13 (18.3%) | 8 (15.4%) | 19 (18.3%) | 2 (10.5%) | |||
| Ic | 1 (1.4%) | 3 (5.8%) | 3(2.9%) | 1 (5.3%) | |||
| II | 21 (29.6%) | 9 (17.3%) | 28 (26.9%) | 2 (10.5%) | |||
| IIc | 1 (1.4%) | 3 (5.8%) | 3 (2.9%) | 1 (5.3%) | |||
| III | 29 (40.8%) | 26 (50%) | 45 (43.3%) | 10 (52.6%) | |||
| IV | 5 (7%) | 4(3.8%) | 1 (5.3%) | ||||
| IVc | 1 (1.4%) | 1 (5.3%) | |||||
| Flap type | |||||||
| Fibula | 64 (90.1%) | 46 (88.5%) | 92 (88.5%) | 18 (94.7%) | |||
| DCIA | 6 (8.5%) | 5 (9.6%) | 10 (9.6%) | 1 (5.3%) | |||
| Scapula | 1 (1.4%) | 1 (1.9%) | 2 (1.9%) | ||||
| Bone Segments | n | 1.9 ± 0.8 | 2.1 ± 0.8 | 1.9 ± 0.8 | 2.4 ± 0.7 | 0.03 | |
| Flap Revision | Yes | 4 (5.6%) | 16 (30.8%) | <0.001 | 17 (16.3%) | 3 (15.8%) | |
| Flap success | Yes | 64 (90.1%) | 40 (76.9%) | 0.05 | 87 (83.7%) | 17 (89.5%) | |
| Partial Flap Loss | Yes | 23 (32.4%) | 19 (36.5%) | 35 (33.7%) | 7 (36.8%) | ||
| Donor Site Complication | Yes | 17 (23.6%) | 16 (30.8%) | 30 (28.8%) | 3 (15.8%) | ||
| Flap Site Complication | Yes | 35 (49.3%) | 35 (67.3%) | 0.05 | 60 (57.7%) | 10 (52.6%) | |
| Delirium | Yes | 5 (7%) | 14 (26.9%) | 0.003 | 12 (11.5%) | 7 (36.8%) | 0.005 |
| Anaesthesia induction time | Ø min | 73.2± 22.8 | 64.5 ± 23.1 | 0.04 | 67.6 ± 23 | 80.2 ± 22.2 | 0.03 |
| Length of surgery | Ø min | 504 ± 120.3 | 589.9 ± 184.6 | 541.5 ± 149.9 | 533.6 ± 190.7 | ||
| Preoperative Radiotherapy | Yes | 30 (42.3%) | 22 (42.3%) | 42 (40.4%) | 10 (52.6%) | ||
| Cumulative dose | Gray | 62.2 ±9.5 | 64.9 ± 7.5 | 62.9 ± 8.5 | 64.7 ± 11.1 | ||
| Normal ward | days | 16.4 ± 8.5 | 18.1 ± 10 | 17.4 ± 8.5 | 15.4 ± 12.3 | ||
| Intensive Care Unit | days | 2.3 ± 1.1 | 8 ± 5.6 | <0.001 | 3.9 ± 3.6 | 7.6 ± 6.3 | <0.001 |
| Length of stay | days | 18.7 ± 8.6 | 25.1 ± 9.6 | <0.001 | 21.1 ±9.1 | 23.1 ±2.6 | |
| Postoperative ventilation time | days | 1.8 ± 0.4 | 4.6 ± 1.8 | 2.7 ± 1.6 | 4.3 ± 2.6 | 0.001 | |
| Ventilation > 48 h | y/n | 40 (38.5%) | 12 (63.2%) | ||||
| BMI | kg/m2 | 24.2 ± 4.7 | 23.3 ± 4.5 | 23.6 ± 4.3 | 24.7 ± 6.4 | ||
| Smoking | Yes | 49 (69%) | 38 (73.1%) | 73 (70.2%) | 14 (73.3%) | ||
| Alcohol | Yes | 37 (52.1%) | 32 (61.5%) | 60 (57.7%) | 9 (47.4%) | ||
| Coronary heart disease | Yes | 7 (9.9%) | 6 (11.5%) | 11 (10.6%) | 2 (10.5%) | ||
| Hypertension | Yes | 32 (45.1%) | 22 (42.3%) | 44 (42.3%) | 10 (52.6%) | ||
| Charlson Comorbidity Index | Points | 2.9 ± 1.9 | 3.33 ± 2.1 | 3.1 ± 2 | 3 ± 1.9 | ||
| COPD | Yes | 8 (11.3%) | 8 (15.4%) | 13 (12.5%) | 3 (15.8%) | ||
| HAP | Yes | 7 (9.9%) | 12 (23.1%) | 0.04 |
| Elective Tracheotomy Yes N = 70 | Unplanned Tracheotomy Yes N = 12 | p-Value | ||
|---|---|---|---|---|
| Sex | Men | 46 (65.7%) | 8 (66.7%) | |
| Women | 24 (34.3%) | 4 (33.3%) | ||
| Age | Ø years | 59.5 ± 10.8 | 56.6 ± 13.4 | |
| Previous surgery head neck | Yes | 32 (45.7%) | 8 (66.7%) | |
| Diagnosis | ||||
| Tumor | 53 (75.7%) | 7 (58.3%) | ||
| RONJ | 12 (17.1%) | 4 (33.3%) | ||
| MRONJ | 1 (1.4%) | 1 (8.3%) | ||
| Osteomyelitis | 4 (5.7%) | |||
| Trauma | - | |||
| Closure of tracheotomy | days | 17.3 ± 9.1 | 28.9 ± 32.6 | 0.03 |
| Complication Tracheostoma site | Yes | 16 (22.9%) | 5 (41.7%) | |
| Difficult airway | Yes | 18 (25.7%) | 7 (58.3%) | 0.02 |
| Oral intake after surgery | days | 14.8 ± 6.7 | 16.4 ± 7.3 | |
| Neck dissection | Yes | 58 (82.9%) | 9 (75%) | |
| Brown Classification | ||||
| I | 7 (10%) | |||
| Ic | 1 (1.4%) | 1 (8.3%) | ||
| II | 14 (20%) | 3 (25%) | ||
| IIc | 3 (4.3%) | 1 (8.3%) | ||
| III | 39 (55.7%) | 5 (41.7%) | ||
| IV | 5 (7.1%) | |||
| IVc | 1 (1.4%) | |||
| Flap type | ||||
| Fibula | 65 (92.9%) | 9 (75%) | ||
| DCIA | 4 (5.7%) | 3 (25%) | ||
| Scapula | 1 (1.4%) | |||
| Bone Segments | n | 2.1 ± 0.8 | 2.1 ± 0.8 | |
| Flap Revision | Yes | 16 (22.9%) | 2 (16.7%) | |
| Flap success | Yes | 56 (80%) | 9 (75%) | |
| Partial Flap Loss | Yes | 26 (37.1%) | 4 (33.3%) | |
| Donor Site Complication | Yes | 24 (34.3%) | 2 (16.7%) | |
| Flap Site Complication | Yes | 44 (62.9%) | 9 (75%) | |
| Delirium | Yes | 13 (18.6%) | 6 (50%) | 0.02 |
| Anaesthesia induction time | min | 68.2 ± 23.8 | 68.3 ± 24.5 | |
| Length of surgery | min | 598.1 ± 149.3 | 523.3 ± 148.4 | |
| Preoperative Radiotherapy | Yes | 20 (28.6%) | 5 (41.7%) | |
| Cumulative dose | Gray | 64.6 ± 6.6 | 65.4 ± 3.1 | |
| Normal ward | days | 21 ± 9.2 | 14.8 ± 9.4 | 0.03 |
| Intensive Care Unit | days | 4.4 ± 3.9 | 9.7 ± 6 | <0.001 |
| Length of stay | days | 25 ± 9.5 | 24.2 ± 8.6 | |
| Postoperative ventilation time | days | 2.7 ± 1.4 | 5.7 ± 2.2 | <0.001 |
| Ventilation > 48 h | y/n | 26 (37.1%) | 11 (91.7%) | <0.001 |
| BMI | kg/m2 | 23.8 ± 4.5 | 24.2 ± 6.2 | |
| Smoking | Yes | 51 (72.9%) | 9 (75%) | |
| Alcohol | Yes | 43 (61.4%) | 7 (85.3%) | |
| Coronary heart disease | Yes | 8 (11.4%) | 2 (16.7%) | |
| Hypertension | Yes | 33 (47.1%) | 7 (58.3%) | |
| Charlson Comorbidity Index | Points | 3.6 ± 1.9 | 3.2 ± 2.3 | |
| COPD | Yes | 10 (14.3%) | ||
| HAP | Yes | 10 (14.3%) | 4 (33.3%) |
| No Tracheotomy N= 41 | Tracheotomy N = 82 | p-Value | ||
|---|---|---|---|---|
| Age | years | 52.9 ± 14.6 | 59.1 ± 11.1 | 0.01 |
| Previous surgery head neck | Yes | 33 (80.5%) | 40 (48.8%) | |
| Diagnosis | 0.03 | |||
| Tumor | 19 (46.3%) | 60 (73.2%) | ||
| RONJ | 15 (36.6%) | 16 (19.5%) | ||
| MRONJ | 1 (2.4%) | 2 (2.4%) | ||
| Osteomyelitis | 5 (12.2%) | 4 (4.9%) | ||
| Trauma | 1 (2.4%) | |||
| Neck dissection | Yes | 22 (53.7%) | 67 (81.7%) | 0.001 |
| Brown Classification | 0.001 | |||
| I | 14 (34.1%) | 7 (8.8%) | <0.001 | |
| III | 11 (26.8%) | 44 (55%) | 0.04 | |
| Bone Segments | n | 1.73 ± 0.8 | 2.1 ± 0.8 | 0.01 |
| Flap success | Yes | 39 (95.1%) | 65 (79.3%) | 0.02 |
| Flap Site Complication | Yes | 17 (41.5%) | 53 (64.6%) | 0.01 |
| Length of surgery | min | 446.8 ± 122 | 587.2 ± 150.6 | <0.001 |
| Preoperative Radiotherapy | Yes | 27 (65.9%) | 25 (30.5%) | |
| Normal ward | days | 11.2 ± 4.8 | 20.1 ± 9.4 | <0.001 |
| Intensive Care Unit | days | 3.9 ± 4.8 | 4.8 ± 4.4 | 0.05 |
| Ventilation > 48 h | y/n | 15 (36.6%) | 37 (45.1%) | |
| Charlson Comorbidity Index | Points | 2.2 ±1.9 | 3.6 ± 1.9 | <0.001 |
| Variable | Coding | p-Value | OR | CI | |
|---|---|---|---|---|---|
| Ventilation > 48 h | |||||
| Operation time | Ø min | 0.005 | 1.004 | 1.001–1.007 | |
| Difficult airway | y/n | 0.02 | 2.869 | 1.161–7.088 | |
| Elective tracheotomy | y/n | 0.006 | 0.054 | 0.007–0.440 | |
| HAP | |||||
| Intensive Care Unit | Ø days | 0.001 | 1.268 | 1.101–1.461 | |
| Induction time | Ø min | 0.009 | 1.039 | 1.009–1.069 | |
| Elective tracheotomy | y/n | 0.047 | 0.32 | 0.104–0.983 | |
| Elective Tracheotomy | |||||
| Preoperative Radiation | y/n | 0.018 | 2.81 | 1.184–6.257 | |
| Charlson Comorbidity Index | Ø points | 0.017 | 1.325 | 1.052–1.668 | |
| Brown Class III | y/n | 0.038 | 2.646 | 1.055–6.637 | |
| Unplanned Tracheotomy | |||||
| Difficult airway | y/n | 0.03 | 4.711 | 1.145–19.389 | |
| Delirium | y/n | 0.01 | 6.761 | 1.594–29.229 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schuderer, J.G.; Reider, L.; Wunschel, M.; Spanier, G.; Spoerl, S.; Gottsauner, M.J.; Maurer, M.; Meier, J.K.; Kummer, P.; Reichert, T.E.; et al. Elective Tracheotomy in Patients Receiving Mandibular Reconstructions: Reduced Postoperative Ventilation Time and Lower Incidence of Hospital-Acquired Pneumonia. J. Clin. Med. 2023, 12, 883. https://doi.org/10.3390/jcm12030883
Schuderer JG, Reider L, Wunschel M, Spanier G, Spoerl S, Gottsauner MJ, Maurer M, Meier JK, Kummer P, Reichert TE, et al. Elective Tracheotomy in Patients Receiving Mandibular Reconstructions: Reduced Postoperative Ventilation Time and Lower Incidence of Hospital-Acquired Pneumonia. Journal of Clinical Medicine. 2023; 12(3):883. https://doi.org/10.3390/jcm12030883
Chicago/Turabian StyleSchuderer, Johannes G., Leonie Reider, Michael Wunschel, Gerrit Spanier, Steffen Spoerl, Maximilian Josef Gottsauner, Michael Maurer, Johannes K. Meier, Peter Kummer, Torsten E. Reichert, and et al. 2023. "Elective Tracheotomy in Patients Receiving Mandibular Reconstructions: Reduced Postoperative Ventilation Time and Lower Incidence of Hospital-Acquired Pneumonia" Journal of Clinical Medicine 12, no. 3: 883. https://doi.org/10.3390/jcm12030883
APA StyleSchuderer, J. G., Reider, L., Wunschel, M., Spanier, G., Spoerl, S., Gottsauner, M. J., Maurer, M., Meier, J. K., Kummer, P., Reichert, T. E., & Ettl, T. (2023). Elective Tracheotomy in Patients Receiving Mandibular Reconstructions: Reduced Postoperative Ventilation Time and Lower Incidence of Hospital-Acquired Pneumonia. Journal of Clinical Medicine, 12(3), 883. https://doi.org/10.3390/jcm12030883








