Impact of the 3% Oxygen Desaturation Index via Overnight Pulse Oximetry on Cardiovascular Events and Death in Patients Undergoing Hemodialysis: A Retrospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Measurements and Definitions
2.3. ODI
2.4. Patient and Data Collections
2.5. Study Endpoints
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics
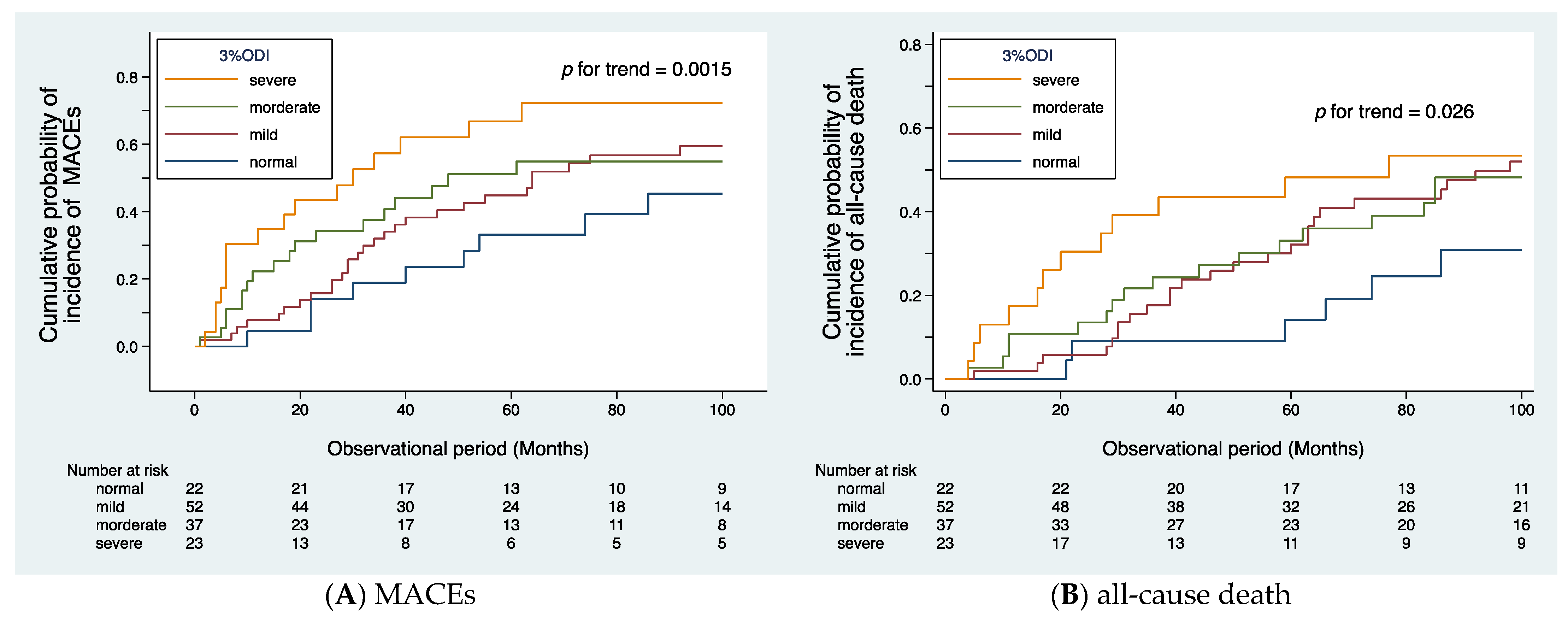
3.2. Primary Endpoints
3.3. Secondary Endpoints
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Somers, V.K.; Dyken, M.E.; Clary, M.P.; Abboud, F.M. Sympathetic neural mechanisms in obstructive sleep apnea. J. Clin. Investig. 1995, 96, 1897–1904. [Google Scholar] [CrossRef] [PubMed]
- Pensuksan, W.C.; Chen, X.; Lohsoonthorn, V.; Lertmaharit, S.; Gelaye, B.; Williams, M.A. High risk for obstructive sleep apnea in relation to hypertension among southeast Asian young adults: Role of obesity as an effect modifier. Am. J. Hypertens. 2014, 27, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Marin, J.M.; Agusti, A.; Villar, I.; Forner, M.; Nieto, D.; Carrizo, S.J.; Barbe, F.; Vicente, E.; Wei, Y.; Nieto, F.J.; et al. Association between treated and untreated obstructive sleep apnea and risk of hypertension. JAMA 2012, 307, 2169–2176. [Google Scholar] [CrossRef]
- Yaggi, H.K.; Concato, J.; Kernan, W.N.; Lichtman, J.H.; Brass, L.M.; Mohsenin, V. Obstructive sleep apnea as a risk factor for stroke and death. N. Engl. J. Med. 2005, 353, 2034–2041. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.G.; Johnson, D.C. Obstructive sleep apnea is a risk factor for stroke and atrial fibrillation. Chest 2010, 138, 239. [Google Scholar] [CrossRef] [PubMed]
- King, S.; Cuellar, N. Obstructive Sleep Apnea as an Independent Stroke Risk Factor: A Review of the Evidence, Stroke Prevention Guidelines, and Implications for Neuroscience Nursing Practice. J. Neurosci. Nurs. 2016, 48, 133–142. [Google Scholar] [CrossRef]
- Gottlieb, D.J.; Yenokyan, G.; Newman, A.B.; O’Connor, G.T.; Punjabi, N.M.; Quan, S.F.; Redline, S.; Resnick, H.E.; Tong, E.K.; Diener-West, M.; et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: The sleep heart health study. Circulation 2010, 122, 352–360. [Google Scholar] [CrossRef]
- Yumino, D.; Redolfi, S.; Ruttanaumpawan, P.; Su, M.C.; Smith, S.; Newton, G.E.; Mak, S.; Bradley, T.D. Nocturnal rostral fluid shift: A unifying concept for the pathogenesis of obstructive and central sleep apnea in men with heart failure. Circulation 2010, 121, 1598–1605. [Google Scholar] [CrossRef]
- Kasai, T.; Bradley, T.D. Obstructive sleep apnea and heart failure: Pathophysiologic and therapeutic implications. J. Am. Coll. Cardiol. 2011, 57, 119–127. [Google Scholar] [CrossRef]
- Gami, A.S.; Howard, D.E.; Olson, E.J.; Somers, V.K. Day-night pattern of sudden death in obstructive sleep apnea. New Engl. J. Med. 2005, 352, 1206–1214. [Google Scholar] [CrossRef]
- Gami, A.S.; Olson, E.J.; Shen, W.K.; Wright, R.S.; Ballman, K.V.; Hodge, D.O.; Herges, R.M.; Howard, D.E.; Somers, V.K. Obstructive sleep apnea and the risk of sudden cardiac death: A longitudinal study of 10,701 adults. J. Am. Coll. Cardiol. 2013, 62, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Young, T.; Palta, M.; Dempsey, J.; Skatrud, J.; Weber, S.; Badr, S. The occurrence of sleep-disordered breathing among middle-aged adults. N. Engl. J. Med. 1993, 328, 1230–1235. [Google Scholar] [CrossRef] [PubMed]
- Sanner, B.M.; Tepel, M.; Esser, M.; Klewer, J.; Hoehmann-Riese, B.; Zidek, W.; Hellmich, B. Sleep-related breathing disorders impair quality of life in haemodialysis recipients. Nephrol. Dial. Transplant. 2002, 17, 1260–1265. [Google Scholar] [CrossRef] [PubMed]
- Mucsi, I.; Molnar, M.Z.; Rethelyi, J.; Vamos, E.; Csepanyi, G.; Tompa, G.; Barotfi, S.; Marton, A.; Novak, M. Sleep disorders and illness intrusiveness in patients on chronic dialysis. Nephrol. Dial. Transplant. 2004, 19, 1815–1822. [Google Scholar] [CrossRef] [PubMed]
- Merlino, G.; Gigli, G.L.; Valente, M. Sleep disturbances in dialysis patients. J. Nephrol. 2008, 21 (Suppl. S13), S66–S70. [Google Scholar]
- Al-Jahdali, H. A comparison of sleep disturbances and sleep apnea in patients on hemodialysis and chronic peritoneal dialysis. Saudi J. Kidney Dis. Transplant. 2011, 22, 922–930. [Google Scholar]
- Roumelioti, M.E.; Buysse, D.J.; Sanders, M.H.; Strollo, P.; Newman, A.B.; Unruh, M.L. Sleep-disordered breathing and excessive daytime sleepiness in chronic kidney disease and hemodialysis. Clin. J. Am. Soc. Nephrol. CJASN 2011, 6, 986–994. [Google Scholar] [CrossRef]
- Young, T.; Shahar, E.; Nieto, F.J.; Redline, S.; Newman, A.B.; Gottlieb, D.J.; Walsleben, J.A.; Finn, L.; Enright, P.; Samet, J.M.; et al. Predictors of sleep-disordered breathing in community-dwelling adults: The Sleep Heart Health Study. Arch. Intern. Med. 2002, 162, 893–900. [Google Scholar] [CrossRef]
- Laratta, C.R.; Ayas, N.T.; Povitz, M.; Pendharkar, S.R. Diagnosis and treatment of obstructive sleep apnea in adults. CMAJ 2017, 189, E1481–E1488. [Google Scholar] [CrossRef]
- Jung, H.H.; Han, H.; Lee, J.H. Sleep apnea, coronary artery disease, and antioxidant status in hemodialysis patients. Am. J. Kidney Dis. 2005, 45, 875–882. [Google Scholar] [CrossRef]
- Masuda, T.; Murata, M.; Honma, S.; Iwazu, Y.; Sasaki, N.; Ogura, M.; Onishi, A.; Ando, Y.; Muto, S.; Shimada, K.; et al. Sleep-disordered breathing predicts cardiovascular events and mortality in hemodialysis patients. Nephrol. Dial. Transplant. 2011, 26, 2289–2295. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.H.; Lee, J.H.; Baek, H.J.; Kim, S.J.; Lee, J.J. Nocturnal hypoxemia and periodic limb movement predict mortality in patients on maintenance hemodialysis. Clin. J. Am. Soc. Nephrol. CJASN 2010, 5, 1607–1613. [Google Scholar] [CrossRef] [PubMed]
- Unruh, M.L.; Sanders, M.H.; Redline, S.; Piraino, B.M.; Umans, J.G.; Hammond, T.C.; Sharief, I.; Punjabi, N.M.; Newman, A.B. Sleep apnea in patients on conventional thrice-weekly hemodialysis: Comparison with matched controls from the Sleep Heart Health Study. J. Am. Soc. Nephrol. JASN 2006, 17, 3503–3509. [Google Scholar] [CrossRef] [PubMed]
- Jhamb, M.; Ran, X.; Abdalla, H.; Roumelioti, M.-E.; Hou, S.; Davis, H.; Patel, S.R.; Yabes, J.; Unruh, M. Association of Sleep Apnea with Mortality in Patients with Advanced Kidney Disease. Clin. J. Am. Soc. Nephrol. 2020, 15, 182–190. [Google Scholar] [CrossRef]
- Masuda, T.; Murata, M.; Honma, S.; Iwazu, Y.; Ogura, M.; Onishi, A.; Shimada, K.; Kusano, E.; Asano, Y. Pulse oximetry is useful for screening sleep apnoea syndrome in dialysis patients. NDT Plus 2008, 1, 378–379. [Google Scholar] [CrossRef]
- Tuohy, C.V.; Montez-Rath, M.E.; Turakhia, M.; Chang, T.I.; Winkelman, J.W.; Winkelmayer, W.C. Sleep disordered breathing and cardiovascular risk in older patients initiating dialysis in the United States: A retrospective observational study using medicare data. BMC Nephrol. 2016, 17, 16. [Google Scholar] [CrossRef]
- Wizemann, V.; Tong, L.; Satayathum, S.; Disney, A.; Akiba, T.; Fissell, R.B.; Kerr, P.G.; Young, E.W.; Robinson, B.M. Atrial fibrillation in hemodialysis patients: Clinical features and associations with anticoagulant therapy. Kidney Int. 2010, 77, 1098–1106. [Google Scholar] [CrossRef]
- Mitsuma, W.; Matsubara, T.; Hatada, K.; Imai, S.; Tamura, M.; Tsubata, Y.; Ikarashi, K.; Morioka, T.; Saito, N.; Shimada, H.; et al. Atrial Fibrillation Had Less Impact on the Risk of Ischemic Stroke in Non-anticoagulated Patients Undergoing Hemodialysis: Insight from the RAKUEN study. Intern. Med. 2018, 57, 2295–2300. [Google Scholar] [CrossRef]
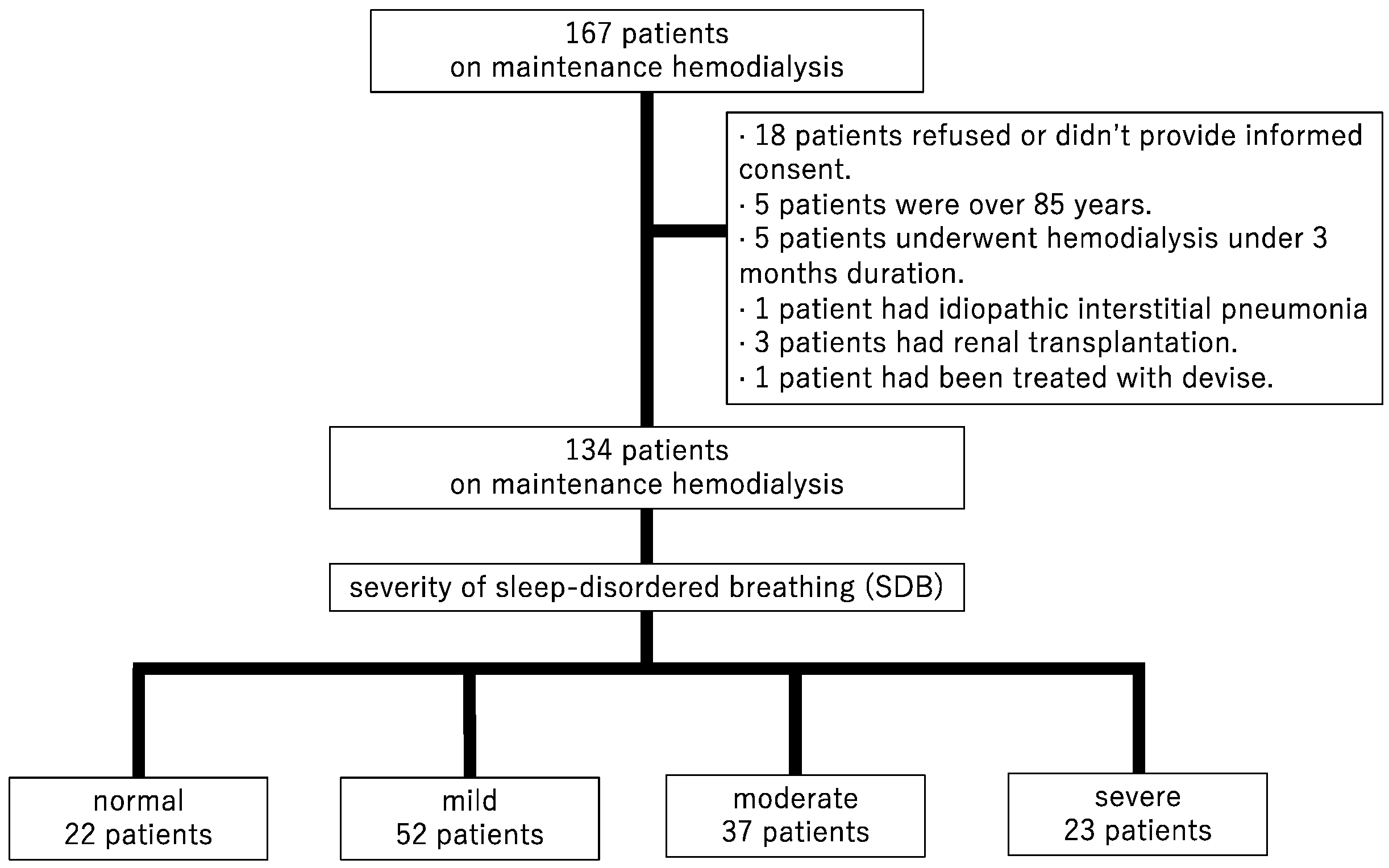
| Total | Normal | Mild | Moderate | Severe | p for Trend | |
|---|---|---|---|---|---|---|
| N = 134 | n = 22 | n = 52 | n = 37 | n = 23 | ||
| 3% ODI, times/h | 11.3 (6.2–25.5) | 3.7 (1.7–4.7) | 8.6 (6.4–10.2) | 21.0 (19.0–25.8) | 48.2 (35.8–56.5) | <0.001 |
| SpO2 at rest, % | 98 (97–99) | 98 (98–99) | 98 (98–99) | 98 (97–98) | 98 (96–99) | 0.065 |
| Age, y | 67 (59–75) | 64 (61–72) | 71 (59–75) | 70 (63–76) | 67 (59–75) | 0.50 |
| Men, % | 86 (64.2%) | 11 (50.0%) | 25 (48.1%) | 31 (83.8%) | 19 (82.6%) | <0.001 |
| BMI, kg/m2 | 20.5 (18.5–23.0) | 18.9 (17.8–21.0) | 19.8 (18.5–22.3) | 20.9 (19.4–23.9) | 22.5 (20.5–25.1) | 0.021 |
| Duration of HD, months | 69 (29–132) | 120 (43–236) | 83 (31–132) | 49 (13–115) | 61 (35–105) | 0.019 |
| Vascular access | ||||||
| AVF | 124 (92.5%) | 22 (100.0%) | 45 (86.5%) | 34 (91.9%) | 23 (100.0%) | 0.095 |
| AVG | 10 (7.5%) | 0 (0.0%) | 7 (13.5%) | 3 (8.1%) | 0 (0.0%) | |
| CVC | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| DM, % | 50 (37.3%) | 8 (36.4%) | 23 (44.2%) | 13 (35.1%) | 6 (26.1%) | 0.29 |
| History of CVD, % | 63 (47.0%) | 8 (36.4%) | 25 (48.1%) | 15 (40.5%) | 15 (65.2%) | 0.17 |
| History of IHD, % | 31 (23.1%) | 1 (4.5%) | 16 (30.8%) | 9 (24.3%) | 5 (21.7%) | 0.42 |
| History of CBVD, % | 24 (17.9%) | 4 (18.2%) | 6 (11.5%) | 5 (13.5%) | 9 (39.1%) | 0.079 |
| History of PAD, % | 23 (17.2%) | 1 (4.5%) | 10 (19.2%) | 6 (16.2%) | 6 (26.1%) | 0.14 |
| AF, % | 21 (15.7%) | 2 (9.1%) | 9 (17.3%) | 4 (10.8%) | 6 (26.1%) | 0.33 |
| SBP, mmHg | 150 (130–160) | 150 (140–160) | 150 (130–160) | 150 (130–160) | 150 (130–160) | 0.69 |
| DBP, mmHg | 80 (70–85) | 80 (65–85) | 75 (70–85) | 80 (70–80) | 85 (70–90) | 0.35 |
| Hb, g/dL | 11.3 (10.4–12.2) | 11.4 (10.8–11.9) | 11.3 (10.3–11.9) | 11.3 (10.8–12.8) | 11.5 (10.2–12.4) | 0.57 |
| Alb, g/dL | 3.7 (3.5–3.9) | 3.8 (3.6–3.9) | 3.7 (3.5–3.9) | 3.8 (3.5–3.9) | 3.7 (3.5–4) | 0.94 |
| P, mg/dL | 5.3 (4.6–6.1) | 5.1 (4.3–6.4) | 5.5 (4.6–6.2) | 5.1 (4.7–5.9) | 5.4 (4.7–6.9) | 0.52 |
| PTH, pg/mL | 160 (104–285) | 191 (105–299) | 159 (108–273) | 144 (80–255) | 236 (120–378) | 0.77 |
| HDL, mg/dL | 48 (40–59) | 57 (50–64) | 46 (38–55) | 49 (39–59) | 46 (38–61) | 0.34 |
| LDL, mg/dL | 77 (59–94) | 73 (54–79) | 77 (65–94.5) | 83 (57–96) | 74 (63–120) | 0.16 |
| GA, % | 15.2 (13.5–17.7) | 15.2 (13.4–17.9) | 16.1 (14.6–18.3) | 14.6 (13.1–17) | 14.9 (12.4–17.6) | 0.12 |
| CRP (mg/dL) | 0.10 (0.04–0.23) | 0.05 (0.03–0.09) | 0.11 (0.05–0.22) | 0.13 (0.07–0.41) | 0.16 (0.05–0.23) | 0.010 |
| LVMI, g/m2 | 127 (104–146) | 138 (117–161) | 123 (103–139) | 120 (101–149) | 127 (106–142) | 0.29 |
| EF, % | 63 (60–67) | 65 (62–67) | 63 (60–66.5) | 63 (60–66) | 63 (53–68) | 0.20 |
| E/e’ | 14.4 (11.7–18.8) | 14.9 (12.6–19.9) | 14.1 (11.8–17.9) | 13.4 (11.2–17.5) | 15.2 (11.3–19.7) | 0.67 |
| (A) MACEs | ||||||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | |||||
| HR | 95% CI | p | HR | 95% CI | p | |
| SDB severity | ||||||
| Normal | ref | ref | ||||
| Mild | 1.63 | 0.76–3.47 | 0.207 | 1.41 | 0.66–3.05 | 0.369 |
| Moderate | 1.81 | 0.79–4.17 | 0.162 | 1.75 | 0.74–4.13 | 0.199 |
| Severe | 3.91 | 1.61–9.47 | 0.003 | 4.66 | 1.87–11.61 | 0.001 |
| Age, ×10 year | 1.25 | 0.96–1.61 | 0.10 | 1.25 | 0.96–1.64 | 0.090 |
| Men | 1.29 | 0.76–2.19 | 0.339 | 1.15 | 0.69–1.94 | 0.608 |
| BMI, kg/m2 | 0.91 | 0.84–0.99 | 0.003 | 0.89 | 0.82–0.97 | 0.009 |
| Duration of HD, year | 1.00 | 0.99–1.00 | 0.050 | |||
| DM | 1.80 | 1.08–2.99 | 0.023 | |||
| CRP, mg/dL | 0.92 | 0.61–1.37 | 0.685 | |||
| (B)All-Cause Mortality | ||||||
| Model 1 | Model 2 | |||||
| HR | 95% CI | p | HR | 95% CI | p | |
| SDB severity | ||||||
| Normal | ref | - | - | ref | - | - |
| Mild | 2.25 | 0.92–5.52 | 0.077 | 2.05 | 0.83–5.07 | 0.119 |
| Moderate | 1.90 | 0.73–4.97 | 0.187 | 1.70 | 0.62–4.47 | 0.307 |
| Severe | 4.75 | 1.69–13.32 | 0.003 | 5.74 | 1.92–16.70 | 0.001 |
| Age, ×10 year | 1.81 | 1.33–2.47 | <0.001 | 1.77 | 1.30–2.41 | <0.001 |
| Men | 1.36 | 0.77–2.47 | 0.285 | 1.11 | 0.61–2.01 | 0.741 |
| BMI, kg/m2 | 0.85 | 0.77–0.94 | 0.002 | 0.84 | 0.75–0.93 | 0.001 |
| duration of HD, year | 0.97 | 0.93–1.01 | 0.118 | |||
| DM | 1.20 | 0.87–2.69 | 0.139 | |||
| CRP, mg/dL | 1.33 | 1.04–1.62 | 0.020 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mochida, Y.; Ohtake, T.; Ishioka, K.; Oka, M.; Maesato, K.; Moriya, H.; Hidaka, S.; Kobayashi, S. Impact of the 3% Oxygen Desaturation Index via Overnight Pulse Oximetry on Cardiovascular Events and Death in Patients Undergoing Hemodialysis: A Retrospective Cohort Study. J. Clin. Med. 2023, 12, 858. https://doi.org/10.3390/jcm12030858
Mochida Y, Ohtake T, Ishioka K, Oka M, Maesato K, Moriya H, Hidaka S, Kobayashi S. Impact of the 3% Oxygen Desaturation Index via Overnight Pulse Oximetry on Cardiovascular Events and Death in Patients Undergoing Hemodialysis: A Retrospective Cohort Study. Journal of Clinical Medicine. 2023; 12(3):858. https://doi.org/10.3390/jcm12030858
Chicago/Turabian StyleMochida, Yasuhiro, Takayasu Ohtake, Kunihiro Ishioka, Machiko Oka, Kyoko Maesato, Hidekazu Moriya, Sumi Hidaka, and Shuzo Kobayashi. 2023. "Impact of the 3% Oxygen Desaturation Index via Overnight Pulse Oximetry on Cardiovascular Events and Death in Patients Undergoing Hemodialysis: A Retrospective Cohort Study" Journal of Clinical Medicine 12, no. 3: 858. https://doi.org/10.3390/jcm12030858
APA StyleMochida, Y., Ohtake, T., Ishioka, K., Oka, M., Maesato, K., Moriya, H., Hidaka, S., & Kobayashi, S. (2023). Impact of the 3% Oxygen Desaturation Index via Overnight Pulse Oximetry on Cardiovascular Events and Death in Patients Undergoing Hemodialysis: A Retrospective Cohort Study. Journal of Clinical Medicine, 12(3), 858. https://doi.org/10.3390/jcm12030858







