Non-Alcoholic Fatty Liver Disease in Patients with Polycystic Ovary Syndrome: A Systematic Review, Meta-Analysis, and Meta-Regression
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Inclusion Criteria and Exclusion Criteria
2.2.1. Types of Studies
2.2.2. Participants
2.3. Outcomes
2.4. Electronic Search Strategies
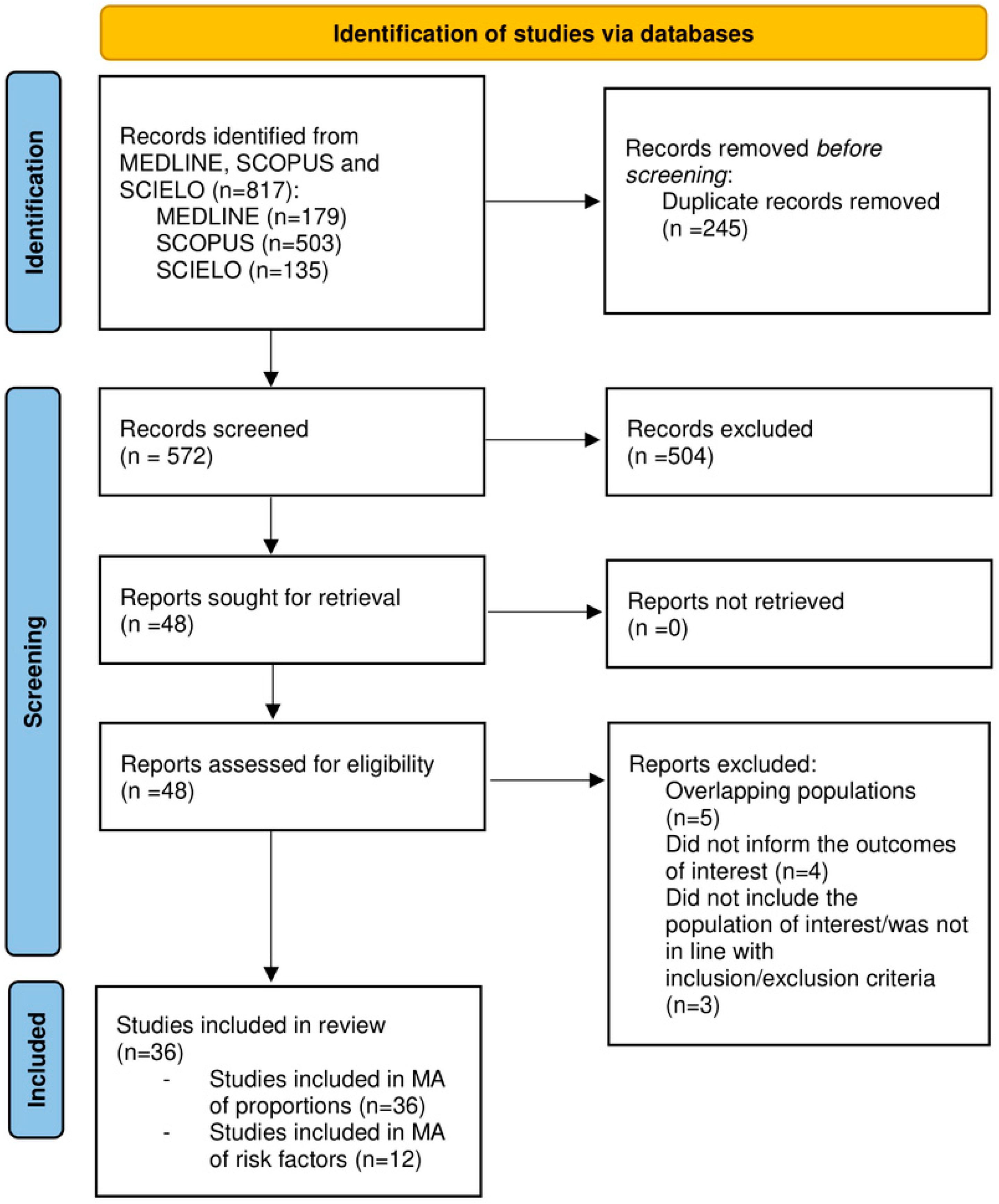
2.5. Study Selection
2.6. Data Collection
2.7. Risk of Bias
2.8. Data Synthesis and Meta-Analysis
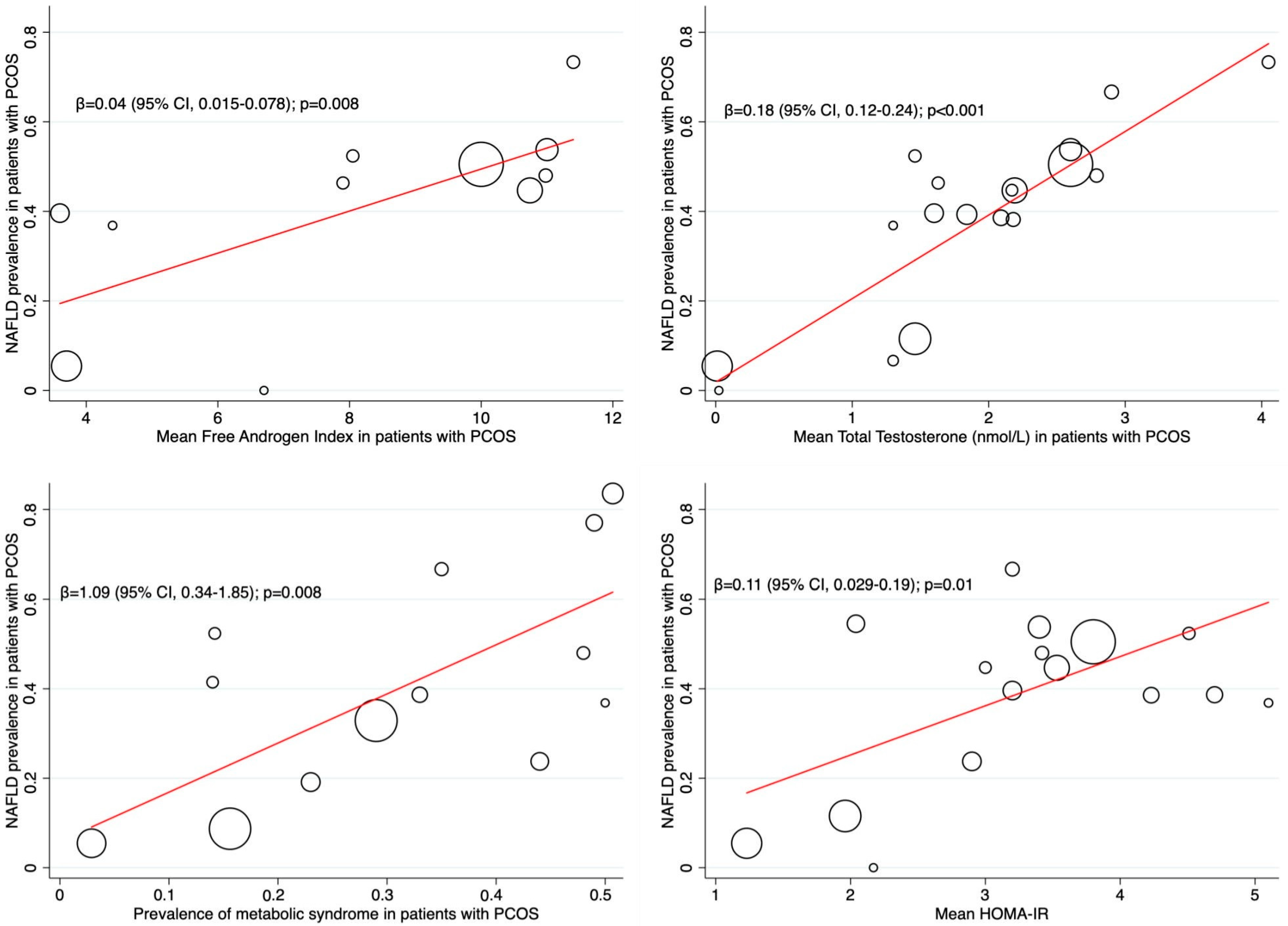
2.9. Subgroup Analyses and Meta-Regression
3. Results
3.1. Characteristics of the Included Studies
3.2. Characteristics of Participants
3.3. NAFLD Assessment
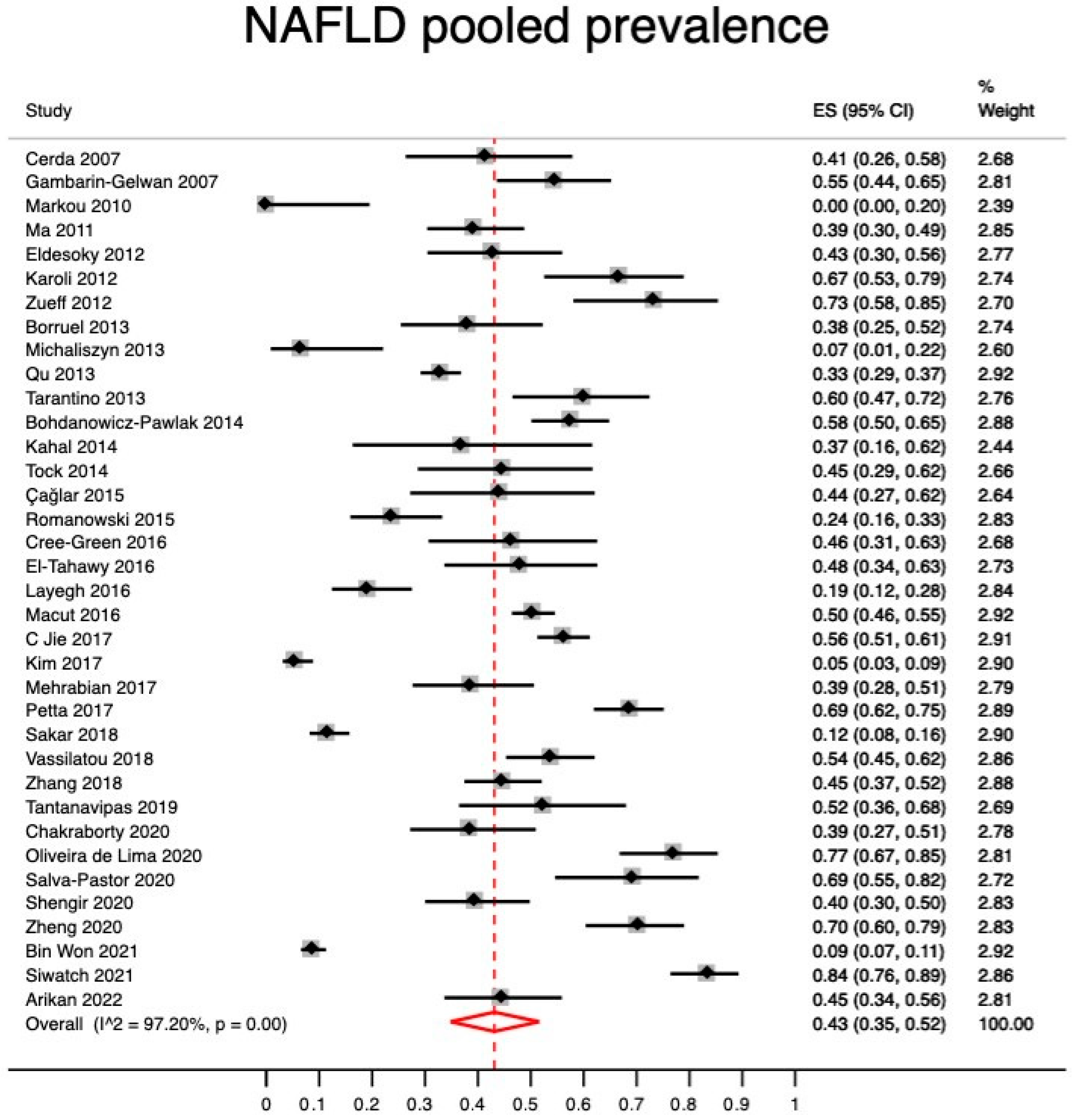
Quantitative Synthesis (Meta-Analysis)
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global Epidemiology of Nonalcoholic Fatty Liver Disease-Meta-Analytic Assessment of Prevalence, Incidence, and Outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, A.J.; Van Natta, M.L.; Clark, J.; Neuschwander-Tetri, B.A.; Diehl, A.; Dasarathy, S.; Loomba, R.; Chalasani, N.; Kowdley, K.; Hameed, B.; et al. Prospective Study of Outcomes in Adults with Nonalcoholic Fatty Liver Disease. N. Engl. J. Med. 2021, 385, 1559–1569. [Google Scholar] [CrossRef] [PubMed]
- McCartney, C.R.; Marshall, J.C. Polycystic Ovary Syndrome. N. Engl. J. Med. 2016, 375, 54–64. [Google Scholar] [CrossRef]
- Zeng, X.; Xie, Y.-J.; Liu, Y.-T.; Long, S.-L.; Mo, Z.-C. Polycystic Ovarian Syndrome: Correlation between Hyperandrogenism, Insulin Resistance and Obesity. Clin. Chim. Acta. 2020, 502, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, D.; Guo, H.; Li, M. Hyperandrogenemia and Insulin Resistance: The Chief Culprit of Polycystic Ovary Syndrome. Life Sci. 2019, 236, 116940. [Google Scholar] [CrossRef]
- Harsha Varma, S.; Tirupati, S.; Pradeep, T.V.S.; Sarathi, V.; Kumar, D. Insulin Resistance and Hyperandrogenemia Independently Predict Nonalcoholic Fatty Liver Disease in Women with Polycystic Ovary Syndrome. Diabetes Metab. Syndr. 2019, 13, 1065–1069. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. J. Clin. Epidemiol. 2009, 62, 1006–1012. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; John Wiley & Sons: Chichester, UK, 2019. [Google Scholar]
- Stroup, D.F.F.; Berlin, J.A.A.; Morton, S.C.C.; Olkin, I.; Williamson, G.D.D.; Rennie, D.; Moher, D.; Becker, B.J.J.; Sipe, T.A.A.; Thacker, S.B.B. Meta-Analysis of Observational Studies in Epidemiology: A Proposal for Reporting. Meta-Analysis of Observational Studies in Epidemiology (MOOSE) Group. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef]
- EASL. EASL Clinical Practice Guidelines on Non-Invasive Tests for Evaluation of Liver Disease Severity and Prognosis-2021 Update. J. Hepatol. 2021, 75, 659–689. [Google Scholar] [CrossRef]
- Wong, V.W.-S.; Adams, L.A.; de Lédinghen, V.; Wong, G.L.-H.; Sookoian, S. Noninvasive Biomarkers in NAFLD and NASH—Current Progress and Future Promise. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 461–478. [Google Scholar] [CrossRef]
- Robinson, K.A.; Dickersin, K. Development of a Highly Sensitive Search Strategy for the Retrieval of Reports of Controlled Trials Using PubMed. Int. J. Epidemiol. 2002, 31, 150–153. [Google Scholar] [CrossRef] [PubMed]
- Cooper, C.; Booth, A.; Varley-Campbell, J.; Britten, N.; Garside, R. Defining the Process to Literature Searching in Systematic Reviews: A Literature Review of Guidance and Supporting Studies. BMC Med. Res. Methodol. 2018, 18, 85. [Google Scholar] [CrossRef] [PubMed]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A Web and Mobile App for Systematic Reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [PubMed]
- Slim, K.; Nini, E.; Forestier, D.; Kwiatkowski, F.; Panis, Y.; Chipponi, J. Methodological Index for Non-Randomized Studies (MINORS): Development and Validation of a New Instrument. ANZ J. Surg. 2003, 73, 712–716. [Google Scholar] [CrossRef] [PubMed]
- Hunter, J.P.; Saratzis, A.; Sutton, A.J.; Boucher, R.H.; Sayers, R.D.; Bown, M.J. In Meta-Analyses of Proportion Studies, Funnel Plots Were Found to Be an Inaccurate Method of Assessing Publication Bias. J. Clin. Epidemiol. 2014, 67, 897–903. [Google Scholar] [CrossRef]
- Barker, T.H.H.; Migliavaca, C.B.B.; Stein, C.; Colpani, V.; Falavigna, M.; Aromataris, E.; Munn, Z. Conducting Proportional Meta-Analysis in Different Types of Systematic Reviews: A Guide for Synthesisers of Evidence. BMC Med. Res. Methodol. 2021, 21, 189. [Google Scholar] [CrossRef]
- Nyaga, V.N.; Arbyn, M.; Aerts, M. Metaprop: A Stata Command to Perform Meta-Analysis of Binomial Data. Arch. Public Health 2014, 72, 39. [Google Scholar] [CrossRef]
- Eldesoky, A.E.E.; Gad, Y.Z.; Ahmed, N. Nonalcoholic Fatty Liver Disease in Young Adult Egyptian Women with Polycystic Ovary Syndrome. Egypt. Liver J. 2013, 3, 15–19. [Google Scholar] [CrossRef]
- Arikan, D.; Önmez, A.; Aksu, E.; Tasdemir, N. Predictivity of Fatty Liver Index for Non-Alcoholic Fatty Liver Disease in Lean Females with Polycystic Ovary Syndrome. Afr. Health Sci. 2022, 22, 648–656. [Google Scholar] [CrossRef]
- Kahal, H.; Abouda, G.; Rigby, A.S.; Coady, A.M.; Kilpatrick, E.S.; Atkin, S.L. Glucagon-like Peptide-1 Analogue, Liraglutide, Improves Liver Fibrosis Markers in Obese Women with Polycystic Ovary Syndrome and Nonalcoholic Fatty Liver Disease. Clin. Endocrinol. 2014, 81, 523–528. [Google Scholar] [CrossRef]
- Karoli, R.; Fatima, J.; Chandra, A.; Gupta, U.; Islam, F.U.; Singh, G. Prevalence of Hepatic Steatosis in Women with Polycystic Ovary Syndrome. J. Hum. Reprod. Sci. 2013, 6, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J.; Kim, D.; Yim, J.Y.; Kang, J.H.; Han, K.H.; Kim, S.M.; Hwang, K.R.; Ku, S.Y.; Suh, C.S.; Kim, S.H.; et al. Polycystic Ovary Syndrome with Hyperandrogenism as a Risk Factor for Non-Obese Non-Alcoholic Fatty Liver Disease. Aliment. Pharmacol. Ther. 2017, 45, 1403–1412. [Google Scholar] [CrossRef] [PubMed]
- Layegh, P.; Mousavi, Z.; Tehrani, D.F.; Parizadeh, S.M.R.; Khajedaluee, M. Insulin Resistance and Endocrine-Metabolic Abnormalities in Polycystic Ovarian Syndrome: Comparison between Obese and Non-Obese PCOS Patients. Int. J. Reprod. Biomed. 2016, 14, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Macut, D.; Tziomalos, K.; Božić-Antić, I.; Bjekić-Macut, J.; Katsikis, I.; Papadakis, E.; Andrić, Z.; Panidis, D. Non-Alcoholic Fatty Liver Disease Is Associated with Insulin Resistance and Lipid Accumulation Product in Women with Polycystic Ovary Syndrome. Hum. Reprod. 2016, 31, 1347–1353. [Google Scholar] [CrossRef]
- Markou, A.; Androulakis, I.I.; Mourmouris, C.; Tsikkini, A.; Samara, C.; Sougioultzis, S.; Piaditis, G.; Kaltsas, G. Hepatic Steatosis in Young Lean Insulin Resistant Women with Polycystic Ovary Syndrome. Fertil. Steril. 2010, 93, 1220–1226. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Ganie, M.A.; Masoodi, I.; Jana, M.; Gupta, N.; Sofi, N.Y. Fibroscan as a Non-Invasive Predictor of Hepatic Steatosis in Women with Polycystic Ovary Syndrome. Indian J. Med. Res. 2020, 151, 333–341. [Google Scholar] [CrossRef]
- Cai, J.; Wu, C.H.; Zhang, Y.; Wang, Y.Y.; Xu, W.D.; Lin, T.C.; Li, S.X.; Wang, L.H.; Zheng, J.; Sun, Y.; et al. High-Free Androgen Index Is Associated with Increased Risk of Non-Alcoholic Fatty Liver Disease in Women with Polycystic Ovary Syndrome, Independent of Obesity and Insulin Resistance. Int. J. Obes. 2017, 41, 1341–1347. [Google Scholar] [CrossRef]
- Ma, R.C.W.; Liu, K.H.; Lam, P.M.; Cheung, L.P.; Tam, W.H.; Ko, G.T.C.; Chan, M.H.M.; Ho, C.S.; Lam, C.W.K.; Chu, W.C.W.; et al. Sonographic Measurement of Mesenteric Fat Predicts Presence of Fatty Liver among Subjects with Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2011, 96, 799–807. [Google Scholar] [CrossRef]
- Mehrabian, F.; Jahanmardi, R. Nonalcoholic Fatty Liver Disease in a Sample of Iranian Women with Polycystic Ovary Syndrome. Int. J. Prev. Med. 2017, 8, 79. [Google Scholar] [CrossRef]
- Won, Y.B.; Seo, S.K.; Yun, B.H.; Cho, S.H.; Choi, Y.S.; Lee, B.S. Non-Alcoholic Fatty Liver Disease in Polycystic Ovary Syndrome Women. Sci. Rep. 2021, 11, 7085. [Google Scholar] [CrossRef]
- Michaliszyn, S.F.; Lee, S.; Tfayli, H.; Arslanian, S. Polycystic Ovary Syndrome and Nonalcoholic Fatty Liver in Obese Adolescents: Association with Metabolic Risk Profile. Fertil. Steril. 2013, 100, 1745–1751. [Google Scholar] [CrossRef] [PubMed]
- Oliveira de Lima, D.; Guimarães, T.C.M.; Couto, C.A.; Cândido, A.L.; Azevedo, R.C.S.; Mattos, F.S.; Elias, M.L.C.; Reis, F.M.; Rocha, A.L.L.; Faria, L.C. Nonalcoholic Fatty Liver Disease in Women with Polycystic Ovary Syndrome: Associated Factors and Noninvasive Fibrosis Staging in a Single Brazilian Center. Arch. Endocrinol. Metab. 2020, 64, 235–242. [Google Scholar] [CrossRef]
- Petta, S.; Ciresi, A.; Bianco, J.; Geraci, V.; Boemi, R.; Galvano, L.; Magliozzo, F.; Merlino, G.; Craxì, A.; Giordano, C. Insulin Resistance and Hyperandrogenism Drive Steatosis and Fibrosis Risk in Young Females with PCOS. PLoS ONE 2017, 12, e0186136. [Google Scholar] [CrossRef] [PubMed]
- Qu, Z.; Zhu, Y.; Jiang, J.; Shi, Y.; Chen, Z. The Clinical Characteristics and Etiological Study of Nonalcoholic Fatty Liver Disease in Chinese Women with PCOS. Int. J. Reprod. Biomed. 2013, 11, 725–732. [Google Scholar]
- Romanowski, M.D.; Parolin, M.B.; Freitas, A.C.T.; Piazza, M.J.; Basso, J.; Urbanetz, A.A. Prevalence of Non-Alcoholic Fatty Liver Disease in Women with Polycystic Ovary Syndrome and Its Correlation with Metabolic Syndrome. Arq. Gastroenterol. 2015, 52, 117–123. [Google Scholar] [CrossRef]
- Salva-Pastor, N.; López-Sánchez, G.N.; Chávez-Tapia, N.C.; Audifred-Salomón, J.R.; Niebla-Cárdenas, D.; Topete-Estrada, R.; Pereznuñez-Zamora, H.; Vidaltamayo-Ramírez, R.; Báez-Arellano, M.E.; Uribe, M.; et al. Polycystic Ovary Syndrome with Feasible Equivalence to Overweight as a Risk Factor for Non-Alcoholic Fatty Liver Disease Development and Severity in Mexican Population. Ann. Hepatol. 2020, 19, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, M.; Terrault, N.; Duwaerts, C.C.; Tien, P.; Cedars, M.; Huddleston, H. The Association of Hispanic Ethnicity with Nonalcoholic Fatty Liver Disease in Polycystic Ovary Syndrome. Curr. Opin. Gynecol. Obstet. 2018, 1, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Shengir, M.; Krishnamurthy, S.; Ghali, P.; Deschenes, M.; Wong, P.; Chen, T.; Sebastiani, G. Prevalence and Predictors of Nonalcoholic Fatty Liver Disease in South Asian Women with Polycystic Ovary Syndrome. World J. Gastroenterol. 2020, 26, 7046–7060. [Google Scholar] [CrossRef]
- Siwatch, S.; Singh, V.; Dhaliwal, L.K.; Kumari, S.; Singh, K. Non-Alcoholic Fatty Liver Disease in Polycystic Ovarian Syndrome in Indian Women. J. Obstet. Gynaecol. 2021, 42, 957–961. [Google Scholar] [CrossRef]
- Tantanavipas, S.; Vallibhakara, O.; Sobhonslidsuk, A.; Phongkitkarun, S.; Vallibhakara, S.A.O.; Promson, K.; Sophonsritsuk, A. Abdominal Obesity as a Predictive Factor of Nonalcoholic Fatty Liver Disease Assessed by Ultrasonography and Transient Elastography in Polycystic Ovary Syndrome and Healthy Women. Biomed Res. Int. 2019, 2019, 9047324. [Google Scholar] [CrossRef]
- Bohdanowicz-Pawlak, A.; Lenarcik-Kabza, A.; Brona, A.; Kuliczkowska-Płaksej, J.; Łaczmański, Ł.; Zaleska-Dorobisz, U.; Milewicz, A. Non-Alcoholic Fatty Liver Disease in Women with Polycystic Ovary Syndrome-Clinical and Metabolic Aspects and Lipoprotein Lipase Gene Polymorphism. Endokrynol. Pol. 2014, 65, 416–421. [Google Scholar] [CrossRef]
- Tarantino, G.; Di Somma, C.; Pizza, G.; Brancato, V.; Nedi, V.; Valentino, R.; Orio, F.; Pivonello, C.; Colao, A.; Savastano, S. Polycystic Ovary Syndrome and Hepatic Steatosis: Could Low-Grade Chronic Inflammation Be Mediated by the Spleen? Eur. J. Inflamm. 2013, 11, 179–191. [Google Scholar] [CrossRef]
- Tock, L.; Carneiro, G.; Togeiro, S.M.; Hachul, H.; Pereira, A.Z.; Tufik, S.; Zanella, M.T. Obstructive Sleep Apnea Predisposes to Nonalcoholic Fatty Liver Disease in Patients with Polycystic Ovary Syndrome. Endocr. Pract. 2014, 20, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Vassilatou, E.; Lafoyianni, S.; Vassiliadi, D.A.; Ioannidis, D.; Paschou, S.A.; Mizamtsidi, M.; Panagou, M.; Vryonidou, A. Visceral Adiposity Index for the Diagnosis of Nonalcoholic Fatty Liver Disease in Premenopausal Women with and without Polycystic Ovary Syndrome. Maturitas 2018, 116, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hu, J.; Zhang, C.; Jiao, Y.; Kong, X.; Wang, W. Analyses of Risk Factors for Polycystic Ovary Syndrome Complicated with Non-Alcoholic Fatty Liver Disease. Exp. Ther. Med. 2018, 15, 4259–4264. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Tong, M.; Dong, L.; Du, C.; Zheng, X.; Wang, L.; Huang, P.; Liu, W.; Lin, M.; Liu, C. Lipid Accumulation Product Independently Correlate with Hepatic Steatosis Quantified by Controlled Attenuation Parameter in Women with Polycystic Ovary Syndrome. Endocr. Connect. 2020, 9, 154–162. [Google Scholar] [CrossRef]
- Zueff, L.F.N.; Martins, W.P.; Vieira, C.S.; Ferriani, R.A. Ultrasonographic and Laboratory Markers of Metabolic and Cardiovascular Disease Risk in Obese Women with Polycystic Ovary Syndrome. Ultrasound Obstet. Gynecol. 2012, 39, 341–347. [Google Scholar] [CrossRef]
- Borruel, S.; Fernández-Durán, E.; Alpañés, M.; Martí, D.; Álvarez-Blasco, F.; Luque-Ramírez, M.; Escobar-Morreale, H.F. Global Adiposity and Thickness of Intraperitoneal and Mesenteric Adipose Tissue Depots Are Increased in Women with Polycystic Ovary Syndrome (PCOS). J. Clin. Endocrinol. Metab. 2013, 98, 1254–1263. [Google Scholar] [CrossRef]
- Caglar, G.-S.; Kiseli, M.; Seker, R.; Ozdemir, E.-D.; Karadag, D.; Demirtas, S. Atherogenic Dyslipidemia, Subclinical Atherosclerosis, Non-Alcoholic Fatty Liver Disease and Insulin Resistance in Polycystic Ovarian Syndrome. Turkish J. Biochem. 2015, 40, 24–30. [Google Scholar] [CrossRef]
- Cerda, C.; Pérez-Ayuso, R.M.; Riquelme, A.; Soza, A.; Villaseca, P.; Sir-Petermann, T.; Espinoza, M.; Pizarro, M.; Solis, N.; Miquel, J.F.; et al. Nonalcoholic Fatty Liver Disease in Women with Polycystic Ovary Syndrome. J. Hepatol. 2007, 47, 412–417. [Google Scholar] [CrossRef]
- Cree-Green, M.; Bergman, B.C.; Coe, G.V.; Newnes, L.; Baumgartner, A.D.; Bacon, S.; Sherzinger, A.; Pyle, L.; Nadeau, K.J. Hepatic Steatosis Is Common in Adolescents with Obesity and PCOS and Relates to De Novo Lipogenesis but Not Insulin Resistance. Obesity 2016, 24, 2399–2406. [Google Scholar] [CrossRef]
- Tahawy, M.E.; Almassry, H. Non-Alcoholic Fatty Liver Disease in Egyptian Females with Polycystic Ovary Syndrome: Clinical Characteristics and Etiological Factors. Br. J. Med. Health Res. 2016, 3, 17–38. [Google Scholar]
- Gambarin-Gelwan, M.; Kinkhabwala, S.V.; Schiano, T.D.; Bodian, C.; Yeh, H.C.; Futterweit, W. Prevalence of Nonalcoholic Fatty Liver Disease in Women with Polycystic Ovary Syndrome. Clin. Gastroenterol. Hepatol. 2007, 5, 496–501. [Google Scholar] [CrossRef]
- Ge, X.; Zheng, L.; Wang, M.; Du, Y.; Jiang, J. Prevalence Trends in Non-Alcoholic Fatty Liver Disease at the Global, Regional and National Levels, 1990–2017: A Population-Based Observational Study. BMJ Open 2020, 10, e036663. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yao, X.-Y.; Shi, R.-X.; Liu, S.-F.; Wang, X.-Y. A Potential Link between Polycystic Ovary Syndrome and Non-Alcoholic Fatty Liver Disease: An Update Meta-Analysis. Reprod. Health 2018, 15, 77. [Google Scholar] [CrossRef] [PubMed]
- Rocha, A.L.L.; Faria, L.C.; Guimarães, T.C.M.; Moreira, G.V.; Cândido, A.L.; Couto, C.A.; Reis, F.M. Non-Alcoholic Fatty Liver Disease in Women with Polycystic Ovary Syndrome: Systematic Review and Meta-Analysis. J. Endocrinol. Investig. 2017, 40, 1279–1288. [Google Scholar] [CrossRef] [PubMed]
- Lonardo, A.; Bellentani, S.; Argo, C.K.; Ballestri, S.; Byrne, C.D.; Caldwell, S.H.; Cortez-Pinto, H.; Grieco, A.; Machado, M.V.; Miele, L.; et al. Epidemiological Modifiers of Non-Alcoholic Fatty Liver Disease: Focus on High-Risk Groups. Dig. Liver Dis. 2015, 47, 997–1006. [Google Scholar] [CrossRef]
- Benedict, M.; Zhang, X. Non-Alcoholic Fatty Liver Disease: An Expanded Review. World J. Hepatol. 2017, 9, 715–732. [Google Scholar] [CrossRef]
- Sarkar, M.; Terrault, N.; Chan, W.; Cedars, M.I.; Huddleston, H.G.; Duwaerts, C.C.; Balitzer, D.; Gill, R.M. Polycystic Ovary Syndrome (PCOS) Is Associated with NASH Severity and Advanced Fibrosis. Liver Int. 2020, 40, 355–359. [Google Scholar] [CrossRef]
- O’Reilly, M.W.; Taylor, A.E.; Crabtree, N.J.; Hughes, B.A.; Capper, F.; Crowley, R.K.; Stewart, P.M.; Tomlinson, J.W.; Arlt, W. Hyperandrogenemia Predicts Metabolic Phenotype in Polycystic Ovary Syndrome: The Utility of Serum Androstenedione. J. Clin. Endocrinol. Metab. 2014, 99, 1027–1036. [Google Scholar] [CrossRef]
- Lerchbaum, E.; Schwetz, V.; Rabe, T.; Giuliani, A.; Obermayer-Pietsch, B. Hyperandrogenemia in Polycystic Ovary Syndrome: Exploration of the Role of Free Testosterone and Androstenedione in Metabolic Phenotype. PLoS ONE 2014, 9, e108263. [Google Scholar] [CrossRef] [PubMed]
- Jamil, A.S.; Alalaf, S.K.; Al-Tawil, N.G.; Al-Shawaf, T. A Case–Control Observational Study of Insulin Resistance and Metabolic Syndrome among the Four Phenotypes of Polycystic Ovary Syndrome Based on Rotterdam Criteria. Reprod. Health 2015, 12, 7. [Google Scholar] [CrossRef] [PubMed]
- Mu, L.; Li, R.; Lai, Y.; Zhao, Y.; Qiao, J. Adipose Insulin Resistance Is Associated with Cardiovascular Risk Factors in Polycystic Ovary Syndrome. J. Endocrinol. Investg. 2019, 42, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Blum, M.R.; Popat, R.A.; Nagy, A.; Cataldo, N.A.; McLaughlin, T.L. Using Metabolic Markers to Identify Insulin Resistance in Premenopausal Women with and without Polycystic Ovary Syndrome. J. Endocrinol. Investg. 2021, 44, 2123–2130. [Google Scholar] [CrossRef] [PubMed]
- Melsen, W.G.; Bootsma, M.C.J.; Rovers, M.M.; Bonten, M.J.M. The Effects of Clinical and Statistical Heterogeneity on the Predictive Values of Results from Meta-Analyses. Clin. Microbiol. Infect. 2014, 20, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Goodman, S.; Dickersin, K. Metabias: A Challenge for Comparative Effectiveness Research. Ann. Intern. Med. 2011, 155, 61–62. [Google Scholar] [CrossRef]
| Author/y | Study Design | Country | PCOS Dx | NAFLD Dx | N Total | N PCOS | N NAFLD in PCOS | Age | BMI kg/m2 | TG mg/dL | Metabolic Syndrome, n (%) | Obesity, n (%) | T2DM, n (%) | Weight Circumference (cm) | HOMA-IR | FAI | Total Testosterone nmol/L |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [51] Cerda 2007 | Cohort | Chile | Rotterdam | US | 72 | 41 | 17 | 24.6 (7.2) | 30.3 (7.07) | 125.5 (96.6) | 6 (14.6) | 24 (58.5) | 3 (7.3) | NR | NR | NR | NR |
| [54] Gambarin-Gelwan 2007 | Case-series | USA | NIH | US | 88 | 88 | 48 | 31.4 | 26.9 | 97 | NR | NR | NR | NR | 2.04 | NR | NR |
| [26] Markou 2010 | Case-control | Greece | Rotterdam | CT-Scan | 34 | 17 | 0 | 25.1 (1) | 20.9 (0.5) | 55.7 (5.2) | NR | NR | NR | 73.1 (2.3) | 2.17 (0.29) | 6.7 (1.1) | 0.023 (0.0027) |
| [29] Ma 2011 | Cross-sectional | China | Rotterdam | US | 117 | 117 | 46 | NR | NR | NR | NR | NR | NR | NR | NR | NR | 1.84 (0.9) |
| [19] Eldesoky 2012 | Case-series | Egypt | Rotterdam | US | 63 | 63 | 27 | NR | NR | NR | NR | 46 (73) | 18 (28.5) | NR | NR | NR | NR |
| [22] Karoli 2012 | Cross-sectional | India | Rotterdam | US | 109 | 54 | 36 | 28.5 (6.2) | 27.2 (5.4) | 136 (24) | 19 (35) | NR | NR | NR | 3.2 (1.7) | NR | 2.9 (0.7) |
| [48] Zueff 2012 | Case-control | Brasil | Rotterdam | US | 90 | 45 | 33 | 31.6 (4.1) | 34.7 (2.9) | 102.5 (80.5–163) | NR | NR | NR | 103.71 (8.83) | NR | 10.4 (6.9–16.9) | 2.97 (2.13–7.06) |
| [49] Borruel 2013 | Case-control | Spain | NIH | US | 106 | 55 | 21 | NR | NR | 83 (39) | NR | 23 (42) | NR | 88 (20) | NR | NR | 2.18 (0.86) |
| [32] Michaliszyn 2013 | Case-series | USA | NIH | CT-Scan | 30 | 30 | 2 | 16.1 (03) | 37.1 (1.3) | NR | NR | NR | NR | 104.5 (3.3) | NR | NR | 1.3 (0.1) |
| [35] Qu 2013 | Case-series | China | Rotterdam | US | 602 | 602 | 198 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| [43] Tarantino 2013 | Cross-sectional | Italy | Rotterdam | US | 80 | 60 | 36 | 25.5 (16–38) | 25.2 (18.2–46.6) | 158.5 (52–230) | NR | 30 (50) | NR | 85 (67–118) | 2.6 (0.7–11.1) | 9.1 (3.4–16.9) no sirve | 3.8 (1.8–5.48) |
| [42] Bohdanowicz-Pawlak 2014 | Cross-sectional | Poland | Rotterdam | US | 184 | 184 | 106 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| [21] Kahal 2014 | Case-control | UK | Rotterdam | US | 36 | 19 | 7 | 33.9 (6.7) | 37.9 (5) | NR | 10 (50) | 19 (100) | NR | 112 (12.6) | 5.1 (2.6) | 4.4 (2.2) | 1.3 (0.4) |
| [44] Tock 2014 | Case-series | Brasil | Rotterdam | US | 38 | 38 | 17 | 28.3 (6.8) | 32.9 (7.7) | 108 (48.4) | NR | NR | NR | 103.2 (19.2) | 3 (2.3) | NR | 2.17 (1.25) |
| [50] Çağlar 2015 | Case-control | Turkey | Rotterdam | US | 55 | 34 | 15 | 26 (2.5) | 22 (1.1) | 80 (34–233) | NR | NR | NR | NR | 2 (0.8–14) | NR | 0.01 (0.001–0.045) |
| [36] Romanowski 2015 | Case-control | Brasil | AE and PCOS society | US | 131 | 101 | 24 | 26.8 (5) | 28.5 (6) | 103.3 (60) | 45 (44.6) | NR | NR | 91.6 (16) | 2.9 (2) | NR | NR |
| [52] Cree-Green 2016 | Cohort | USA | NIH | MRI | 71 | 41 | 19 | 15 (13–16) | 35.2 (0.61) | 122 (76–158) | NR | 19 (100) | NR | 103 (96–111) | NR | 7.9 (6.6–14.6) si sirve | 1.63 (1.17–2.11) |
| [53] El-Tahawy 2016 | Case-control | Egypt | Rotterdam | US | 105 | 50 | 24 | 28.3 (5.4) | 29.8 (6.7) | 130.3 (18.9) | 24 (48) | NR | 2 (4) | NR | 3.42 (0.87) | 10.98 (5.73) | 2.79 (0.87) |
| [24] Layegh 2016 | Cross-sectional | Iran | Rotterdam | US | 115 | 115 | 22 | 24.5 (5.4) | NR | NR | 27 (23.4) | 70 (60.8) | NR | NR | NR | NR | NR |
| [25] Macut 2016 | Cross-sectional | Serbia | Rotterdam | NAFLD fatty liver score | 725 | 600 | 303 | 25.6 (25.1–26.1) | 30.7 (30.1–31.3) | NR | NR | Nr | NR | 91.8 (90.6–92.9) | 3.8 (3.6–4) | 10 (9.4–10.6) | 2.6 (2.5–2.7) |
| [28] C Jie 2017 | Cross-sectional | China | Rotterdam | Ultrasound | 500 | 400 | 225 | 25.8 | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| [23] Kim 2017 | Case-control | Korea | Rotterdam | Ultrasound | 1167 | 275 | 15 | 30.4 (5.2) | 20.3 (2.1) | 66 (51–86) | 8 (2.9) | 0 | 0 | 75 (6) | 1.23 (1.09–1.37) | 3.7 (2.5–4.9) si sirve | 0.011 (0.008–0.014) |
| [30] Mehrabian 2017 | Cross-sectional | Iran | Rotterdam | US | 150 | 75 | 29 | NR | 24.7 (1.7) | 149.7 (37.4) | 25 (33.3) | NR | NR | 86.5 (8.8) | 4.7 (1.8) | NR | NR |
| [34] Petta 2017 | Case-control | Italy | Rotterdam | Hepatic steatosis index | 303 | 202 | 139 | 33.2 (5.5) | 25.7 (2.9) | 112.6 (44) | NR | 97 (48.2) | NR | 87.5 (22) | NR | NR | NR |
| [38] Sarkar 2018 | Cohort | USA | Rotterdam | TE | 303 | 303 | 35 | 28.2 (6.8) | 27.6 (10.7) | 81 (69) | NR | NR | NR | 83.82 (25.4) | 1.96 (2.4) | NR | 1.46 (1.79) |
| [45] Vassilatou 2018 | Case-control | Greece | Rotterdam | US | 290 | 145 | 78 | 27.5 (7.1) | 31.8 (6.9) | NR | NR | NR | NR | 93.7 (15.4) | 3.4 (2.1) | 11 (6.7) | 2.6 (0.9) |
| [46] Zhang 2018 | Case-control | China | Rotterdam | US | 253 | 188 | 84 | 27.1 (5.2) | 25.1 (3.2) | NR | NR | 74 (39.3) | NR | NR | 3.53 (0.64) | 10.82 (8.89–12.51) si sirve | 2.19 (0.88) |
| [41] Tantanavipas 2019 | Case-control | Thailand | Rotterdam | US | 63 | 42 | 22 | 27.7 (5.2) | 27.05 (6.5) | 104.3 (71.9) | 6 (14.2) | NR | NR | 84.81 (14.7) | 4.51 (4.97) | 8.05 (8.17) | 1.46 (0.74) |
| [27] Chakraborty 2020 | Cross-sectional | India | Rotterdam | US | 130 | 70 | 27 | 20.4 (2.4) | 25.1 (4.6) | 98.9 (37.6) | NR | NR | NR | NR | 4.23 (5.1) | NR | 2.09 (0.81) |
| [33] Oliveira de Lima 2020 | Case-series | Brasil | Rotterdam | US | 127 | 87 | 67 | 34.4 (5.7) | 34.7 (4.7) | 134 (49–373) | 43 (49.9) | 75 (86.2) | 11 (12.6) | 103 (67–128) no sirve | NR | NR | NR |
| [37] Salva-Pastor 2020 | Case-control | Mexico | Rotterdam | TE | 98 | 49 | 34 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| [39] Shengir 2020 | Cross-sectional | Canada | Rotterdam | TE | 101 | 101 | 40 | 36.3 | 27.6 (5) | NR | 97 (96) | 18 (17.8) | 101.1 (12.3) | 3.2 (2.9) | 3.6 (3.7) | 1.6 (0.7) | |
| [47] Zheng 2020 | Cross-sectional | China | Rotterdam | TE | 101 | 101 | 71 | NR | NR | NR | NR | 77 (76.2) | NR | NR | NR | NR | NR |
| [31] Bin Won 2021 | Cross-sectional | Korea | Rotterdam | US/TE/MRI | 586 | 586 | 51 | NR | 23.8 | NR | 92 (15.6) | 198 (33.7) | NR | NR | NR | NR | NR |
| [40] Siwatch 2021 | Cross-sectional | India | Rotterdam | US | 210 | 140 | 117 | 27.4 (3.5) | 25.6 (4.1) | NR | 71 (50.7) | 76 (54.3) | NR | NR | NR | NR | NR |
| [20] Arikan 2022 | Case-control | Turkey | Rotterdam | US | 141 | 83 | 37 | 24.7 (6.2) | 24.5 (4.7) | 107 (69.2) | NR | NR | NR | 83.6 (13.1) | NR | NR | NR |
| Part 1. Subgroup Analyses (Meta-Analysis of Proportions) | ||
|---|---|---|
| Subgroups | Pooled Prevalence (95% CI) | Heterogeneity (I2) |
| Study design | ||
| Case-Control (14 studies) | 41% (27–57) | 96.50% |
| Cross-Sectional (13 studies) | 48% (33–63) | 98.14% |
| Case-Series (6 studies) | 43% (20–60) | 94.54% |
| Cohort (3 studies) | 31% (8–60) | Not estimable |
| Region | ||
| Asia (15 studies) | 42% (28–56) | 98.15% |
| Europe (8 studies) | 48% (38–58) | 89.36% |
| Latin America (6 studies) | 55% (35–75) | 93.41% |
| USA and Canada (5 studies) | 30% (11–52) | 95.81% |
| Africa (2 studies) | 45% (36–54) | Not estimable |
| NAFLD assessment method | ||
| Ultrasound (26 studies) | 46% (38–55) | 95.61% |
| Transient elastography ( 4studies) | 46% (15–79) | 98.23% |
| CT-Scan (2 studies) | 3% (0–11) | Not estimable |
| Non-invasive blood biomarkers/Panels (2 studies) | 55% (52–59) | Not estimable |
| MRI (1 study) | 46% (31–53) | Not estimable |
| PCOS diagnostic criteria | ||
| Rotterdam (31 studies) | 45% (36–54) | 97.52% |
| NIH (4 studies) | 35% (17–57) | 89.09% |
| AE and PCOS society (1 study) | 24% (16–33) | Not estimable |
| Part 2. Risk Factors Meta-analysis | ||
| Factor | Odds Ratio (95% CI) | Heterogeneity, I2 |
| BMI (8 studies) | 1.35 (1.28–1.430) | 70.00% |
| Waist circumference (5 studies) | 1.016 (1.006–1.027) | 71.60% |
| ALT (4 studies) | 1.007 (1.001–1.014) | 81.40% |
| HOMA -IR (4 studies) | 1.21 (1.09–1.24) | 36.50% |
| HDL (2 studies) | 0.99 (0.96–1.03) | 72.10% |
| Free Androgen Index (2 studies) | 1.06 (1.03–1.1) | 82.30% |
| Hyperandrogenism (2 studies) | 10.3 (4.2–25.2) | 58.10% |
| Triglycerides (2 studies) | 1.002 (1.001–1.004) | 63.60% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manzano-Nunez, R.; Santana-Dominguez, M.; Rivera-Esteban, J.; Sabiote, C.; Sena, E.; Bañares, J.; Tacke, F.; Pericàs, J.M. Non-Alcoholic Fatty Liver Disease in Patients with Polycystic Ovary Syndrome: A Systematic Review, Meta-Analysis, and Meta-Regression. J. Clin. Med. 2023, 12, 856. https://doi.org/10.3390/jcm12030856
Manzano-Nunez R, Santana-Dominguez M, Rivera-Esteban J, Sabiote C, Sena E, Bañares J, Tacke F, Pericàs JM. Non-Alcoholic Fatty Liver Disease in Patients with Polycystic Ovary Syndrome: A Systematic Review, Meta-Analysis, and Meta-Regression. Journal of Clinical Medicine. 2023; 12(3):856. https://doi.org/10.3390/jcm12030856
Chicago/Turabian StyleManzano-Nunez, Ramiro, Marta Santana-Dominguez, Jesus Rivera-Esteban, Clara Sabiote, Elena Sena, Juan Bañares, Frank Tacke, and Juan M. Pericàs. 2023. "Non-Alcoholic Fatty Liver Disease in Patients with Polycystic Ovary Syndrome: A Systematic Review, Meta-Analysis, and Meta-Regression" Journal of Clinical Medicine 12, no. 3: 856. https://doi.org/10.3390/jcm12030856
APA StyleManzano-Nunez, R., Santana-Dominguez, M., Rivera-Esteban, J., Sabiote, C., Sena, E., Bañares, J., Tacke, F., & Pericàs, J. M. (2023). Non-Alcoholic Fatty Liver Disease in Patients with Polycystic Ovary Syndrome: A Systematic Review, Meta-Analysis, and Meta-Regression. Journal of Clinical Medicine, 12(3), 856. https://doi.org/10.3390/jcm12030856







