Gender Differences in the Association between Physical Activity and Mortality in Chronic Kidney Disease: Results from the National Health and Nutrition Examination Survey (2011–2018)
Abstract
1. Introduction
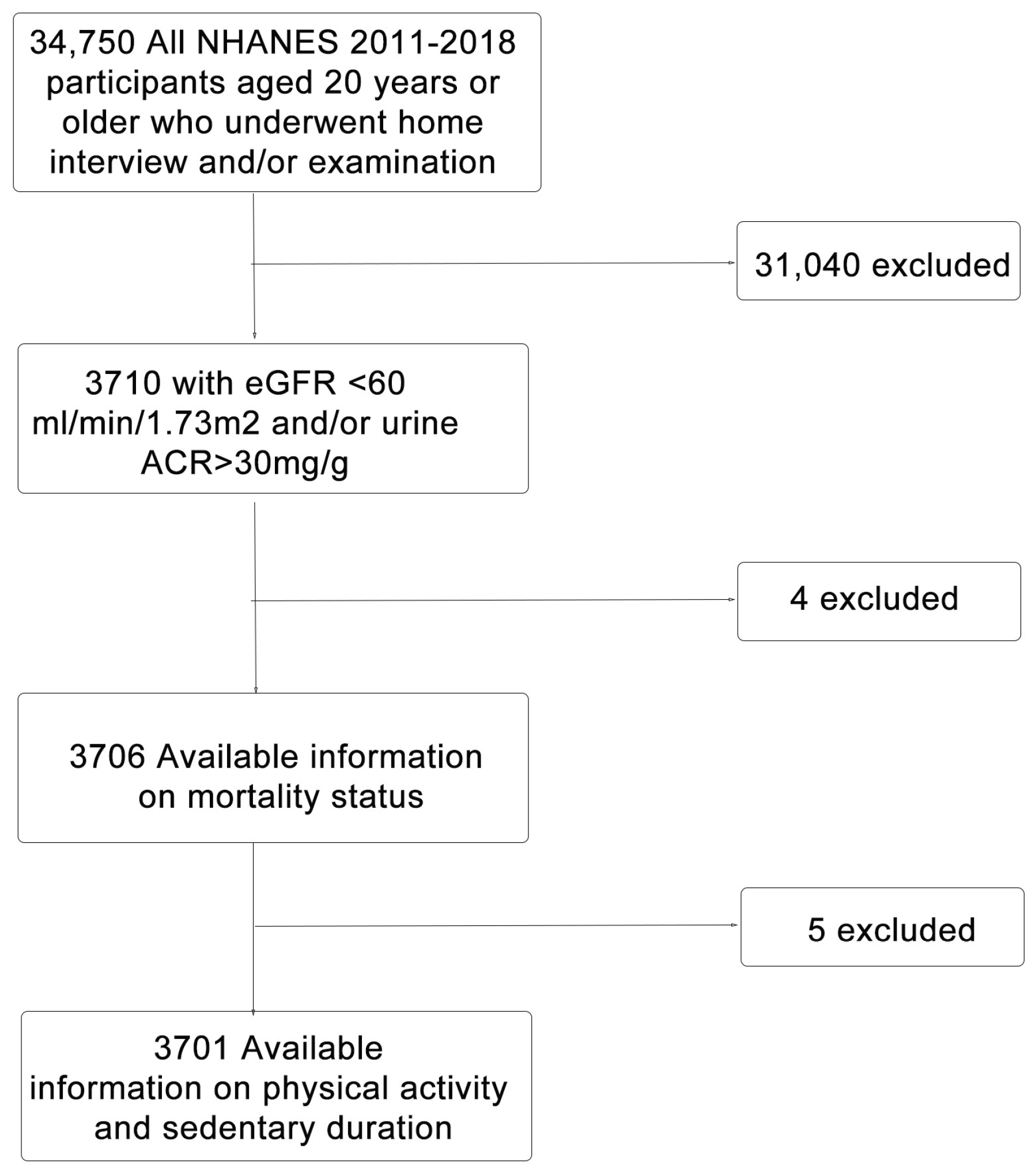
2. Materials & Methods
2.1. Data Source
2.2. Assessment of Renal Function
2.3. Assessment of Physical Activity
2.4. Follow-Up Data
2.5. Assessment of Demographic, Clinical, and Laboratory Measures
2.6. Statistical Analysis
3. Results
3.1. Participant Characteristics
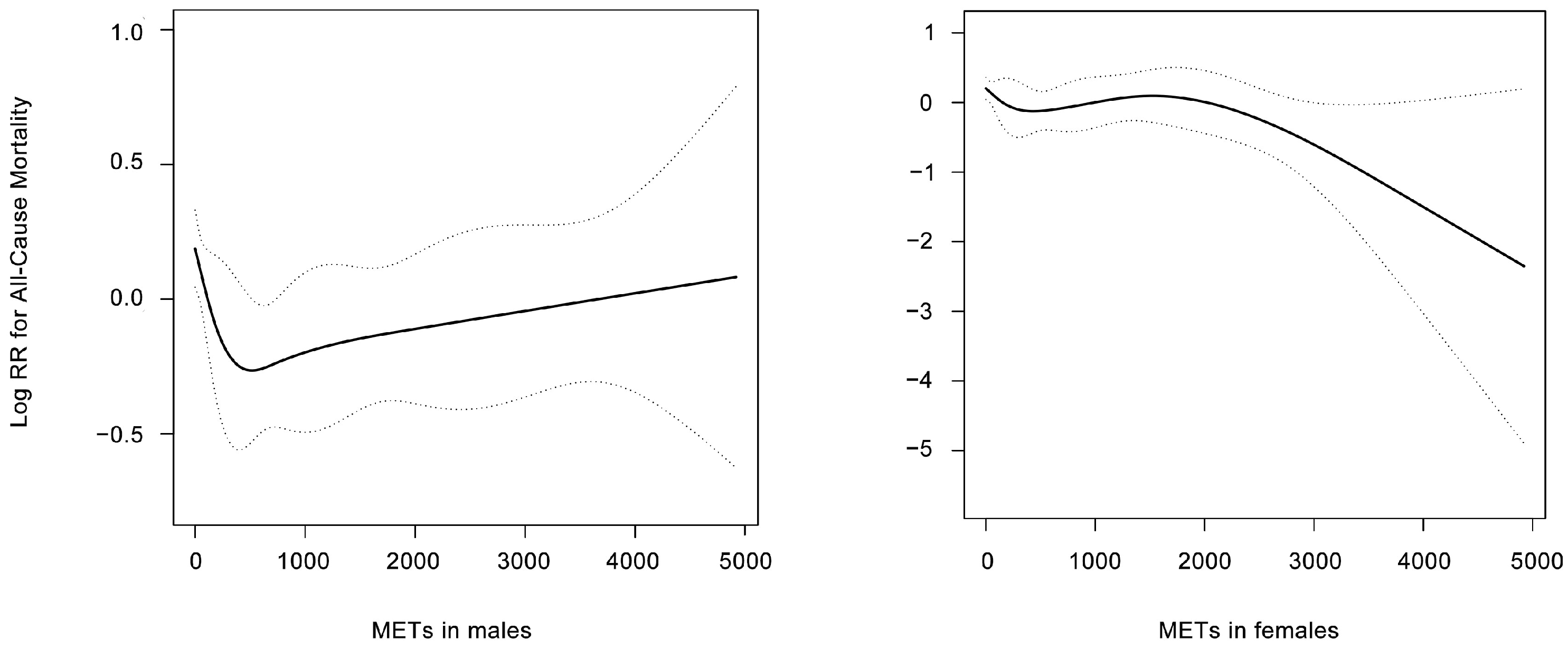
3.2. Association between Physical Activity and Mortality According to Gender
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fletcher, B.R.; Damery, S.; Aiyegbusi, O.L.; Anderson, N.; Calvert, M.; Cockwell, P.; Ferguson, J.; Horton, M.; Paap, M.C.S.; Sidey-Gibbons, C.; et al. Symptom burden and health-related quality of life in chronic kidney disease: A global systematic review and meta-analysis. PLoS Med. 2022, 19, e1003954. [Google Scholar] [CrossRef]
- Zelle, D.M.; Klaassen, G.; van Adrichem, E.; Bakker, S.J.; Corpeleijn, E.; Navis, G. Physical inactivity: A risk factor and target for intervention in renal care. Nat. Rev. Nephrol. 2017, 13, 152–168. [Google Scholar] [CrossRef]
- Rampersad, C.; Brar, R.; Connelly, K.; Komenda, P.; Rigatto, C.; Prasad, B.; Bohm, C.; Tangri, N. Association of Physical Activity and Poor Health Outcomes in Patients With Advanced CKD. Am. J. Kidney Dis. 2021, 78, 391–398. [Google Scholar] [CrossRef]
- Tentori, F.; Elder, S.J.; Thumma, J.; Pisoni, R.L.; Bommer, J.; Fissell, R.B.; Fukuhara, S.; Jadoul, M.; Keen, M.L.; Saran, R.; et al. Physical exercise among participants in the Dialysis Outcomes and Practice Patterns Study (DOPPS): Correlates and associated outcomes. Nephrol. Dial. Transplant. 2010, 25, 3050–3062. [Google Scholar] [CrossRef]
- Stevens, P.E.; Levin, A. Evaluation and management of chronic kidney disease: Synopsis of the kidney disease: Improving global outcomes 2012 clinical practice guideline. Ann. Intern. Med. 2013, 158, 825–830. [Google Scholar] [CrossRef]
- García, G.G.; Iyengar, A.; Kaze, F.; Kierans, C.; Padilla-Altamira, C.; Luyckx, V.A. Sex and gender differences in chronic kidney disease and access to care around the globe. Semin. Nephrol. 2022, 42, 101–113. [Google Scholar] [CrossRef]
- Melsom, T.; Norvik, J.V.; Enoksen, I.T.; Stefansson, V.; Mathisen, U.D.; Fuskevåg, O.M.; Jenssen, T.G.; Solbu, M.D.; Eriksen, B.O. Sex Differences in Age-Related Loss of Kidney Function. J. Am. Soc. Nephrol. 2022, 33, 1891–1902. [Google Scholar] [CrossRef]
- Kirkman, D.L.; Ramick, M.G.; Muth, B.J.; Stock, J.M.; Townsend, R.R.; Edwards, D.G. Sex differences in microvascular function and arterial hemodynamics in nondialysis chronic kidney disease. Am. J. Physiol. Heart Circ. Physiol. 2022, 323, H1130–H1136. [Google Scholar] [CrossRef]
- Baylis, C. Sexual Dimorphism of the Aging Kidney: Role of Nitric Oxide Deficiency. Physiology 2008, 23, 142–150. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A New Equation to Estimate Glomerular Filtration Rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Andrassy, K.M. Comments on ‘KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease’. Kidney Int. 2013, 84, 622–623. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, T.; Bull, F. Development of the World Health Organization Global Physical Activity Questionnaire (GPAQ). J. Public Health 2006, 14, 66–70. [Google Scholar] [CrossRef]
- Ainsworth, B.E.; Haskell, W.L.; Whitt, M.C.; Irwin, M.L.; Swartz, A.M.; Strath, S.J.; O’Brien, W.L.; Bassett, D.R.; Schmitz, K.H.; Emplaincourt, P.O.; et al. Compendium of physical activities: An update of activity codes and MET intensities. Med. Sci. Sport Exerc. 2000, 32 (Suppl. 9), S498–S504. [Google Scholar] [CrossRef] [PubMed]
- Kalyani, R.R.; Saudek, C.D.; Brancati, F.L.; Selvin, E. Association of diabetes, comorbidities, and A1C with functional disability in older adults: Results from the National Health and Nutrition Examination Survey (NHANES), 1999–2006. Diabetes Care 2010, 33, 1055–1060. [Google Scholar] [CrossRef] [PubMed]
- Beddhu, S.; Baird, B.C.; Zitterkoph, J.; Neilson, J.; Greene, T. Physical Activity and Mortality in Chronic Kidney Disease (NHANES III). Clin. J. Am. Soc. Nephrol. 2009, 4, 1901–1906. [Google Scholar] [CrossRef]
- Watkinson, C.; van Sluijs, E.M.; Sutton, S.; Hardeman, W.; Corder, K.; Griffin, S.J. Overestimation of physical activity level is associated with lower BMI: A cross-sectional analysis. Int. J. Behav. Nutr. Phys. Act. 2010, 7, 68. [Google Scholar] [CrossRef] [PubMed]
- Mandrup, C.M.; Egelund, J.; Nyberg, M.; Slingsby, M.H.; Andersen, C.B.; Løgstrup, S.; Bangsbo, J.; Suetta, C.; Stallknecht, B.M.; Hellsten, Y. Effects of high-intensity training on cardiovascular risk factors in premenopausal and postmenopausal women. Am. J. Obstet. Gynecol. 2017, 216, 384.e1–384.e11. [Google Scholar] [CrossRef]
- Asikainen, T.M.; Kukkonen-Harjula, K.; Miilunpalo, S. Exercise for health for early postmenopausal women: A systematic review of randomised controlled trials. Sports Med. 2004, 34, 753–778. [Google Scholar] [CrossRef] [PubMed]
- Miele, E.M.; Headley, S.A.; Germain, M.; Joubert, J.; Herrick, S.; Milch, C.; Evans, E.; Cornelius, A.; Brewer, B.; Taylor, B.; et al. High-density lipoprotein particle pattern and overall lipid responses to a short-term moderate-intensity aerobic exercise training intervention in patients with chronic kidney disease. Clin. Kidney J. 2017, 10, 524–531. [Google Scholar] [CrossRef]
- Molsted, S.; Eidemak, I.; Aadahl, M. Sex Difference in the Association between Physical Activity and All-Cause Mortality in Ambulatory Patients with Chronic Kidney Disease. Int. J. Environ. Res. Public Health 2021, 18, 3698. [Google Scholar] [CrossRef]
- Riesco, E.; Tessier, S.; Lacaille, M.; Pérusse, F.; Côté, M.; Després, J.P.; Bergeron, J.; Weisnagel, J.S.; Doré, J.; Mauriège, P. Impact of a moderate-intensity walking program on cardiometabolic risk markers in overweight to obese women: Is there any influence of menopause? Menopause 2013, 20, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Carr, M.C. The Emergence of the Metabolic Syndrome with Menopause. J. Clin. Endocrinol. Metab. 2003, 88, 2404–2411. [Google Scholar] [CrossRef] [PubMed]
- Nyberg, M.; Seidelin, K.; Andersen, T.R.; Overby, N.N.; Hellsten, Y.; Bangsbo, J. Biomarkers of vascular function in premenopausal and recent postmenopausal women of similar age: Effect of exercise training. Am. J. Physiol. Integr. Comp. Physiol. 2014, 306, R510–R517. [Google Scholar] [CrossRef] [PubMed]
- Parker, B.A.; Kalasky, M.J.; Proctor, D.N. Evidence for sex differences in cardiovascular aging and adaptive responses to physical activity. Eur. J. Appl. Physiol. 2010, 110, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Moreau, K.L.; Stauffer, B.; Kohrt, W.M.; Seals, D.R. Essential Role of Estrogen for Improvements in Vascular Endothelial Function With Endurance Exercise in Postmenopausal Women. J. Clin. Endocrinol. Metab. 2013, 98, 4507–4515. [Google Scholar] [CrossRef] [PubMed]
- Shiels, M.S.; Rohrmann, S.; Menke, A.; Selvin, E.; Crespo, C.J.; Rifai, N.; Dobs, A.; Feinleib, M.; Guallar, E.; Platz, E.A. Association of cigarette smoking, alcohol consumption, and physical activity with sex steroid hormone levels in US men. Cancer Causes Control. 2009, 20, 877–886. [Google Scholar] [CrossRef]
- Bertone-Johnson, E.R.; Tworoger, S.S.; Hankinson, S.E. Recreational Physical Activity and Steroid Hormone Levels in Postmenopausal Women. Am. J. Epidemiol. 2009, 170, 1095–1104. [Google Scholar] [CrossRef]
- Sevre, K.; Lefrandt, J.D.; Nordby, G.; Os, I.; Mulder, M.; Gans, R.O.; Rostrup, M.; Smit, A.J. Autonomic function in hypertensive and normotensive subjects: The importance of gender. Hypertension 2001, 37, 1351–1356. [Google Scholar] [CrossRef]
- Narkiewicz, K.; Phillips, B.G.; Kato, M.; Hering, D.; Bieniaszewski, L.; Somers, V.K. Gender-Selective Interaction Between Aging, Blood Pressure, and Sympathetic Nerve Activity. Hypertension 2005, 45, 522–525. [Google Scholar] [CrossRef]
- Okura, H.; Takada, Y.; Yamabe, A.; Kubo, T.; Asawa, K.; Ozaki, T.; Yamagishi, H.; Toda, I.; Yoshiyama, M.; Yoshikawa, J.; et al. Age- and gender-specific changes in the left ventricular relaxation: A Doppler echocar-diographic study in healthy individuals. Circ. Cardiovasc. Imaging 2009, 2, 41–46. [Google Scholar] [CrossRef]
- Kirkman, D.L.; Bohmke, N.; Carbone, S.; Garten, R.S.; Rodriguez-Miguelez, P.; Franco, R.L.; Kidd, J.M.; Abbate, A. Exercise intolerance in kidney diseases: Physiological contributors and therapeutic strategies. Am. J. Physiol. Physiol. 2021, 320, F161–F173. [Google Scholar] [CrossRef] [PubMed]
- Sprick, J.D.; Downey, R.M.; Morison, D.L.; Fonkoue, I.; Li, Y.; Dacosta, D.; Rapista, D.; Park, J. Functional sympatholysis is impaired in end-stage renal disease. Am. J. Physiol. Integr. Comp. Physiol. 2019, 316, R504–R511. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Total n = 3701 | Physical Activity MET-Min/Week | p Value | |||
|---|---|---|---|---|---|---|
| Inactive 0 (n = 1466) | Low-Active >0, ≤600 (n = 612) | Highly Active >600, ≤1500 (n = 501) | Extremely Highly Active >1500 (n = 1122) | |||
| Demographics | ||||||
| Age, years | 60.8 (0.4) | 66.1 (0.6) | 61.8 (0.7) | 60.6 (1.1) | 54.6 (0.8) | <0.001 |
| Gender | <0.001 | |||||
| Male | 43.2 | 38.1 | 36.5 | 35.5 | 55.5 | |
| Female | 56.8 | 61.9 | 63.5 | 64.5 | 44.5 | |
| Race | <0.001 | |||||
| Mexican American | 7.3 | 8.0 | 6.3 | 4.3 | 8.4 | |
| Other Hispanic | 5.0 | 5.6 | 3.6 | 3.7 | 5.5 | |
| Non-Hispanic White | 67.6 | 67.8 | 64.6 | 72.3 | 66.8 | |
| Non-Hispanic Black | 12.2 | 11.8 | 14.1 | 11.5 | 12.1 | |
| Other race | 7.9 | 6.7 | 11.2 | 8.2 | 7.2 | |
| PIR | 2.7 (0.1) | 2.4 (0.1) | 2.8 (0.1) | 3.1 (0.1) | 2.7 (0.1) | <0.001 |
| Education | <0.001 | |||||
| <High school | 19.8 | 26.8 | 16.5 | 13.6 | 16.6 | |
| High school | 25.2 | 26.0 | 24.6 | 25.7 | 24.6 | |
| >High school | 54.8 | 46.9 | 59.0 | 60.7 | 58.6 | |
| Marital status | <0.001 | |||||
| Married/living with partner | 57.0 | 53.1 | 57.2 | 60.0 | 59.7 | |
| Divorced/separated/widowed | 31.6 | 38.2 | 32.1 | 30.6 | 24.6 | |
| Never married | 11.3 | 8.5 | 10.7 | 9.3 | 15.7 | |
| Smoking | 0.071 | |||||
| Never smoker | 50.6 | 47.2 | 54.1 | 52.0 | 51.6 | |
| Ex-smoker | 33.7 | 37.0 | 32.2 | 35.1 | 30.5 | |
| Current smoker | 15.7 | 15.8 | 13.7 | 12.9 | 17.9 | |
| BMI, kg/m2 | 30.5 (0.2) | 31.2 (0.3) | 30.7 (0.4) | 29.8 (0.5) | 30.0 (0.3) | 0.018 |
| Sedentary activity, min/day | 405.4 (5.6) | 471.3 (9.3) | 414.7 (10.4) | 405.9 (12.5) | 332.2 (7.4) | <0.001 |
| CKD awareness | 11.0 | 13.5 | 11.7 | 11.1 | 8.0 | 0.036 |
| CKD | <0.001 | |||||
| Stage 1 or 2 | 51.8 | 43.2 | 48.9 | 48.9 | 63.4 | |
| Stage 3 | 44.1 | 49.9 | 47.6 | 48.0 | 34.6 | |
| Stage 4 | 2.9 | 4.9 | 1.9 | 2.0 | 1.5 | |
| Stage 5 | 1.3 | 2.0 | 1.6 | 1.1 | 0.5 | |
| Comorbidity | ||||||
| Diabetes | 31.6 | 39.5 | 31.3 | 31.3 | 23.4 | <0.001 |
| Hypertension | 60.6 | 70.1 | 64.2 | 60.3 | 48.8 | <0.001 |
| Dyslipidaemia | 67.4 | 70.1 | 70.0 | 64.9 | 64.3 | 0.057 |
| Congestive heart failure | 9.4 | 14.7 | 7.9 | 9.3 | 4.7 | <0.001 |
| Cardiovascular disease | ||||||
| Coronary artery disease | 10.3 | 13.1 | 10.2 | 10.6 | 7.2 | 0.002 |
| Angina pectoris | 6.0 | 7.4 | 6.5 | 6.4 | 3.9 | 0.008 |
| Myocardial infraction | 9.3 | 12.0 | 7.4 | 10.1 | 6.8 | 0.003 |
| Stroke | 8.5 | 14.3 | 6.8 | 5.6 | 4.3 | 0.001 |
| Cancer | 19.6 | 23.0 | 20.8 | 21.0 | 14.5 | 0.002 |
| Biochemical data | ||||||
| Systolic blood pressure, mmHg | 119.4 (0.5) | 120.8 (0.8) | 118.6 (1.1) | 118.1 (1.6) | 119.0 (0.9) | 0.287 |
| Diastolic blood pressure, mmHg | 66.2 (0.4) | 66.6 (0.5) | 66.8 (0.9) | 65.8 (1.0) | 65.8 (0.5) | 0.601 |
| Hemoglobin, g/dL | 13.7 (0.0) | 13.4 (0.1) | 13.7 (0.1) | 13.6 (0.1) | 14.1 (0.1) | <0.001 |
| Albumin, g/L | 41.0 (0.2) | 40.7 (0.2) | 41.4 (0.2) | 41.5 (0.2) | 42.2 (0.2) | <0.001 |
| Total cholesterol, mg/dL | 191.7 (1.2) | 188.1 (1.5) | 190.9 (3.1) | 194.1 (2.9) | 194.5 (2.3) | 0.124 |
| Triglyceride, mg/dL | 136.1 (3.6) | 184.1(5.9) | 175.5 (7.3) | 160.5 (8.3) | 166.1 (5.3) | 0.086 |
| LDL-C, mg/dL | 107.7 (1.1) | 105.5 (1.8) | 106.6 (3.2) | 109.7 (3.0) | 109.6 (2.7) | 0.514 |
| HDL-C, mg/dL | 53.1 (0.5) | 51.5 (0.9) | 52.9 (1.0) | 55.2 (1.1) | 53.9 (0.8) | 0.074 |
| HbA1c | 6.2 (0.1) | 6.4 (0.1) | 6.1 (0.1) | 6.2 (0.1) | 6.1 (0.1) | <0.001 |
| Physical Activity, Met (Mins/Week) | ||||
|---|---|---|---|---|
| 0 (n = 1461) | >0, ≤600 (n = 607) | >600, ≤1500 (n = 496) | >1500 (n = 1117) | |
| Male | ||||
| All-cause mortality | ||||
| Event, n | 206 | 57 | 38 | 84 |
| HR (95% CI) | Ref | 0.67 (0.48, 0.93) | 0.60 (0.41, 0.88) | 0.65 (0.48, 0.88) |
| Cardiovascular mortality | ||||
| Event, n | 65 | 18 | 12 | 32 |
| HR (95% CI) | Ref | 0.81 (0.46, 1.44) | 0.68 (0.34, 1.37) | 0.93 (0.55, 1.55) |
| Non-cardiovascular mortality | ||||
| Event, n | 141 | 39 | 26 | 52 |
| HR (95% CI) | Ref | 0.62 (0.41, 0.92) | 0.57 (0.36, 0.90) | 0.56 (0.39, 0.81) |
| Female | ||||
| All-cause mortality | ||||
| Event, n | 205 | 41 | 29 | 34 |
| HR (95% CI) | Ref | 0.73 (0.50, 1.05) | 0.81 (0.54, 1.23) | 0.59 (0.39, 0.89) |
| Cardiovascular mortality | ||||
| Event, n | 76 | 8 | 8 | 7 |
| HR (95% CI) | Ref | 0.50 (0.23, 1.06) | 0.63 (0.29, 1.37) | 0.40 (0.17, 0.96) |
| Non-cardiovascular mortality | ||||
| Event, n | 129 | 33 | 21 | 27 |
| HR (95% CI) | Ref | 0.85 (0.55, 1.30) | 0.87 (0.53, 1.43) | 0.67 (0.42, 1.07) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, W.; Han, M.; Xu, G. Gender Differences in the Association between Physical Activity and Mortality in Chronic Kidney Disease: Results from the National Health and Nutrition Examination Survey (2011–2018). J. Clin. Med. 2023, 12, 779. https://doi.org/10.3390/jcm12030779
Peng W, Han M, Xu G. Gender Differences in the Association between Physical Activity and Mortality in Chronic Kidney Disease: Results from the National Health and Nutrition Examination Survey (2011–2018). Journal of Clinical Medicine. 2023; 12(3):779. https://doi.org/10.3390/jcm12030779
Chicago/Turabian StylePeng, Wei, Min Han, and Gang Xu. 2023. "Gender Differences in the Association between Physical Activity and Mortality in Chronic Kidney Disease: Results from the National Health and Nutrition Examination Survey (2011–2018)" Journal of Clinical Medicine 12, no. 3: 779. https://doi.org/10.3390/jcm12030779
APA StylePeng, W., Han, M., & Xu, G. (2023). Gender Differences in the Association between Physical Activity and Mortality in Chronic Kidney Disease: Results from the National Health and Nutrition Examination Survey (2011–2018). Journal of Clinical Medicine, 12(3), 779. https://doi.org/10.3390/jcm12030779





