Effect of Neuro-Adaptive Electrostimulation Therapy versus Sham for Refractory Urge Urinary Incontinence Due to Overactive Bladder: A Randomized Single-Blinded Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Outcomes Evaluated
2.3. Procedures and Intervention
2.4. Statistical Analysis
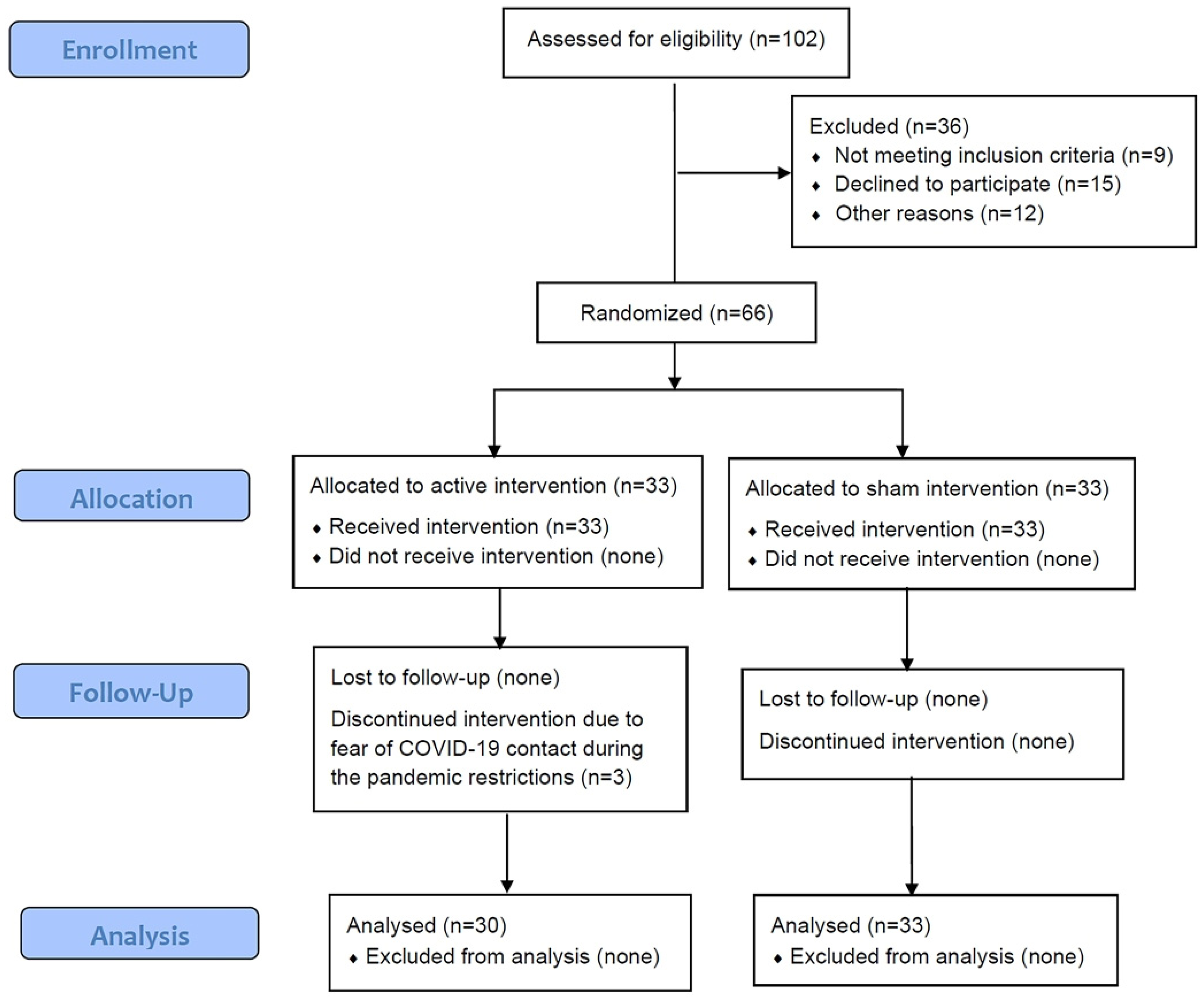
3. Results
3.1. Clinical Data
3.2. Response to Treatment
3.3. Self-Administered Questionnaires
3.4. Self-Assessed Satisfaction with the Procedure
3.5. Prediction of 6-Months Response
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bo, K.; Frawley, H.C.; Haylen, B.T.; Abramov, Y.; Almeida, F.G.; Berghmans, B.; Bortolini, M.; Dumoulin, C.; Gomes, M.; McClurg, D.; et al. An International Urogynecological Association (IUGA) /International Continence Society (ICS) joint report on the terminology for the conservative and nonpharmacological management of female pelvic floor dysfunction. Int. Urogynecol. J. 2017, 28, 191–213. [Google Scholar] [CrossRef] [PubMed]
- Teleman, P.M.; Lidfeldt, J.; Nerbrand, C.; Samsioe, G.; Mattiasson, A. Overactive bladder: Prevalence, risk factors and relation to stress incontinence in middle-aged women. BJOG 2004, 111, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Gormley, E.A.; Lightner, D.J.; Faraday, M.; Vasavada, S.P. Diagnosis and treatment of overactive bladder (Non neurogenic) in adults: AUA/SUFU guideline amendment. J. Urol. 2015, 193, 1572–1580. [Google Scholar] [CrossRef]
- Khullar, V.; Amarenco, G.; Angulo, J.C.; Cambronero, J.; Høye, K.; Milsom, I.; Radziszewski, P.; Rechberger, T.; Boerrigter, P.; Drogendijk, T.; et al. Efficacy and tolerability of mirabegron, a β(3)-adrenoceptor agonist, in patients with overactive bladder: Results from a randomised European-Australian phase 3 trial. Eur. Urol. 2013, 63, 283–295. [Google Scholar] [CrossRef]
- Nitti, V.W.; Khullar, V.; Kerrebroeck, P.; Herschorn, S.; Cambronero, J.; Angulo, J.C.; Blauwet, M.B.; Dorrepaal, C.; Siddiqui, E.; Martin, N.E. Mirabegron for the treatment of overactive bladder: A prespecified pooled efficacy analysis and pooled safety analysis of three randomised, double-blind, placebo-controlled, phase III studies. Int. J. Clin. Pract. 2013, 67, 619–632. [Google Scholar] [CrossRef]
- Khullar, V.; Cambronero, J.; Angulo, J.C.; Wooning, M.; Blauwet, M.B.; Dorrepaal, C.; Martin, N.E. Efficacy of mirabegron in patients with and without prior antimuscarinic therapy for overactive bladder: A post hoc analysis of a randomized European-Australian Phase 3 trial. BMC Urol. 2013, 13, 45. [Google Scholar] [CrossRef]
- Castro, D.; Espuña, M.; Prieto, M.; Badia, X. Prevalence of overactive bladder in Spain: A population-based study. Arch. Esp. Urol. 2005, 58, 131–138. [Google Scholar]
- Angulo, J.; Calderín, M.; Fernández, Y.; González, M.; Gómez, E.; Herreros, M.; Peñasco, P.; Zapatero, M.; Dorado, J. Comparative study of the B-SAQ, OAB-V8 and OAB-V3 questionnaires as screening tools for overactive bladders in clinical practice. Actas Urol. Esp. 2017, 41, 383–390. [Google Scholar] [CrossRef]
- Nazir, J.; Hakimi, Z.; Guelfucci, F.; Khemiri, A.; Fatoye, F.; Blázquez, A.M.M.; González, M.H. A retrospective study of treatment persistence and adherence to mirabegron versus antimuscarinics, for the treatment of overactive bladder in Spain. BMC Urol. 2018, 18, 76–86. [Google Scholar] [CrossRef]
- Rai, B.P.; Cody, J.D.; Alhasso, A.; Stewart, L. Anticholinergic drugs versus non-drug active therapies for non-neurogenic overactive bladder syndrome in adults. Cochrane Database Syst. Rev. 2012, 12, CD003193. [Google Scholar] [CrossRef]
- Abrams, P.; Andersson, K.E.; Birder, L.; Brubaker, L.; Cardozo, L.; Chapple, C.; Cottenden, A.; Davila, W.; de Ridder, D.; Dmochowski, R.; et al. Fourth international consultation on incontinence recommendations of the International Scientific Committee: Evaluation and treatment of urinary incontinence, pelvic organ prolapse and fecal incontinence. Neurourol. Urodyn. 2010, 29, 213–240. [Google Scholar] [CrossRef]
- Tutolo, M.; Ammirati, E.; Van der Aa, F. What is new in neuromodulation for overactive bladder. Eur. Urol. Focus 2018, 4, 49–53. [Google Scholar] [CrossRef]
- Siegel, S.; Noblett, K.; Mangel, J.; Griebling, T.L.; Sutherland, S.E.; Bird, E.T.; Comiter, C.; Culkin, D.; Bennett, J.; Zylstra, S.; et al. Results of a prospective, randomized, multicenter study evaluating sacral neuromodulaton with InterStim therapy compared to standard medical therapy al 6 months in subjects with mild symptoms of overactive bladder. Neurourol. Urodyn. 2015, 34, 224–230. [Google Scholar] [CrossRef]
- Amundsen, C.L.; Richter, H.E.; Menefee, S.A.; Komesu, Y.M.; Arya, L.A.; Gregory, W.T.; Myers, D.L.; Zyczynski, H.M.; Vasavada, S.; Nolen, T.L.; et al. OnabotulinumtoxinA vs sacral neuromodulation on refractory urgency urinary incontinence in women. JAMA 2016, 182, 1055–1061. [Google Scholar] [CrossRef]
- Weil, E.H.; Ruiz Cerda, J.L.; Eerdmans, P.H.; Janknegt, R.A.; Bemelmans, B.L.; van Kerrebroeck, P.E. Sacral root neuromodulation in the treatment of refractary uriinary urge incontinence: A prospective randomized clinical trial. Eur. Urol. 2000, 37, 161–171. [Google Scholar] [CrossRef]
- Hassouna, M.M.; Siegel, S.W.; Ànÿeholt, A.L.; Elhilali, M.M.; van Kerrebroeck, P.E.; DAS, A.K.; Gajewski, J.B.; Janknegt, R.A.; Rivas, D.A.; Dijkema, H.; et al. Sacral neuromodulation in the treatment of urgency frequency symptoms: A multicenter study on efficacy and safety. J. Urol. 2000, 163, 1849–1854. [Google Scholar] [CrossRef]
- Raju, R.; Linder, B.J. Evaluation and treatment of overactive bladder in Women. Mayo Clin. Proc. 2020, 95, 370–377. [Google Scholar] [CrossRef]
- Peters, K.M.; MacDiarmid, S.A.; Wooldridge, L.S.; Leong, F.C.; Shobeiri, S.A.; Rovner, E.S.; Siegel, S.W.; Tate, S.B.; Jarnagin, B.K.; Rosenblatt, P.L.; et al. Randomized trial of percutaneous tibial nerve stimulation versus extended -release tolterodine: Results from the overactive bladder innovative therapy trial. J. Urol. 2009, 182, 1055–1061. [Google Scholar] [CrossRef]
- Peters, K.M.; Carrico, D.; Perez-Marrero, R.A.; Khan, A.U.; Wooldridge, L.S.; Davis, G.L.; MacDiarmid, S.A. Randomized trial of percutaneous tibial nerve stimulation versus sham efficacy in the treatment of overactive bladder syndrome from SumiT trial. J. Urol. 2010, 183, 1438–1443. [Google Scholar] [CrossRef]
- Finazzi-Agro, E.; Petta, F.; Sciobica, F.; Pasquqletti, P.; Musco, S.; Bove, P. Percutaneous tibial nerve stimulation effects on detrusor overactivity incontinence are not due to a placebo effect: A randomized, doubled-blind, placebo cotrolled trial. J. Urol. 2010, 184, 2001–2006. [Google Scholar] [CrossRef]
- Deaf, M.A.; Eldosoky, M.A.A.; El-Garhy, A.M.; Gomma, H.W.; El-Azab, A.S. Parasympathetic nervous signal damping using the adaptive neuro-fuzzy inference system method to control overactive bladder. J. Clin. Eng. 2015, 40, 197–201. [Google Scholar] [CrossRef]
- Avery, K.; Donovan, J.; Peters, T.J.; Shaw, C.; Gotoh, M.; Abrams, P. ICIQ: A brief and robust measure for evaluating symptoms and impact of urinary incontinence. Neurourol. Urodyn. 2004, 23, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Pons, M.E.; Alvarez, P.R.; Clota, M.P. Validation of the Spanish version of the International Consultation on Incontinence Questionnaire-Short Form. A questionnaire for assessing the urinary incontinence. Med. Clin. 2004, 122, 288–292. [Google Scholar] [CrossRef]
- Sandvik, H.; Espuña, M.; Hunskaar, S. Validity of the incontinence severity index: Comparison with pad-weighing tests. Int. Urogynecol. J. Pelvic. Floor Dysfunct 2006, 17, 520–524. [Google Scholar] [CrossRef]
- Ugurlucan, F.G.; Onal, M.; Asian, E.; Erkan, H.A.; Beji, N.K.; Yalcin, O. Comparison of the effects of electrical stimulation and posterior tibial nerve stimulation in the treatmentof overactive bladder síndrome. Gynecol. Obstet. Investig. 2013, 75, 46–52. [Google Scholar] [CrossRef]
- Komesu, Y.M.; Amundsen, C.L.; Richter, H.E.; Erickson, S.W.; Ackenbom, M.F.; Andy, U.U.; Sung, V.W.; Albo, M.; Gregory, W.T.; Paraiso, M.F.; et al. Refractory urgency urinary incontinence treatment in women: Impact of age on outcomes and complications. Am. J. Obs. Gynecol. 2018, 218, e1–e111. [Google Scholar] [CrossRef] [PubMed]
- Krusche-Mandl, I.; Kaider, A.; Starlinger, J.; Preschitz, M.; Schuster, R.; Kefurt, R.; Marhofer, P.; Kasparek, M.; Hajdu, S.; Sator-Katzenschlager, S. Implementation of Electrical Auricular Acupuncture and Low Frequency Modulated Electric Current Therapy in Pain Management of Patients with Knee Osteoarthritis: A Randomized Pilot Trial. J. Clin. Med. 2019, 8, 1229. [Google Scholar] [CrossRef]
- Udina-Cortés, C.; Fernández-Carnero, J.; Romano, A.A.; Cuenca-Zaldívar, J.N.; Villafañe, J.H.; Castro-Marrero, J.; Alguacil-Diego, I.M. Effects of neuro-adaptive electrostimulation therapy on pain and disability in fibromyalgia: A prospective, randomized, double-blind study. Medicine 2020, 99, e23785. [Google Scholar] [CrossRef]
- Michel-Cherqui, M.; Guirimand, A.; Szekely, B.; Kennel, T.; Fischler, M.; Le Guen, M. Effects of a Single Application of ScenarTM, a Low-Frequency Modulated Electric Current Therapy, for Pain Relief in Patients with Low Back and Neck Pain: A Randomized Single Blinded Trial. J. Clin. Med. 2021, 10, 5570. [Google Scholar] [CrossRef]

| Variables | Active (n = 33) | Sham (n = 33) | p Value |
|---|---|---|---|
| Age (year) | 61.4 ± 11.4 | 60.9 ± 11.9 | 0.89 |
| Weight (kg) | 73.3 ± 14.5 | 69.7 ± 11.9 | 0.41 |
| Height (cm) | 160.1 ± 6.1 | 161.7 ± 4.9 | 0.21 |
| Body mass index | 28.5 ± 5.3 | 26.4 ± 3.8 | 0.08 |
| Previous pelvic surgery, n (%) | 8 (24) | 8 (24) | 0.99 |
| Stress incontinence 1, n (%) | 4 (12) | 7 (21) | 0.5 |
| Urge incontinence episodes 2, n | 1.7 ± 1.7 | 1.6 ± 1.9 | 0.45 |
| Urgency episodes 2, n | 2.1 ± 1.9 | 2.3 ± 1.9 | 0.56 |
| Nocturia 3, n | 1.8 ± 1.5 | 2 ± 1.2 | 0.87 |
| ICIQ-SF score | 14.4 ± 3 | 14.9 ± 3.2 | 0.68 |
| Sandvik score | 5.2 ± 3.1 | 4.6 ± 2.7 | 0.45 |
| Outcome | Active NAT Group, n (%) | Sham Group, n (%) | ||||
|---|---|---|---|---|---|---|
| Month 1 | Month 3 | Month 6 | Month 1 | Month 3 | Month 6 | |
| Response # | 23 (70) * | 19 (58) ** | 18 (55) *** | 16 (48) * | 14 (42) ** | 11 (33) *** |
| Complete Partial | 20 | 15 | 9 | 16 | 11 | 8 |
| 3 | 4 | 9 | - | 3 | 3 | |
| Failure | 7 (21) | 11 (33) | 12 (36) | 17 (52) | 19 (58) | 22 (67) |
| Abandoned | 3 (9) | 3 (9) | 3 (9) | - | - | - |
| Questionnaire | Treatment Group | Baseline | Month 1 | Month 3 | Month 6 | Dif. * (95% CI) |
|---|---|---|---|---|---|---|
| ICIQ-SF (0–21) | Active | 14.4 ± 3 | 3.5 ± 5.7 | 5.7 ± 6.4 | 7.5 ± 6.1 | 2.2 (0.6, 5.0) |
| Sham | 14.9 ± 3.2 | 7.7 ± 6.7 | 7.9 ± 6.7 | 9.5 ± 6.2 | ||
| Sandvik (0–12) | Active | 5.2 ± 3.1 | 1.6 ± 2.7 | 2.8 ± 3.3 | 3.8 ± 3.2 | 0.6 (−0.9, 2.1) |
| Sham | 4.6 ± 2.7 | 3.5 ± 3.3 | 3.6 ± 3.3 | 4.3 ± 3.2 | ||
| Satisfaction (0–10) | Active | - | 8.4 ± 2.5 | 7.2 ± 3.1 | 6.5 ± 2.8 | −1 (−2.2, 0.2) |
| Sham | - | 5.8 ± 3.1 | 6.1 ± 3.2 | 5.5 ± 2.8 |
| Questionnaire | Group Effect | Time Effect | Group–Time Effect |
|---|---|---|---|
| ICIQ-SF (0–21) | 0.002 | <0.001 | 0.062 |
| Sandvik (0–12) | 0.072 | <0.001 | 0.049 |
| Satisfaction (0–10) | <0.001 | 0.016 | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zapico, Á.; Ercilla, J.; Angulo, J.C.; Pérez, V.; Cuenca, J.N.; Barreira-Hernández, D.; Udina-Cortés, C. Effect of Neuro-Adaptive Electrostimulation Therapy versus Sham for Refractory Urge Urinary Incontinence Due to Overactive Bladder: A Randomized Single-Blinded Trial. J. Clin. Med. 2023, 12, 759. https://doi.org/10.3390/jcm12030759
Zapico Á, Ercilla J, Angulo JC, Pérez V, Cuenca JN, Barreira-Hernández D, Udina-Cortés C. Effect of Neuro-Adaptive Electrostimulation Therapy versus Sham for Refractory Urge Urinary Incontinence Due to Overactive Bladder: A Randomized Single-Blinded Trial. Journal of Clinical Medicine. 2023; 12(3):759. https://doi.org/10.3390/jcm12030759
Chicago/Turabian StyleZapico, Álvaro, Julia Ercilla, Javier C. Angulo, Vicente Pérez, Juan Nicolás Cuenca, Diana Barreira-Hernández, and Carlos Udina-Cortés. 2023. "Effect of Neuro-Adaptive Electrostimulation Therapy versus Sham for Refractory Urge Urinary Incontinence Due to Overactive Bladder: A Randomized Single-Blinded Trial" Journal of Clinical Medicine 12, no. 3: 759. https://doi.org/10.3390/jcm12030759
APA StyleZapico, Á., Ercilla, J., Angulo, J. C., Pérez, V., Cuenca, J. N., Barreira-Hernández, D., & Udina-Cortés, C. (2023). Effect of Neuro-Adaptive Electrostimulation Therapy versus Sham for Refractory Urge Urinary Incontinence Due to Overactive Bladder: A Randomized Single-Blinded Trial. Journal of Clinical Medicine, 12(3), 759. https://doi.org/10.3390/jcm12030759








